Abstract
Purpose
To compare febrile neutropenia (FN) incidence and hospitalization among breast cancer patients on docetaxel with no granulocyte colony-stimulating factors (GCSF) primary prophylaxis (PP), 4/5-day PP, or 7-day PP.
Methods
We identified 3916 breast cancer patients using docetaxel-cyclophosphamide (TC), doxorubicin-cyclophosphamide then docetaxel (AC-T), fluorouracil-epirubicin-cyclophosphamide then docetaxel (FEC-T), docetaxel-carboplatin-trastuzumab (TJH), or docetaxel-doxorubicin-cyclophosphamide (TAC) from a hospital pharmacy dispensing database in Hong Kong between 2014 and 2016. Patients were offered GCSF within 5 days since administering docetaxel. Outcomes included FN incidence, time to first hospitalization, hospitalization rate, and duration.
Results
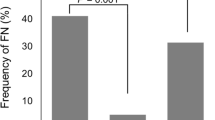
In TC regimen, FN incidence (with odds ratio, OR) of patients with no PP, 4/5-day PP, and 7-day PP was 21.69%, 7.95% (OR 0.31, p < 0.001), and 5.33% (OR 0.20, p < 0.001), respectively. In TJH regimen, FN incidence of patients with no PP, 4/5-day PP, and 7-day PP was 38.26%, 8.33% (OR 0.15, p < 0.001), and 8.57% (OR 0.15, p < 0.001), respectively. FN incidence of patients on AC-T regimen with no PP and 4/5-day PP was 20.93% and 6.84%, respectively (OR 0.28, p = 0.005); with FEC-T regimen, the incidence was 9.91% and 4.77%, respectively (OR 0.46, p = 0.035). Only 3.27% FN cases were not hospitalized. Mean (±standard deviation, SD) time to first hospitalization was 8.21 ± 2.44 days. Mean (±SD) duration of hospitalization for patients with no PP, 4/5-day PP, and 7-day PP was 4.66 ± 2.60, 4.37 ± 2.85, and 5.12 ± 2.97 days, respectively.
Conclusion
GCSF prophylaxis in breast cancer patients on docetaxel could reduce FN incidence and hospitalization. 4/5-day PP demonstrated similar efficacy to 7-day PP with superior saving benefits on healthcare expenditure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Febrile neutropenia is a common adverse drug reaction in breast cancer patients on docetaxel [1,2,3]. Patients on regimens including TC (docetaxel, cyclophosphamide), TJH (docetaxel, carboplatin, trastuzumab), and TAC (docetaxel, cyclophosphamide, doxorubicin) are in high risk of febrile neutropenia (> 20%) [2]. Risk of febrile neutropenia in AC-T (doxorubicin, cyclophosphamide then docetaxel) and FEC-T (fluorouracil, epirubicin, cyclophosphamide then docetaxel) is intermediate (10–20%) [2]. Patient factors including advanced age, higher cancer staging, poor performance status, and nutritional status also increase risk of febrile neutropenia [3].
Prophylactic medications including antibiotics and granulocyte colony-stimulating factors (GCSF) can reduce risk of febrile neutropenia. However, the use of antibiotics remains controversial due to potential increase in bacterial resistance and adverse effects [1, 4]. Administration of GCSF is more common to reduce risk of febrile neutropenia. In TC regimen, incidence of febrile neutropenia is only 1.2%–10.6% in population with GCSF [5,6,7,8,9,10,11]. The riskFootnote 1 of febrile neutropenia in FEC-T and TJH regimens with primary GCSF prophylaxis is also reduced to 5.3% and 9.7%, respectively [5, 9]. Incidence of febrile neutropenia in TAC patients is 16.4% with GCSF prophylaxis [12]. In patients aged > 65 years, GCSF prophylaxis could have a 4% absolute risk reduction [13]. In terms of hospitalization due to febrile neutropenia, GCSF prophylaxis reduces the risk by 5.1–5.8% [5].
