Abstract
Purpose
Febrile neutropenia (FN) incidence during docetaxel and cyclophosphamide (TC) chemotherapy, known as a high-risk regimen, differs among countries. The role of prophylactic granulocyte colony-stimulating factor (G-CSF) in FN is unclear. This study aimed to investigate FN frequency and relative dose intensity (RDI) of TC chemotherapy in patients with breast cancer and identify the correct population requiring prophylactic G-CSF.
Methods
In total, 205 patients with breast cancer were scheduled for TC chemotherapy (docetaxel/cyclophosphamide 75/600 mg/m2, every 3 weeks, 4 cycles) as adjuvant chemotherapy. Trastuzumab (8 mg/kg; continued with 6 mg/kg) was administrated intravenously for human epidermal growth factor receptor 2 (HER2)-positive cancer. Fifty-five patients received primary prophylactic measures (G-CSF: 20 and antibiotics: 35). We investigated the frequency of FN and hospitalization, RDI, and the factors related to FN, adverse events, hospitalization, and RDI.
Results
FN occurred in 70 patients (35.7%). FN incidence was noted in 41.1% without any prophylactic measures and in 5.0% with prophylactic G-CSF. In multivariate analysis, the independent risk factors of FN were older age (≥ 60 years, P = 0.017) and without primary prophylactic G-CSF (P = 0.011). Eleven patients (5.6%) were hospitalized of which 8 (72.7%) were elderly. The median RDIs of docetaxel and cyclophosphamide were 96.7% and 99.7%, respectively.
Conclusion
FN frequency during TC chemotherapy was high, and primary prophylactic G-CSF reduced FN incidence. Primary prophylactic G-CSF is an effective therapy for preventing FN during TC chemotherapy. However, prophylactic G-CSF should be considered for elderly patients based on the low hospitalization rate and the high RDI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Docetaxel and cyclophosphamide (TC) chemotherapy improves disease-free and overall survival after breast cancer surgery when compared with doxorubicin and cyclophosphamide (AC) chemotherapy. It is also one of the standard regimens of adjuvant chemotherapy for breast cancer [1, 2]. The incidence of febrile neutropenia (FN) caused by TC chemotherapy varies widely in clinical studies (5% in a US Oncology Research trial [3] and 68.8% in a Japanese trial [4]). This difference might be due to race or clinical trial settings such as frequent temperature measurements and blood tests. In addition, Asian patients are known to show strong hematological toxicity [5].
FN is one of the major life-threatening adverse events in chemotherapy. A high FN incidence rate increases infection, leading to mortality. G-CSF has been shown efficacy of reduction FN by production of granulocytes and enhancement of neutrophil function [6]. Therefore, the American Society of Clinical Oncology (ASCO), the European Organisation for Research and Treatment of Cancer (EORTC), and the National Comprehensive Cancer Network (NCCN) guidelines recommend the administration of prophylactic granulocyte colony-stimulating factor (G-CSF) in high-risk FN (> 20%) treatment regimens [7,8,9]. In addition, the FN incidence might lead to a reduction in treatment intensity [10]. High relative dose intensity (RDI) of perioperative chemotherapy is reported to be important to improve prognosis [10, 11].
G-CSF causes adverse events associated with bone pain, fever, leukocytosis, and allergic reactions [12,13,14]. In addition, the use of G-CSF adds considerable cost to adjuvant chemotherapy of breast cancer [15]. Therefore, it is important to select breast cancer patients who would benefit from prophylactic G-CSF treatment for TC chemotherapy.
Therefore, we aimed to retrospectively investigate FN frequency, RDI, and the potential risk factors related to FN onset in Japanese patients receiving TC chemotherapy in the clinical setting.
Patients and methods
Patients
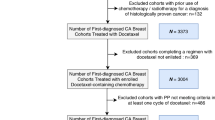
A total of 205 patients with breast cancer who received adjuvant TC chemotherapy between April 2009 and June 2017 at Hiroshima University Hospital were retrospectively reviewed. Nine patients who discontinued the therapy due to allergic reaction during initial treatment were excluded from FN and RDI assessment. We evaluated the frequency and risk factors of FN, adverse events, hospitalization, and RDI.
