Abstract
US Oncology Research Trial 9735 reported that TC improved overall survival when compared to doxorubicin and cyclophosphamide in early-stage breast cancer. Despite 61% grades 3–4 neutropenia in the TC arm, only 5% of patients developed febrile neutropenia (FN) without primary prophylactic GCSF (ppGCSF). TC has risen in popularity, particularly in older patients or in those where an anthracycline is contraindicated. Other studies examining the toxicity of TC without ppGCSF reported a higher incidence of FN between 23 and 46%. We reviewed our institutional experience with ppGCSF and the TC regimen. Women treated with adjuvant TC and pegfilgrastim at Roswell Park Cancer Institute were identified from the pharmacy database between 8/2006 and 11/2010. Patient characteristics and comorbidities were abstracted. Endpoints included incidence of FN, hematologic toxicities, relative dose intensity (RDI), and other acute complications. Docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 were given every 21 day/cycle for a planned four cycles. All patients received pegfilgrastim 6 mg on day 3. One hundred and eleven women with median age of 56 years (27–79) were identified. Twenty-two percent of patients were ≥65 at diagnosis. Eight patients developed FN (7%). Ninety-five patients (86%) were able to complete four cycles. Completion rate was significantly lower in patients with age ≥65 (71% vs. 90%; P = 0.02). Incidence of hospitalization, delay, RDI <85%, and dose reduction were not significantly different between the age groups. The overall incidence of FN was 7%. Older patients were significantly less likely to complete four cycles of TC as planned. ppGCSF should be strongly considered in breast cancer patients receiving adjuvant TC chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
US Oncology Research Trial 9735 reported that docetaxel and cyclophosphamide (TC) improved disease-free survival (DFS) and overall survival (OS) when compared to doxorubicin and cyclophosphamide (AC) in early-stage breast cancer [1]. TC has increased in popularity due to its suitability for older women or in those where an anthracycline-based regimen is not preferred. Anthracycline-based chemotherapy has known cardiac toxicity, lifetime dose limitations, and potential leukemogenesis [2]. Additionally, the anthracyclines appear to be poorly tolerated in older patients (age ≥ 65), due to acute complications resulting in higher rates of delay or early termination of planned chemotherapy [3]. Therefore, non-anthracycline-based regimens, such as TC, have been recommended as an alternative in these particular patients [2].
Despite the development of 61% grades 3–4 neutropenia in the TC arm on US Oncology Research Trial 9735, only 5% of patients developed febrile neutropenia (FN) [4]. The use of oral prophylactic antibiotics was permitted at the discretion of the treating physician in this study, but not required. However, contemporary supportive care practice does not advise the routine use of oral antibiotics for prophylaxis of FN with chemotherapy because of emerging antibiotic resistance [5]. The rate of febrile neutropenia reported in US Oncology Research Trial 9735 may not be reflective of rates seen in patients treated with this regimen off protocol.
Other studies examining the toxicity of TC without primary prophylactic granulocyte colony-stimulating factor (ppGCSF) have reported a higher incidence of FN between 23 and 46% [6–11]. One study reported that when primary prophylactic GCSF was added to the TC regimen, FN decreased from 25 to 6.3%, providing an absolute benefit of 18.7% [6]. However, ppGCSF support with either pegfilgrastim or standard filgrastim dosing during TC administration is not yet recommended in standard guidelines [12, 13]. Nonetheless, ppGCSF has been adopted in some clinical practices as other studies report a much higher rate of FN [6–11]. We reviewed our institutional experience using primary prophylactic pegfilgrastim (PEG) with the TC regimen at a comprehensive cancer center.
Patients and methods
Patients
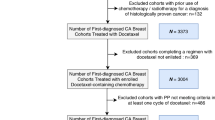
We identified breast cancer patients who were treated with adjuvant TC with primary PEG from the pharmacy database at Roswell Park Cancer Institute (RPCI) between 8/2006 and 11/2010. Patient characteristics, comorbidities, Charlson comorbidity index (CCI) [14], ECOG performance status (PS), stage at diagnosis, estrogen receptor (ER), progesterone receptor (PR), HER-2 status, surgery type, and radiation treatment were abstracted. Relative dose intensity (RDI) of docetaxel (T) and cyclophosphamide (C) was calculated by the amount of drug administered per unit time expressed as a percentage of the standard regimen [15]. Patients who received TC for local recurrence, previous chemotherapy, neoadjuvant chemotherapy, and planned chemotherapy for more than four cycles of TC were excluded.