However, the clinical outcome on GCSF varies among studies due to different study design, recruitment criteria, and types of GCSF used. The start date and duration of GCSF were different among cohorts, and GCSF prophylaxis was not offered to all cohorts. The selection criteria in offering GCSF had not been mentioned in these studies. Most studies adopted use of PEGylated GCSF, while some studies used GCSF for evaluation. Correlation between duration of GCSF prophylaxis versus incidence of febrile neutropenia and its hospitalization outcomes was not evaluated. In Hong Kong, different hospitals adopt different regimens of GCSF prophylaxis. Fixed duration of GCSF prophylaxis, either in 4/5 days or 7 days, is often initiated within 5 days after chemotherapy to reduce risk of febrile neutropenia. Some patients are not given prophylactic GCSF, either due to patient preference or local clinical policy. Consensus has not been reached on how GCSF prophylaxis shall be given to offer the greatest protection against febrile neutropenia with the best utilization of healthcare resources.
To bridge the study gap, this study aims at evaluating febrile neutropenia outcomes retrospectively in breast cancer patients on docetaxel. Outcomes are compared among patients with no primary prophylaxis (PP), 4/5 days PP, and 7 days PP. Primary outcome refers to the incidence of febrile neutropenia. Secondary outcomes include (1) febrile neutropenia-related hospitalization rate, (2) length of stay in hospital due to febrile neutropenia, and (3) time to first hospitalization due to febrile neutropenia.
Method
Study design and data source
This was a retrospective cohort study conducted in all public hospitals in Hong Kong with the Department of Clinical Oncology and Department of Pharmacy under the governance of Hospital Authority (Research Ethics Committee Reference Number: NTWC/CREC/17099). Cohorts on docetaxel-containing regimens were identified through Query Template System in Pharmacy Management System. A list of patients who had a dispensing record of docetaxel in any formulation was generated within the period from 1 January 2014 to 31 December 2016. Electronic patient record of each patient was reviewed to identify cohorts fitting the enrollment criteria below. Subjects with irretrievable electronic patient records were excluded from analysis.
Study population
Patients who had started chemotherapy containing docetaxel from 1 January 2014 to 31 December 2016 for the treatment of breast cancer were included in the study. Docetaxel-containing regimens used in treatment of breast cancer include TC, TJH, AC-T, TAC, and FEC-T (Table 1). Patients were excluded from study if they did not complete the regimen according to standard treatment protocol as described in Table 1. Patients aged ≥ 18 years old who have been initiated with docetaxel-containing chemotherapy regimens for treatment of histologically proven breast cancer were enrolled. Only subjects who have received (1) no PP, (2) 4/5-day PP, or (3) 7-day PP within 5 days after administration of docetaxel were included. Subjects with a history of receiving chemotherapy and/or radiotherapy due to other cancer diagnosis prior to the administration of docetaxel-containing regimen for breast cancer were excluded from the analysis.
Data collection
Patient demographics including age, gender, regimen used, history of receiving chemotherapy and/or radiotherapy due to other cancer diagnosis, baseline disease burden including TNM staging, ER/PR status, neoadjuvant/adjuvant chemotherapy, and performance status were documented. Regarding the details of chemotherapy and its supportive care protocol, for each docetaxel cycle the start date of chemotherapy, start date of GCSF, duration of GCSF, adjustment of dosage and treatment delay, if any, were also recorded. The reason(s) for dosage adjustment and delay of treatment, if any, were also documented. Regarding the clinical outcomes for investigation, incidence of febrile neutropenia and its hospitalization per cohort were reported. If the patient had an episode of febrile neutropenia and hospitalized for further treatment, time to first hospitalization due to febrile neutropenia and length of hospitalization due to febrile neutropenia were also recorded for further investigation.
Outcome measurement
Incidence of febrile neutropenia
Primary outcome was the incidence of febrile neutropenia for each regimen. Febrile neutropenia is defined as a single oral temperature of 38.3 °C or a temperature of ≥ 38 °C sustained for over an hour with concurrent neutropenia proven in hematological report [2, 4]. Neutropenia is defined as absolute neutrophil count (ANC) < 0.5 × 109/L, or < 1 × 109/L which is anticipated to drop below 0.5 × 109/L within the next 48 hr [2, 4]. Febrile neutropenia occurring 21 days after the administration of docetaxel in the cycle would not be evaluated.