Treatment
Docetaxel (75 mg/m2) and cyclophosphamide (600 mg/m2) were intravenously administered at day 1 of each cycle to all 205 patients. Chemotherapy was repeated every 3 weeks for four cycles. TC therapy was reduced and delayed as needed according to the choice of the physician. Trastuzumab 8 mg/kg (continued with 6 mg/kg after two cycles) was administered intravenously to 15 patients (7.3%) with human epidermal growth factor receptor 2 (HER2)-positive cancer. FN was diagnosed when the patient developed fever (axillary temperature of over 37.5°) and had grade 3/4 neutropenia (< 1.0 × 109/L) or fever during the neutropenic period (days 5-14) [4]. Other adverse events were defined according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 [16].
Statistical analysis
Data are presented as numbers and percentages unless otherwise stated. Frequencies of FN and RDI were compared using Fisher’s exact test for categorical variables. Logistic regression was used to predict FN incidence and low RDI. The cutoff for age was defined as 60 years based on the receiver operating characteristic curves. P < 0.05 was considered to indicate statistical significance in all comparisons. All data were statistically analyzed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [17].
Results
Patient and characteristics
The characteristics of all 205 patients are shown in Table 1. The median patient age was 53 years, and 78 patients (38.0%) were aged ≥ 60 years. Most patients had stage I (33.7%) or stage II (57.6%) disease at diagnosis. Fifty-five patients (26.8%) received primary prophylactic measures (G-CSF 9.7% (n = 20) and antibiotics 17.1% (n = 35)).
Frequency and risk factors for FN
The frequency of FN incidence was 35.7% (70/196) in all patients, 41.1% (58/141) in patients who did not receive primary prophylactic measures, 5.0% (1/20) in patients who received prophylactic G-CSF, and 31.4% (11/35) in patients who received prophylactic antibiotics. Prophylactic G-CSF significantly reduced the frequency of FN incidence (P = 0.001), whereas prophylactic antibiotics did not (P = 0.337) (Fig. 1).
From the multivariate analysis, we found that older age (≥ 60 years) and absence of primary prophylactic G-CSF administration were independent risk factors for FN (P = 0.017 and P = 0.011, respectively) (Table 2); however, hepatic dysfunction, renal dysfunction, and prophylactic antibiotics were not associated with FN incidence. Other adverse events are listed in Supplementary Table S1. No treatment-related death occurred.
Hospitalization
During TC chemotherapy, hospitalization was observed in 5.6% of the patients for the following reasons: 3 (1.5%) were diagnosed with FN, 3 (1.5%) had an infection, 3 (1.5%) had anorexia, and 2 (1.0%) were admitted for other reasons (Table 3). The frequency of overall hospitalization and neutropenia-related hospitalization was low.
Relative dose intensity and risk factors
Discontinuation of TC chemotherapy occurred in 20 patients (9.8%), delay in 23 (11.2%), and dose reduction in 41 (20.0%) (Supplementary Table S2). The major reason for discontinuation of the therapy was an allergic reaction and it occurred in 13 patients. Although there were no cases of discontinuation due to FN, it was the most common cause for delay (8 patients) and dose reduction (15 patients). The median RDIs of docetaxel and cyclophosphamide treatments were 96.7% and 99.7%, respectively (Fig. 2). There was no difference in RDIs regardless of the use of G-CSF (with G-CSF: 99.7% and 99.9%, and without G-CSF: 96.6% and 99.7%). Among patients with a low RDI (< 85%), there were 36 patients (18.4%) in the docetaxel treatment group and 20 (10.2%) in the cyclophosphamide treatment group.
Multivariate analysis revealed that older age (≥ 60 years) was an independent risk factor for a low RDI (odds ratio (OR) 5.16, 95% confidence interval (CI) 2.32-11.5, P = 0.001), whereas primary prophylactic measures were not (G-CSF: OR 0.39, 95% CI 0.08-1.92, P = 0.247 and antibiotics: OR 1.08, 95% CI 0.43-2.72, P = 0.862) (Supplementary Table S3). Hepatic dysfunction and renal dysfunction were also not associated with low RDI.
Discussion
This study demonstrated the high incidence rate of FN and the benefits of primary prophylactic G-CSF in breast cancer patients treated with TC chemotherapy in Japanese clinical practice. We also showed that a high frequency of FN does not always lead to an increased probability for hospitalization or reduction of RDI. Our findings support the importance of an adequate decision for prophylactic G-CSF administration, which should be based on the risk factors in the particular case, such as age.