Treatment
All patients were treated at RPCI with docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 i.v. over 30–60 min given on day 1 of a 21 day/cycle planned for four cycles. Patients were pre-medicated with dexamethasone 8 mg orally twice daily on the day before, day of, and day after chemotherapy. Pegfilgrastim 6 mg was administered subcutaneously on day 3 of each cycle in all patients. No patients received prophylactic antibiotics. In a minority of patients with HER-2-positive disease, trastuzumab 8 mg/kg intravenous infusion given over 90 min was administered on day 1 of cycle 1 and continued with 6 mg/kg intravenous infusion every 3 weeks until the completion at 1 year.
Complications and toxicities
Early termination of chemotherapy, delay (of at least 7 days), dose reductions (of at least 15%), and hospitalizations while receiving chemotherapy were recorded. Febrile neutropenia was defined as body temperature ≥38.2°C and absolute neutrophil count <0.5 × 109/L on the same day of the fever or the day after [16]. Neutropenia and anemia were defined by Common Terminology Criteria for Adverse Events (CTCAE V.4) [17]. Toxicity was assessed retrospectively from medical records at each patient visit and for 3–4 weeks after the last dose of TC.
Statistical analysis
The primary objective was to determine the incidence of febrile neutropenia in all patients. Secondary analyses investigated the association of age (<65 years vs. ≥65 years) with the rate of completion of chemotherapy, hospitalization, delay in chemotherapy, dose reduction, grades 3–4 neutropenia and anemia, and RDI (<85% vs. ≥85%) [18, 19]. Statistical tests for association of categorical variables included the Pearson chi-square and Fisher’s exact tests, where appropriate. All statistical tests were two-sided, with P < 0.05 considered statistically significant. Since this was an exploratory study, no corrections for multiple comparisons were made. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 18.0 (SPSS, Chicago, IL).
Results
Patient characteristics
A total of 144 patients were identified. Thirty-three patients were excluded due to previous chemotherapy treatment (n = 16), treatment for local recurrence (n = 7), neoadjuvant setting (n = 4), plan for six cycles of TC (n = 4), lost follow-up (n = 1), and male gender (n = 1). One hundred and eleven patients remained in the study with a median age of 56 (range 27–79). Twenty-two percent of patients were 65 or older. Table 1 is a summary of patient characteristics. Older patients (age ≥65) had a significantly higher incidence of comorbidities such as hypertension (67% vs. 22%; P < 0.001), renal disease (8% vs. 0%; P = 0.045), COPD/asthma (25% vs. 9%; P = 0.039), and CCI ≥2 (29% vs. 16%; P = 0.003). Older patients presented with a later stage at diagnosis (stages II–III; 67% vs. 25%; P < 0.001) received more axillary lymph node dissections (46% vs. 22%; P = 0.019) and radiation therapy (83% vs. 56%; P = 0.018). There was no significant difference in ECOG PS, other comorbidities, biomarkers, or surgery type.
Acute complications and toxicities
The majority of patients completed four cycles of TC (86%). Older patients (≥age 65) were significantly less likely to complete TC as planned (71% vs. 90%; P = 0.020). Hospitalizations during administration of TC (15%), delays (5%), and dose reductions (5%) were not significantly different among age groups. Any of these events occurred in 25% of all patients (Table 2). Seventeen patients were hospitalized during TC administration. Fourteen patients were hospitalized due to infection/FN despite primary PEG prophylaxis. Ten percent of all patients treated had either a delay in treatment or dose reduction. Early termination of chemotherapy occurred in 16 patients. The reasons for early termination of TC are as follows: infection/FN in 4/16 patients (25%), allergic reaction/anaphylaxis in 3/16 patients (19%), grade 3 fatigue in 2/16 (13%), and steroid-induced psychosis in 2/16 patients (13%). There were no chemotherapy-related deaths observed in the study.
Mean RDI of TC were 91.2% (range 25–100) and 92.6% (range 25–100), respectively. Eighteen of 111 patients (16%) who received docetaxel had an RDI <85%, while 15/111 patients (14%) who received cyclophosphamide had an RDI <85%. Ten of 111 patients (9%) developed grades 3–4 neutropenia, while 3/111 patients (3%) had grades 3–4 anemia. Erythropoietin support was administered in 8/111 patients (7%). Median follow-up of the study was 19.1 months.
Febrile neutropenia characteristics
Febrile neutropenia developed in 8/111 patients (7%). The median age of FN patient was 59 (range 52–70). There was no significant difference in the incidence of FN between age groups (<65 vs. ≥65). Seven of 8 patients were hospitalized due to FN with average length of hospital stay of 6 days (range 2–21 days). Only 1 patient was treated with oral antibiotic as an outpatient. All patients developed FN during the first cycle of TC despite PEG prophylaxis. Median time from PEG administration to FN was 5 days (range 4–6). Five of 8 patients (63%) received dose reduction in TC in the subsequent cycles, while 3/8 patients (38%) were unable to complete four cycles as planned. There were no FN-related deaths observed in the study.