Hospitalization outcomes related to febrile neutropenia
This study also evaluates (1) hospitalization rate due to febrile neutropenia, (2) length of stay in hospital due to febrile neutropenia, and (3) time to first hospitalization due to febrile neutropenia as secondary outcomes. Hospitalization refers to the admission of patient into hospital causing at least 1 day of residence in a non-day-ward setting. Only admissions corresponding to febrile neutropenia as primary or secondary diagnosis during hospitalization within 21 days since administration of docetaxel were counted.
Statistical analysis
SPSS Statistics (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.) was used to perform data collection and statistics analysis. Chi-squared test was used to examine the difference in incidence of febrile neutropenia between patients with different GCSF prophylaxis regimens. Number needed to treat (NNT) analysis was carried out to evaluate the clinical impact of GCSF protocol. Independent samples t-test was adopted for febrile neutropenia hospitalization outcomes including duration and time to first hospitalization. P values < 0.05 were considered statistically significant.
Results
Patient characteristics
Among 3916 patients with breast cancer on docetaxel-containing regimens enrolled in the study, only 2518 cases met the inclusion criteria of the study (Fig. 1). A number of patients receiving no PP, 4/5-day PP, and 7-day PP were 352 (13.98%), 1400 (55.60%), and 766 (30.42%), respectively.
Baseline characteristics of study population were shown in Table 2. The mean age (±standard deviation, SD) of subjects recruited was 53.62 ± 9.12 years old. The mean age of patients on TC with no PP (51.38 ± 10.95 years) was lower than those on 4/5-day PP (54.16 ± 9.48 years, p = 0.031) and 7-day PP (54.23 ± 9.36 years, p = 0.036). In other treatment arms, the mean age of subjects did not differ significantly. Only four cases were male in the study. Tumors were mainly in Stage II (52.90%) and III (28.51%). Regimens including TJH, FEC-T, AC-T, and TAC were mainly used in Stage II and III cases, whereas TC regimen was mainly reserved for Stage I and II cases. There were 15 patients (0.60%) with metastatic tumor upon the start of docetaxel treatment. The need of neoadjuvant chemotherapy did not differ among patients on TC and TJH regimen. More patients on AC-T regimen with 7-day PP (58.95%) had surgery prior to chemotherapy than those with no PP (16.28%) and 4/5-day PP (42.74%). Except 11 patients on FEC-T with 4/5-day PP who received neoadjuvant chemotherapy, all other patients on FEC-T had surgery prior to chemotherapy.
More patients on 4/5-day PP had a performance status ≥ 2 during treatment of docetaxel (TC, 15.34%; TJH, 12.5%; FEC-T, 15.02%; AC-T, 12.82%) than patients with no PP (TC, 0%; TJH. 4.35%; FEC-T, 0.9%; AC-T, 2.33%) and 7-day PP (TC, 0.41%; TJH, 1.43%; FEC-T, 0%; AC-T, 2.11%). In FEC-T regimen, patients with 4/5-day PP were more likely to receive dosage adjustment of docetaxel (13.96%) than those with no PP (6.31%) and 7-day PP (4.84%). However, a number of cases with dosage adjustment due to low ANC did not significantly differ among other GCSF prophylaxis protocols. In other regimens including TC, TJH, and AC-T, difference in number of cases having dosage adjustment among other GCSF prophylaxis protocols was not significant. In TJH regimen, patients on 7-day PP (27.14%) were more likely to experience treatment delay than patients with no PP (8.7%) or 4/5-day PP (25%). But in TC and FEC-T regimens, more patients on 4/5-day PP required treatment delay in at least one cycle of chemotherapy (TC, 17.23%; FEC-T, 21.02%) than patients with no PP (TC, 6.02%; FEC-T, 4.50%) or 7-day PP (TC, 9.43%; FEC-T, 10.48%). In FEC-T regimen, more patients with 4/5-day PP experienced cycle delay due to low ANC (5.65%) than patients with no PP (0%) or 7-day PP (4.44%). The difference in number of cases with treatment delay among different GCSF prophylaxis protocols was not significant in patients on AC-T.
