Abstract
Background
Febrile neutropenia (FN) is one of the most common and most critical adverse effects of chemotherapy. Despite many existing guidelines based on the use of granulocyte-colony stimulating factor (G-CSF), FN continues to impair the quality of life and interfere with the treatment of many patients. The purpose of this study was to assess the incidence and management of FN associated with chemotherapy for early breast cancer in routine clinical practice.
Methods
All patients with early-stage breast cancer (ESBC) treated by chemotherapy at Institut Curie, Hôpital René Huguenin, in 2014 were retrospectively included. The incidence and management of FN were reported. Risk factors associated with FN were studied by robust-error-variance Poisson regression.
Results
A total of 524 patients received either neoadjuvant (N = 130) or adjuvant chemotherapy (N = 394). Most patients (80%) were treated with a combination of 5-fluorouracil, epirubicin, and cyclophosphamide (FEC100; 3 cycles) followed by docetaxel 100 mg/m2 (D; 3 cycles). The overall incidence of FN was 17%. Eighteen percent of patients received primary prophylaxis (PP) for FN with G-CSF, using pegfilgrastim in 64% of cases and 74% of patients over the age of 70 received PP. Less than 5% of patients who received PP experienced FN. Recurrent FN after secondary prophylaxis was observed in 9% of patients. Forty-seven percent of cases of FN occurred after the first cycle and 30% occurred after the fourth cycle, corresponding to D ± trastuzumab (T). The FEC100 regimen was associated with a relative risk of FN of 1.98 (p = 0.09). Autoimmune (AI) and inflammatory diseases were associated with a higher risk of FN (RR 3.08; p < 0.01). No significant difference in the incidence of FN was observed between adjuvant and neoadjuvant chemotherapy. FN was managed on an outpatient basis in 72% of cases. Outpatients with FN were mainly treated by a combination of amoxicillin–clavulanic acid and ciprofloxacin. Dose reduction or chemotherapy regimen modification were necessary in 25% of patients after FN. No toxic death was reported.
Conclusion
The incidence of FN induced by adjuvant/neoadjuvant chemotherapy in ESBC is higher in routine clinical practice than in clinical trials. AI or inflammatory diseases were significant independent risk factors for FN. Primary prophylaxis in patients at risk (elderly, comorbid patients), especially treated with the FEC regimen, is the keystone of management of this adverse effect. Prevention and management of FN to ensure the patient’s safety and quality of life are a major issue for both medical oncologists and supportive care physicians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer in women in France and worldwide, with an estimated 54,062 new cases diagnosed in 2015. [1] As a result of many clinical trials, treatment has improved the overall survival of breast cancer. Most of the drugs used in the treatment of breast cancer can cause blood disorders, such as neutropenia. This common toxicity associated with fever is responsible for substantial morbidity, mortality, and escalating healthcare costs [2] and also leads to chemotherapy dose reductions, which compromise survival outcomes in the curative setting. [3]
A decreased incidence of FN has been clearly established in patients receiving granulocyte-colony stimulating factors (G-CSF), filgrastim and pegfilgrastim. [3, 4] Guidelines for the use of G-CSF based on the risk of FN have been published by several groups, such as the European Organization for Research and Treatment of cancer (EORTC), European Society for Medical Oncology (ESMO), and American Society of Clinical Oncology (ASCO). According to these guidelines, prophylactic use of G-CSF is recommended for patients with a clinically significant risk of FN, based on regimen- and patient-specific risk factors, such as age over 65, advanced disease, history of previous FN, no antibiotic prophylaxis or G-CSF use, poor performance status, and cardiovascular disease. [5,6,7]
However, multiple published studies have shown discrepancies in the reported incidence of FN. In the FNCLCC PACS 01 trial, in early-stage breast cancer (ESBC), the incidence of FN for patients on FEC-D was between 8.4 and 11.2%, [8] but more recent studies have shown that it could be higher than 20%. [9,10,11,12,13]
We therefore conducted a retrospective study in our center to determine the FN rate with FEC-D or other regimens in patients with ESBC. We also described the management of this complication and determined the influence of other risk factors on the incidence of FN.
Materials and methods
Patients with ESBC receiving chemotherapy between November 2013 and October 2014 at Institut Curie, Hôpital René Huguenin, were eligible.
This retrospective study and patient data collection were approved by the institutional breast cancer committee.