Discussion
At RPCI, three of our first 4 patients (age 43, 61, 67, and 71) who received adjuvant TC without primary prophylactic GCSF (75%) developed FN despite 2/4 patients (50%) receiving antibiotic prophylaxis. These patients were not included in this study but prompted the standard administration of prophylactic PEG in our practice. The standard guidelines from both European [13] and US [12] consensus panels recommend ppGCSF to prevent febrile neutropenia in patients who receive high-risk chemotherapy regimens (FN >20%) and recommend its consideration in intermediate-risk regimens (FN 10–20%), particularly in patients with risk factors for developing FN such as old age (≥65), previous chemotherapy and/or radiation, and pre-existing neutropenia. [12, 13]. The current US standard guideline suggests that single-agent docetaxel 100 mg/m2 given every 3 weeks in metastatic breast cancer is considered as an intermediate risk for FN (10–20%), while combination with trastuzumab is considered as a high risk (>20%) and ppGCSF should be administered [12, 20]. In our study, the incidence of FN was observed in 7% of patients despite utilization of pegfilgrastim as primary prophylaxis. Our study and other studies of adjuvant TC in breast cancer are summarized in Table 3, [6–11]. Without ppGCSF, the incidence of FN was reported between 23 and 46% and appears to be related to older age (≥65). When ppGCSF was administered, FN was significantly reduced with an absolute benefit of risk reduction between 18.7 and 46.0%.
In our study, despite primary PEG, infection and febrile neutropenia were the primary reason (>80%) for delay, hospitalization, and dose reduction in TC. In other studies, the incidence of dose reduction was lower in the ppGCSF group [6, 11]. Chan et al. [6] reported that ppGCSF was significantly associated with a lower risk of dose reduction in the TC regimen (16% vs. 3%; P = 0.02). Delay, dose reduction, and early termination of TC may reduce the relative dose intensity (RDI) of the planned chemotherapy. A relative dose intensity of <85% is associated with decreased survival in breast cancer patients who receive adjuvant or neoadjuvant chemotherapy, including those in the older population (age ≥ 65) [3, 18]. Since ppGCSF may improve the RDI [18, 21], it is hypothesized that the ppGCSF may affect the survival of these patients. However, large meta-analyses did not show a benefit of ppGCSF in improvement of response or overall survival rates [21–24].
The overall successful completion rate of four cycles of TC in our study was 86%, compared to 88–94% in other studies. Older patients (age ≥ 65) were significantly less likely to complete four cycles when compared to patients younger than 65 (90% vs. 71%; P = 0.02). We hypothesize this could result from the baseline characteristics of the patients, as older patients presented with more comorbidities and a later stage of diagnosis. A study of older breast cancer patients who received adjuvant or neoadjuvant chemotherapy suggests that comorbidities and older age significantly affected the ability to deliver and complete planned chemotherapy [3]. Being aware of these particular comorbidities (such as cardiac disease, hypertension, and CCI ≥1) as they relate to the delivery of adjuvant chemotherapy should increase the physician’s awareness and maximize successful chemotherapy delivery in elderly patients.
Our study is limited by a small sample size and lack of comparison with patients who did not receive ppGCSF as a control arm. Other toxicities (such as neuropathy, nausea, vomiting, fatigue, etc.) are not described because of the retrospective nature of the study. Hospitalization for the treatment of FN appears to be expensive. A Canadian study of direct medical costs of hospitalization for febrile neutropenia demonstrated that a mean overall cost per episode was 6,324 ± 4,783 in 2007 Canadian dollars [25]. A cost-effectiveness analysis of ppGCSF in the TC regimen is warranted in a further study.
Conclusion
Despite primary PEG, the overall incidence of FN during adjuvant TC was 7%. Although they received ppGCSF, older patients (≥age 65) were significantly less likely to complete four cycles of TC as planned. Primary prophylactic growth factor support should be strongly considered in breast cancer patients receiving adjuvant TC chemotherapy.
References
Jones S, Holmes FA, O’Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US oncology research trial 9735. J Clin Oncol. 2009;27(8):1177–83. doi:10.1200/JCO.2008.18.4028.
Aapro M, Bernard-Marty C, Brain EG, Batist G, Erdkamp F, Krzemieniecki K, et al. Anthracycline cardiotoxicity in the elderly cancer patient: a SIOG expert position paper. Ann Oncol. 2011;22(2):257–67. doi:10.1093/annonc/mdq609.
Ngamphaiboon N, Edge SB, Groman A, Wilding GE, Ademuyiwa FO, Levine EG, Watroba N, O’Connor TL. Factors affecting delivery of planned chemotherapy for breast cancer in older women. In: The 33rd Annual San Antonio Breast Cancer Symposium. 2010;abstract#851603.