Incidence of febrile neutropenia and NNT analysis
Febrile neutropenic outcomes were shown in Table 3. In TC regimen, incidence of febrile neutropenia (with odds ratio, OR) of patients with no PP, 4/5-day PP, and 7-day PP are 21.69%, 7.95% (OR 0.31, 95% Confidence Interval (CI): 0.17–0.57, p < 0.001), and 5.33% (OR 0.20, 95% CI: 0.09–0.44, p < 0.001), respectively. Patients with 7-day PP had a slightly lower risk of febrile neutropenia than those with 4/5-day PP (OR: 0.65, 95% CI: 0.34–1.24, p = 0.19). Treating seven patients (95% CI: 4–22) with 4/5-day PP or six patients (95% CI: 4–14) with 7-day PP could prevent a case of febrile neutropenia. In TJH regimen, incidence of febrile neutropenia in patients with no PP, 4/5-day PP, and 7-day PP is 38.26%, 8.33% (OR 0.15, 95% CI: 0.06–0.37, p < 0.001), and 8.57% (OR 0.15, 95% CI: 0.06–0.38, p < 0.001), respectively. Treating three patients, either with 4/5-day (95% CI: 2–5) or 7-day PP (95% CI: 2–5), could prevent a case of febrile neutropenia.
In AC-T regimen, incidence of febrile neutropenia in patients with no PP, 4/5-day PP, and 7-day PP were 20.93%, 6.84% (OR 0.28, 95% CI: 0.11–0.68, p = 0.005), and 16.84% (OR: 0.77, 95% CI: 0.31–1.90, p = 0.564), respectively. Treating seven patients with 4/5-day PP (95% CI: 4–66) could prevent a case of febrile neutropenia. The risk for patients on 4/5-day PP was lower than those on 7-day PP (OR: 2.76, 95% CI: 1.32–5.78, p = 0.007). In FEC-T regimen, incidence of febrile neutropenia in patients with no PP, 4/5-day PP, and 7-day PP were 9.91%, 4.77% (OR 0.46, 95% CI: 0.22–0.95, p = 0.035), and 7.26% (OR: 0.71, 95% CI: 0.32–1.56, p = 0.396), respectively. In FEC-T and AC-T regimen, febrile neutropenia incidence was not statistically different among patients with no PP and 7-day PP.
Febrile neutropenia-related hospitalization outcomes
Hospitalization rate of febrile neutropenia was 96.73%. In patients with no PP, 4/5-day PP, and 7-day PP, the mean (±SD) time to first hospitalization due to febrile neutropenia since administration of docetaxel were 9.37 ± 2.39 days, 7.64 ± 2.34 days (p < 0.001), and 7.63 ± 2.15 days (p < 0.001), respectively. However, some chemotherapy regimens showed a different trend. In FEC-T regimen, the mean (±SD) time to first hospitalization for patients on 4/5-day PP, no PP, and 7-day PP were 6.74 ± 1.41 days, 8.82 ± 3.84 days (p = 0.042), and 7.82 ± 1.81 days (p = 0.013), respectively. In TC regimen, the mean (±SD) time to first hospitalization for patients with no PP, 4/5-day, PP and 7-day PP were 9.76 ± 2.46 days, 8.08 ± 2.48 days (p = 0.026), and 6.77 ± 1.17 days (p < .001), respectively. In TJH and AC-T regimens, time to first hospitalization for different GCSF prophylaxis protocols did not differ from each other. For patients with no PP, 4/5-day PP, and 7-day PP, the mean (±SD) duration of hospitalization due to febrile neutropenia were 4.66 ± 2.60 days, 4.37 ± 2.85 days, and 5.12 ± 2.97 days, respectively. The difference was not statistically significant.