Adjuvant chemotherapy is important for reducing recurrence and mortality in breast cancer patients. Although anthracycline- or taxane-based regimens are key treatments in breast cancer chemotherapy [18, 19], regimens excluding anthracycline are being developed as it can cause severe adverse events such as cardiotoxicity and secondary malignancies [20]. TC chemotherapy has been reported to improve disease-free and overall survival compared to AC chemotherapy in the US Oncology Research trial 9735 [2]. Among the chemotherapy regimens used for breast cancer, TC chemotherapy is known to have a high risk for FN. The frequency of FN incidence related to TC chemotherapy was reported to be 68.5% in the Japanese trial [4] and 5% in the US Oncology Research trial [3], showing a significant difference in the results. Population-based studies have shown that the incidence of FN associated with TC chemotherapy was higher (20.9–38.0%) in patients who did not receive primary prophylactic G-CSF than in those who did receive G-CSF (4.2–8.1%) [21,22,23]. A systematic review has shown that the median FN rates with and without primary prophylaxis were 6.6% and 31.3%, respectively [24]. The present study showed that the frequency of FN in TC chemotherapy without any primary prophylactic measures was 41.1% and prophylactic G-CSF reduced the FN incidence by 36.1%. Asian patients who received TC chemotherapy have been reported to develop strong hematological toxicity, and an appropriate neutropenic management was needed [5]. The frequency of hospitalization in US patients treated with TC chemotherapy was reported to be 16.5% despite the use of prophylactic G-CSF in 62.1% of them [25]. In addition, patients older than 65 years had approximately twice the risk of hospitalization compared to patients below the age of 65 years [26]. The present study showed low frequency of hospitalization, with a hospitalization rate of 72.7% for older patients and no treatment-related mortality during TC chemotherapy.
Dose reduction was often performed as a measure against FN before pegfilgrastim had been approved in Japan in 2014. However, reducing RDI of adjuvant chemotherapy worsens the prognosis of breast cancer [11]. Therefore, avoiding discontinuation, treatment delays, and dose reduction is important for maintaining the treatment effect. Dose intensity of TC chemotherapy has been reported to be 99.8% in US Oncology Research trial [3]. A previous study has reported that prophylactic G-CSF prevented dose reduction in chemotherapy [25]. The present study showed a high RDI (docetaxel 96.7% and cyclophosphamide 99.7%) despite the high frequency of FN. Prophylactic G-CSF was not related to RDI. Therefore, primary prophylactic G-CSF is an effective therapy for preventing FN during TC chemotherapy.
G-CSF has some limitations. The most relevant and harmful adverse events of G-CSF are bone and musculoskeletal pain [12]. Among the patients who received chemotherapy with G-CSF for breast cancer, the prevalence of bone pain was reported to be 46.6% [27]. In addition, G-CSF has cost issue. In Japan, the cost of G-CSF is around $1000 per dose and approximately $4000 entire course of four cycles of TC chemotherapy. G-CSF administration in all chemotherapy cycles is associated with high costs; the cost effectiveness of this therapy should be considered [15]. Our findings support a selective use of prophylactic G-CSF in patients with breast cancer who received TC chemotherapy. We propose prophylactic G-CSF administration for elderly patients because older age was the only risk factor for both FN and low RDI.
The limitations of this study arose from its retrospective design. FN might have been overestimated because neutrophil count during fever was not taken owing to outpatient care in many cases. A prospective cohort study in Japan reported that FN was surveyed based on two different definitions, namely, true FN: ≥ 37.5 °C and grade 4 neutropenia, and surrogate FN: ≥ 37.5 °C and oral antibiotic and antipyretic intake [28, 29]. This study showed that no differences were found in the incidence of FN in the two groups (36.6% and 31.5%, respectively). Hence, the definition of FN in this study is reasonable.
Conclusion
FN incidence in TC chemotherapy is high, and primary prophylactic G-CSF was effective in reducing FN events. TC chemotherapy was well-tolerated and administrated at the appropriate dosage in Japanese clinical practice. Primary prophylactic G-CSF should be applied selectively, such as in patients at an older age.
Data availability
Not applicable.