Jones SE, Savin MA, Holmes FA, O’Shaughnessy JA, Blum JL, Vukelja S, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24(34):5381–7. doi:10.1200/JCO.2006.06.5391.
Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34(6):730–51. doi:10.1086/339215.
Chan A, Fu WH, Shih V, Coyuco JC, Tan SH, Ng R. Impact of colony-stimulating factors to reduce febrile neutropenic events in breast cancer patients receiving docetaxel plus cyclophosphamide chemotherapy. Support Care Cancer. 2011;19(4):497–504. doi:10.1007/s00520-010-0843-8.
Kotasek D. Febrile neutropenia rates during docetaxel and cyclophosphamide (TC) adjuvant therapy in early breast cancer (EBC). J Clin Oncol 2011;29(suppl; abstr 1101).
Soni A, Brufsky A, Jankowitz RC, Rastogi P, Vogel VG, Puhalla S Incidence of febrile neutropenia with docetaxel plus cyclophosphamide in a university-based breast oncology clinic. J Clin Oncol. 2011;29(suppl; abstr 9061).
Vandenberg T, Younus J, Al-Khayyat S. Febrile neutropenia rates with adjuvant docetaxel and cyclophosphamide chemotherapy in early breast cancer: discrepancy between published reports and community practice-a retrospective analysis. Curr Oncol. 2010;17(2):2–3.
Takabatake D, Taira N, Hara F, Sien T, Kiyoto S, Takashima S, et al. Feasibility study of docetaxel with cyclophosphamide as adjuvant chemotherapy for Japanese breast cancer patients. Jpn J Clin Oncol. 2009;39(8):478–83. doi:10.1093/jjco/hyp050.
Myers R, Higgins B, Jeffrey M, Mova L, Rajagopal S, Jones G, Stakiw J. Chemotherapy induced febrile neutropenia of docetaxel with cyclophosphamide (TC) for adjuvant therapy of breast cancer in the community-reality check. In: The 32nd Annual San Antonio Breast Cancer Symposium. 2009;Abstract#2092.
NCCN. NCCN Guideline V1.2011 Myeloid Growth Factors. http://wwwnccnorg/professionals/physician_gls/pdf/myeloid_growthpdf (2011).
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32. doi:10.1016/j.ejca.2010.10.013.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol. 1984;2(11):1281–8.
Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas-Figueroa LJ, Wiens BL, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23(6):1178–84. doi:10.1200/JCO.2005.09.102.
NCI. Common Terminology Criteria for Adverse Events (CTCAE V.4). http://evsncinihgov/ftp1/CTCAE/CTCAE_403_2010-06-14_QuickReference_85x11pdf (2009).
Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit Rev Oncol Hematol. 2011;77(3):221–40. doi:S1040-8428(10)00032-6.
Shayne M, Culakova E, Wolff D, Poniewierski MS, Dale DC, Crawford J, et al. Dose intensity and hematologic toxicity in older breast cancer patients receiving systemic chemotherapy. Cancer. 2009;115(22):5319–28. doi:10.1002/cncr.24560.
Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–74. doi:10.1200/JCO.2005.04.173.
Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158–67. doi:10.1200/JCO.2006.08.8823.
Sung L, Nathan PC, Alibhai SM, Tomlinson GA, Beyene J. Meta-analysis: effect of prophylactic hematopoietic colony-stimulating factors on mortality and outcomes of infection. Ann Intern Med. 2007;147(6):400–11.
Bohlius J, Herbst C, Reiser M, Schwarzer G, Engert A. Granulopoiesis-stimulating factors to prevent adverse effects in the treatment of malignant lymphoma. Cochrane Database Syst Rev. 2008;4:CD003189. doi:10.1002/14651858.CD003189.
Lyman GH, Kuderer NM, Djulbegovic B. Prophylactic granulocyte colony-stimulating factor in patients receiving dose-intensive cancer chemotherapy: a meta-analysis. Am J Med. 2002;112(5):406–11.
Lathia N, Mittmann N, DeAngelis C, Knowles S, Cheung M, Piliotis E, et al. Evaluation of direct medical costs of hospitalization for febrile neutropenia. Cancer. 2010;116(3):742–8. doi:10.1002/cncr.24773.
Acknowledgments
We would like to acknowledge the assistance of Theresa E. Hahn, Ph.D. in statistical analysis of the study.
Conflict of interest
All authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ngamphaiboon, N., O’Connor, T.L., Advani, P.P. et al. Febrile neutropenia in adjuvant docetaxel and cyclophosphamide (TC) with prophylactic pegfilgrastim in breast cancer patients: a retrospective analysis. Med Oncol 29, 1495–1501 (2012). https://doi.org/10.1007/s12032-011-0035-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0035-5