Discussion
Addition of taxanes in anthracyclines treatment is common for breast cancer patients due to its superiority in disease-free and overall survival [14]. Our institution reserves doxorubicin-based regimen in advanced breast cancer cases and FEC-T regimen in nodal-positive cases. Despite risk of febrile neutropenia that is intermediate (10–20%), our study showed that incidence of febrile neutropenia in subsequent docetaxel cycles of AC-T is > 20% [2]. The elevated risk in this study could be explained by having more cases on AC-T in advanced stage with greater underlying disease burden [3]. Benefit in administering 7-day PP to AC-T and FEC-T patients was modest when comparing to patients with no GCSF prophylaxis. However, prescribers would lengthen the duration of GCSF in cycles of docetaxel if patients encountered grade 4 neutropenia in previous cycles of AC or FEC. This could lead to a difference in underlying patient demographics during case recruitment. Administering primary GCSF prophylaxis to patients on AC-T and FEC-T was more beneficial than not offering GCSF prophylaxis, albeit requiring further investigation in prioritizing 4/5-day PP versus 7-day PP. Despite offering 7-day PP to patients on TAC regimen, incidence of febrile neutropenia remained 14.68%, showing that GCSF prophylaxis prescribed in TAC is essential for prevention. Taxane, in combination with cyclophosphamide instead of an anthracycline, is commonly used in early stage breast cancer cases. The adverse events, disease-free survival, and overall survival in TC were similar to AC-T, but the risk of cardiotoxicity is much lower in TC [15]. Despite risk of febrile neutropenia in patients on TC and TJH regimens that is high, it could be reduced through administration of GCSF [2]. Treating only three patients on TJH regimen with 4/5-day or 7-day PP could already prevent a case of febrile neutropenia. Administering 4/5-day or 7-day PP to seven or six patients on TC regimen, respectively, could also avoid a case. In terms of febrile neutropenia incidence, difference between patients on 4/5-day PP and 7-day PP was not significant. Limited studies evaluated the clinical impact for duration of GCSF in breast cancer patients. In general, the need of inpatient care for chemotherapy-induced neutropenic complications reduced when the duration of GCSF prophylaxis increased [16]. Weycker et al. stated that breast cancer patients had a greater risk of hospitalization for neutropenia if they were given a shorter course of GCSF in cycle 1 [17]. However, only 13.1% cases were on docetaxel-containing regimens. In the study these cases were not stratified for subgroup analysis. In fact, most studies did not clearly define the protocol of GCSF used among cohorts [18]. Structured comparison on neutropenic outcomes between different GCSF protocols was thus limited.
Immediate admission to hospital is the standard of care in managing patients with febrile neutropenia in Hong Kong unless patient refused hospitalization. Hospitalization due to fever contributed to 10.7% of cases admitted to an oncology ward in an Italian study [19]. These admissions were not scheduled, and duration of stay in hospital could be long [19]. Hospitalization also caused decrease in quality of life in patients who were already suffering from underlying disease burden and other adverse drug reactions. Although administration of GCSF did not alter the pattern of febrile neutropenic hospitalization including time to first hospitalization and length of stay in hospital, incidence of febrile neutropenia was largely reduced. This directly reduced the population requiring hospitalization due to febrile neutropenia. Through offering primary prophylaxis of neutropenic fever in an ambulatory care setting, patients require less admissions throughout treatment. Quality of life during disease management could thus be maintained. Bed occupancy due to emergency admission could also be reduced. The quality of inpatient care service could be enhanced due to a smaller bed-to-nurse ratio. Inpatient resources could thus be reserved for patients with more severe illness including sepsis or in advanced stage of cancer.
The transition of neutropenic fever from tertiary care management of disease to primary care prevention offered large economic benefits to the healthcare system (Figure 2). Injection of GCSF in Hong Kong is mostly handled by General Out-patient Clinics (GOPC). The healthcare expenditure on administering GCSF requires nursing fee (USD12.85 per visit) and cost of GCSF (USD7 per injection) (Table 4) [20]. For those cases clinically documented with an episode of febrile neutropenia, all patients attended Accident and Emergency Unit for assessment. Either patients were admitted to hospital and stayed for a certain period of time or not admitted to hospital due to various reasons. The costs of hospitalization per day and Accident and Emergency Unit attendance are USD653.8 and USD157.7, respectively (Table 4) [20]. The probability of hospitalization and length of stay in hospital for each regimen were shown in Table 3. The following assumptions were made: (1) GCSF were funded by public healthcare system, (2) all patients required GCSF injections in GOPC in every cycle of chemotherapy, and (3) all hospitalized patients were only managed in general wards with no intensive care unit service needed.