References
Gradishar WJ, Anderson BO, Abraham J et al (2020) Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. 18:452. https://doi.org/10.6004/jnccn.2020.0016
Jones S, Holmes FA, O’Shaughnessy J et al (2009) Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol 27:1177–1183. https://doi.org/10.1200/JCO.2008.18.4028
Jones SE, Savin MA, Holmes FA, O'Shaughnessy JA, Blum JL, Vukelja S, McIntyre KJ, Pippen JE, Bordelon JH, Kirby R, Sandbach J, Hyman WJ, Khandelwal P, Negron AG, Richards DA, Anthony SP, Mennel RG, Boehm KA, Meyer WG, Asmar L (2006) Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 24:5381–5387. https://doi.org/10.1200/jco.2006.06.5391
Kosaka Y, Rai Y, Masuda N, Takano T, Saeki T, Nakamura S, Shimazaki R, Ito Y, Tokuda Y, Tamura K (2015) Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy. Support Care Cancer 23:1137–1143. https://doi.org/10.1007/s00520-014-2597-1
Chow LWC, Biganzoli L, Leo AD, Kuroi K, Han HS, Patel J, Huang CS, Lu YS, Zhu L, Chow CYC, Loo WTY, Glück S, Toi M (2017) Toxicity profile differences of adjuvant docetaxel/cyclophosphamide (TC) between Asian and Caucasian breast cancer patients. Asia-Pac J Clin Oncol 13:372–378. https://doi.org/10.1111/ajco.12682
Kaushansky K (2006) Lineage-specific hematopoietic growth factors. N Engl J Med 354:2034–2045. https://doi.org/10.1056/NEJMra052706
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, Goldberg JM, Khatcheressian JL, Leighl NB, Perkins CL, Somlo G, Wade JL, Wozniak AJ, Armitage JO, American Society of Clinical Oncology (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 33:3199–3212. https://doi.org/10.1200/JCO.2015.62.3488
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C, European Organisation for Research and Treatment of Cancer (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32. https://doi.org/10.1016/j.ejca.2010.10.013
Crawford J, Becker PS, Armitage JO, Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I, Griffiths EA, Hough S, Kloth DD, Kuter DJ, Lyman GH, Mably M, Mukherjee S, Patel S, Perez LE, Poust A, Rampal R, Roy V, Rugo HS, Saad AA, Schwartzberg LS, Shayani S, Talbott M, Vadhan-Raj S, Vasu S, Wadleigh M, Westervelt P, Burns JL, Pluchino L (2017) Myeloid growth factors, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 15:1520–1541. https://doi.org/10.6004/jnccn.2017.0175
Wildiers H, Reiser M (2011) Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit Rev Oncol Hematol 77:221–240. https://doi.org/10.1016/j.critrevonc.2010.02.002
Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C (1995) Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer—the results of 20 years of follow-up. N Engl J Med 332:901–906. https://doi.org/10.1056/nejm199504063321401
Lambertini M, Del Mastro L, Bellodi A, Pronzato P (2014) The five “Ws” for bone pain due to the administration of granulocyte-colony stimulating factors (G-CSFs). Crit Rev Oncol Hematol 89:112–128. https://doi.org/10.1016/j.critrevonc.2013.08.006
Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V, Rausch G, Smith R, Gradishar W, Yahanda A, Vincent M, Stewart M, Glaspy J (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325:164–170. https://doi.org/10.1056/nejm199107183250305
Renner P, Milazzo S, Liu JP et al (2012) Primary prophylactic colony-stimulating factors for the prevention of chemotherapy-induced febrile neutropenia in breast cancer patients. Cochrane Database Syst Rev 10:Cd007913. https://doi.org/10.1002/14651858.CD007913.pub2
Aarts MJ, Grutters JP, Peters FP, Mandigers CM, Dercksen MW, Stouthard JM, Nortier HJ, van Laarhoven HW, van Warmerdam LJ, van de Wouw AJ, Jacobs EM, Mattijssen V, van der Rijt CC, Smilde TJ, van der Velden AW, Temizkan M, Batman E, Muller EW, van Gastel SM, Joore MA, Borm GF, Tjan-Heijnen VC (2013) Cost effectiveness of primary pegfilgrastim prophylaxis in patients with breast cancer at risk of febrile neutropenia. J Clin Oncol 31(34):4283–4289. https://doi.org/10.1200/JCO.2012.48.3644
National Cancer Institute. Common Terminology Criteria For Adverse Events (CTCAE) Version4.0.2009. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed 5 Aug 2020