The comparison on direct medical cost of healthcare utilization largely favored the use of 4/5-day PP in TC, TJH, and AC-T regimens (Table 5). For each patient on TC, TJH, and AC-T regimen receiving 4/5-day PP, the mean expected direct medical cost in managing febrile neutropenia is USD90.7, USD376.7, and USD241.7 per patient less than those without GCSF prophylaxis, respectively. The main reason for the cost reduction is a significant drop in febrile neutropenia incidence, leading to a decrease in healthcare resources utilization due to febrile neutropenia. Despite administering 4/5-day PP requires additional cost of GCSF and nursing fee, extra healthcare expenditure required is modest. The savings in healthcare expenditure is especially significant in patients on TJH regimen due to a higher febrile neutropenia incidence, causing greater management expenditure. Giving 4/5-day PP to patients on FEC-T regimen does not provide healthcare expenditure savings when comparing to patients with no prophylaxis. However, quality of life in these patients was enhanced due to a reduced need of hospitalization. Administering 4/5-day PP to patients on FEC-T is still recommended. Administering 7-day PP requires extra healthcare expenditure with modest benefit in reducing the incidence of febrile neutropenia; thus giving 4/5-day PP to patients on docetaxel is preferred over 7-day PP.
Limitations
First, patients were excluded from the study if their GCSF regimens had been adjusted during treatment, either due to neutropenia in previous cycles or adverse drug reactions including bone pain. Thus, results could only reflect the incidence of febrile neutropenia in patients who could follow and complete the GCSF regimen throughout the treatment. Also, physicians may offer a lengthened GCSF prophylaxis regimen to high-risk cases. Some lower-risk cases are allowed to omit GCSF prophylaxis if patient refuses. Patients may also have a choice to omit GCSF prophylaxis if GCSF was not funded by the public healthcare system. Moreover, cases on FEC-T and AC-T may have an episode of neutropenia in previous cycles. Duration of GCSF prophylaxis is often lengthened to 7 days by prescribers when these cases receive docetaxel. An intrinsically uneven distribution of higher-risk cohorts in the group with longer GCSF prophylaxis administered is resulted. Demographics documented in electronic patient record were not complete. Information including use of antibiotics for febrile neutropenia prophylaxis, comorbidities, liver, and renal function could not be retrieved readily. We also assumed that performance status was < 2 if not specified in clinical notes. Risk stratification of cases for further analysis became not feasible. Moreover, higher risk patients who had been succumbed for over 1 year before data collection were not retrievable from electronic patient record. Patients with higher disease burden may not be included in this study.
The calculation on economic benefits was based on the charging fee to ineligible persons not entitled to local medical welfare [20]. The fee represents an average daily hospitalization cost which is not specifically calculated for febrile neutropenia patients. As a result, calculations may not truly reflect the cost on healthcare system. Moreover, ineligible patients for hospital-funded docetaxel injection are required to purchase GCSF as self-financed item. The calculation assumed all GCSF cases were funded by hospital and injected by nurse in GOPCs. As a result, GCSF administration cost was overestimated. Despite having an overestimated cost of GCSF administration and a potentially undercalculated hospitalization expenditure, the outcome still favored use of GCSF prophylaxis, showing that the actual economic benefit of giving GCSF prophylaxis could be even larger.
Conclusion
The use of GCSF prophylaxis reduced risk of febrile neutropenia in docetaxel-containing regimens for treatment of breast cancer. Offering 4/5-day PP to patients demonstrated similar efficacy to 7-day PP with superior saving benefits on healthcare expenditure. However, 7-day PP administered in cycles of docetaxel could not further reduce febrile neutropenia incidence and alter hospitalization outcomes. Utility and economic analysis could be explored for evaluating the association between primary GCSF prophylaxis and febrile neutropenia hospitalization outcomes toward public healthcare system.
References
Aapro MS, Cameron DA, Pettengell R, Bohlius J, Crawford J, Ellis M et al (2006) EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer 42:2433–2453
Crawford J, Becker PS, Armitage JO, Blayney DW, Curtin P, Dinner S et al (2018) NCCN Clinical Practice Guidelines in Oncology - myeloid growth factors. NCCN Clin Pract Guidelines Oncol:1–49
Flower CR, Seidenfeld J, Bow EJ, Karten C, Gleason C, Hawley DK et al (2013) Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 31(6):794–810
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI (2012) Clinical Practice Guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52(4):e56–e93
Agiro A, Ma Q, Acheson AK, Wu S, Patt DA, Barron JJ et al (2016) Risk of neutropenia-related hospitalization in patients who received colony-stimulating factors with chemotherapy for breast cancer. J Clin Oncol 34(32):3872–3972
Ngamphaiboon N, O'Connor TL, Advani PP, Levine EG, Kossoff EB (2012) Febrile neutropenia in adjuvant docetaxel and cyclophosphamide (TC) with prophylactic pegfilgrastim in breast cancer patients: a retrospective analysis. Med Oncol 29:1495–1501
Chan A, Fu WH, Shih V, Coyuco JC, Tan SH, Ng R (2011) Impact of colony-stimulating factors to reduce febrile neutropenic events in breast cancer patients receiving docetaxel plus cyclophosphamide chemotherapy. Support Care Cancer 19:497–504
Yerushalmi R, Goldvaser H, Sulkes A, Ben-Aharon I, Hendler D, Neiman V et al (2014) Adjuvant docetaxel and cyclophosphamide (DC) with prophylactic granulocyte colony-stimulating factor (G-CSF) on days 8 &12 in breast cancer patients: a retrospective analysis. PLOS One 9(10):1–5
Weycker D, Li X, Edelsberg J, BarronR KA, Xu A et al (2014) Risk of febrile neutropenia in patients receiving emerging chemotherapy regimens. Support Care Cancer 22:3275–3285
Younes J, Vandenberg T, Jawaid M, Jawaid MA (2012) Febrile neutropenia rates with adjuvant docetaxel and cyclophosphamide chemotherapy in early breast cancer: discrepancy between published reports and community practice—an updated analysis. Curr Oncol 19(6):332–334
Kosaka Y, Rai Y, Masuda N, Takano T, Saeki T (2015) Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy. Support Care Cancer 23:1137–1143
Lee J, Lee JE, Kim Z, Han SW, Hur SM, Kim SY, Lee MH, Lim CW (2018) Pegfilgrastim for primary prophylaxis of febrile neutropenia in breast cancer patients undergoing TAC chemotherapy. Ann Surg Treat Res 94(5):223–228
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ et al (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33(28):3199–3215
Greene J, Hennessy B (2015) The role of anthracyclines in the treatment of early breast cancer. J Oncol Pharm Pract 21(3):201–212
Fujii T, Le Du F, Xiao L, Kogawa T, Barcenas CH, Alvarez RH et al (2015) Effectiveness of an adjuvant chemotherapy regimen for early-stage breast cancer: a systematic review and network meta-analysis. JAMA Oncol 1(9):1311–1318
Weycker D, Barron R, Edelsberg J, Kartashov A, Legg J, Glass AG (2014) Risk and consequences of chemotherapy-induced neutropenic complications in patients receiving daily filgrastim: the importance of duration of prophylaxis. BMC Health Serv Res 14:189
Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG (2006) Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother 40:402–407
Fernandes R, Mazzarello S, Stober C, Vandermeer L, Dudani S, Ibrahim MFK et al (2017) Optimal primary febrile neutropenia prophylaxis for patients receiving docetaxel-cyclophosphamide chemotherapy for breast cancer: a systematic review. Breast Cancer Res Treat 161:1–10
Numico G, Cristofano A, Mozzicafreddo A, Cursio OE, Franco P, Courthod G et al (2015) Hospital admission of cancer patients: avoidable practice or necessary care? PLoS One 10(3):1–10
Hospital Authority (2019) Hospital Authority: Fees and Charges. http://wwwhaorghk/visitor/ha_visitor_indexasp?Content_ID = 10045&Lang = ENG&Dimension = 100&Parent_ID = 10044&Ver = HTML Assessed 16 April 2019
Author information
Authors and Affiliations
Contributions
Lee CF, Zhou K, Young WM, Wong CS, Ng TY, and Lee SF are involved with the planning of the investigation. Lee CF, Leung K, Wong LKM, So KH, Tang W, Chong G, Chan SK, Yip YTE, Ma VYM, Yeung A, Chin CHY, Kwan MW, and Tsang HT are involved with data collection. Lee CF also involves in writing manuscript, data interpretation, performing data, and statistical analysis. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest that are directly relevant to the content of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, C.F., Zhou, K., Young, W.M. et al. Febrile neutropenia and its associated hospitalization in breast cancer patients on docetaxel-containing regimen: A retrospective cohort study on duration of prophylactic GCSF administration. Support Care Cancer 28, 3801–3812 (2020). https://doi.org/10.1007/s00520-019-05111-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05111-6