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Early Breast Cancer Trialists’ Collaborative Group (1999) Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet 352:930–942. https://doi.org/10.1016/s0140-6736(98)03301-7
De Laurentiis M, Cancello G, D’Agostino D et al (2008) Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 26(1):44–53. https://doi.org/10.1200/JCO.2007.11.3787
Aapro M, Bernard-Marty C, Brain EG et al (2011) Anthracycline cardiotoxicity in the elderly cancer patient: a SIOG expert position paper. Ann Oncol 22:257–267. https://doi.org/10.1093/annonc/mdq609
Li Y, Family L, Yang SJ, Klippel Z, Page JH, Chao C (2017) Risk of febrile neutropenia associated with select myelosuppressive chemotherapy regimens in a large community-based oncology practice. J Natl Compr Cancer Netw 15:1122–1130. https://doi.org/10.6004/jnccn.2017.7010
Younis T, Rayson D, Thompson K (2012) Primary G-CSF prophylaxis for adjuvant TC or FEC-D chemotherapy outside of clinical trial settings: a systematic review and meta-analysis. Support Care Cancer 20:2523–2530. https://doi.org/10.1007/s00520-011-1375-6
Younus J, Vandenberg T, Jawaid M, Jawaid MA (2012) Febrile neutropenia rates with adjuvant docetaxel and cyclophosphamide chemotherapy in early breast cancer: discrepancy between published reports and community practice-an updated analysis. Curr Oncol 19:332–334. https://doi.org/10.3747/co.19.1174
Fernandes R, Mazzarello S, Stober C, Vandermeer L, Dudani S, Ibrahim MFK, Majeed H, Perdrizet K, Shorr R, Hutton B, Fergusson D, Clemons M (2017) Optimal primary febrile neutropenia prophylaxis for patients receiving docetaxel–cyclophosphamide chemotherapy for breast cancer: a systematic review. Breast Cancer Res Treat 161:1–10. https://doi.org/10.1007/s10549-016-4028-0
Ruddy KJ, Van Houten HK, Sangaralingham LR et al (2017) Impact of treatment regimen on acute care use during and after adjuvant chemotherapy for early-stage breast cancer. Breast Cancer Res Treat 164:515–525. https://doi.org/10.1007/s10549-017-4280-y
Barcenas CH, Niu J, Zhang N, Zhang Y, Buchholz TA, Elting LS, Hortobagyi GN, Smith BD, Giordano SH (2014) Risk of hospitalization according to chemotherapy regimen in early-stage breast cancer. J Clin Oncol 32:2010–2017. https://doi.org/10.1200/JCO.2013.49.3676
Kirshner JJ, McDonald MC, Kruter F et al (2018) NOLAN: a randomized, phase 2 study to estimate the effect of prophylactic naproxen or loratadine vs no prophylactic treatment on bone pain in patients with early-stage breast cancer receiving chemotherapy and pegfilgrastim. Support Care Cancer 26:1323–1334. https://doi.org/10.1007/s00520-017-3959-2
Ishikawa T, Sakamaki K, Narui K, Kaise H, Tsugawa K, Ichikawa Y, Mukai H, for Comprehensive Support Project for Oncological Research of Breast Cancer (2016) Prospective cohort study of febrile neutropenia in breast cancer patients with neoadjuvant and adjuvant chemotherapy: CSPOR-BC FN study. Jpn J Clin Oncol 46:692–695. https://doi.org/10.1093/jjco/hyw045
Ishikawa T, Sakamaki K, Narui K et al (2020) Prospective cohort study of febrile neutropenia in breast cancer patients with neoadjuvant and adjuvant chemotherapy: CSPOR-BC FN Study. Cancer Res 80:P5-14-09. https://doi.org/10.1158/1538-7445.sabcs19-p5-14-09
Acknowledgements
This study was supported by grants from the Japan Society for the Promotion of Science (JSPS) KAKENHI (20K17582).
Author information
Authors and Affiliations
Contributions
Yuri Kimura and Shinsuke Sasada contributed to the study conception and design. Yuri Kimura, Shinsuke Sasada, Akiko Emi, Norio Masumoto, and Takayuki Kadoya collected the clinical data. Yuri Kimura and Shinsuke Sasada analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the Medical University of Hiroshima (No. 1157).
Consent to participate
For this type of study, formal patient consent was not required.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kimura, Y., Sasada, S., Emi, A. et al. Febrile neutropenia and role of prophylactic granulocyte colony-stimulating factor in docetaxel and cyclophosphamide chemotherapy for breast cancer. Support Care Cancer 29, 3507–3512 (2021). https://doi.org/10.1007/s00520-020-05868-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05868-1