Data collection
The following data were recorded from the patients’ files: patient demographics, cancer stage according to the UICC TNM classification, hormone and HER2 receptor status, Ki67, medical history, history of breast cancer, chemotherapy and FN, use of G-CSF for primary prophylaxis (PP) or secondary prophylaxis (SP), lifestyle (working or not working, children under the age of18 years, living in couple or alone), hospital admission or outpatient care, length of hospital stay, antibiotics, chemotherapy dose adaptation, and/or regimen modification.
The regimen could be FEC100 (3 to 4 cycles) followed by D (3 to 4 cycles) every 3 weeks, FEC (3 to 4 cycles) every 3 weeks then paclitaxel weekly (9 to 12 cycles), docetaxel–cyclophosphamide (DC) (4 to 6 cycles) every 3 weeks ± T, liposomal-encapsulated doxorubicin citrate or adriamycin–cyclophosphamide (MC-AC) (3 to 6 cycles) every 3 weeks ± paclitaxel (9 to 12 cycles).
FN was defined as an oral temperature > 38.3 °C or two consecutive temperatures > 38 °C for 2 h and an absolute neutrophil count < 0.5 × 109/L or expected to fall below 0.5 × 109/L, in line with the ESMO definition. [7]
PP usually depended on the chemotherapy regimen and individual risk factors, such as age over 70 years and other comorbidities.
G-CSF was administrated by subcutaneous injection 24 to 72 h after chemotherapy and for 4 to 6 days in the case of daily G-CSF.
Cardiovascular risks factors included diabetes or dyslipidemia, and cardiovascular diseases included high blood pressure, coronary heart disease, and cardiac arrhythmias. Patients were considered to be smokers when they were active smokers at the beginning of treatment. Autoimmune (AI) or inflammatory diseases included inflammatory bowel disease; systemic diseases such as lupus, Sharp’s syndrome, sarcoidosis, and mastocytosis; and inflammatory rheumatism such as ankylosing spondylitis and rheumatoid arthritis. Patients treated with antidepressants, anxiolytics, or other psychiatric drugs were considered to have a psychiatric condition.
The management of patients with FN mainly consisted of strictly outpatient management (i.e., no previous visit to an emergency unit) or hospitalization to rapidly treat FN with broad-spectrum antibiotics.
Patients used to have written recommendations about management of FN for their general practitioner: the standard regimen for the therapy of low-risk febrile neutropenic patients was ciprofloxacin plus amoxicillin/clavulanic acid. [14]
Chemotherapy dose reduction was defined as > 15% dose reduction of any agent.
Study objectives
The primary objective of the trial was to determine the incidence of FN.
Secondary objectives were to determine whether certain patient characteristics were associated with an increased risk of FN, to describe the management of patients with FN, and to determine whether management complied with ESMO clinical practice guidelines. [7]
Statistical analysis
Quantitative variables were expressed as the mean ± standard deviation or median (range), and qualitative variables were expressed as number and percentage.
A robust-error-variance Poisson regression model was constructed to determine which patient characteristics were associated with FN; this model was used to compute relative risks. [15] All variables associated with FN with a p value less than 0.2 were included in a multivariate model, except for collinear variables. A stepwise variable selection procedure was performed to determine the final model (adding and removing thresholds of 0.20). Age was forced in the model, because it plays a major role in FN [7], as well PP administered to prevent FN.
A p value less than 0.05 was considered to be statistically significant.
Results
Patient characteristics
From November 2013 to October 2014, 524 patients (including one male) received chemotherapy for ESBC: 394 (75%) in the adjuvant setting, 130 (25%) in the neoadjuvant setting.
Demographic data and tumor characteristics of the study population are presented in Table 1.
About 75% of patients expressed estrogen receptor (ER+) and less than 20% overexpressed human epidermal growth factor receptor 2 (HER2+).
More than one half of patients (304; 58%) presented comorbidities: 55 (10%) were active smokers, 48 (9%) were treated for thyroid disorders, 26 (5%) had chronic obstructive pulmonary disease (COPD) or asthma, and 38 (7%) were treated for anxiety, depression, or another psychiatric disorder.
Twenty-four patients were followed for AI or inflammatory diseases:
Three of them were under immunosuppressive treatment before starting chemotherapy (methotrexate, mycophenolate, or anti TNF drug). Only one patient kept his treatment during chemotherapy for breast cancer (mycophenolate and low dose of steroids for a polyarteritis nodosa). One patient was under fludrocortisone and glucocortisone for Addison’s disease. Five patients were under low-dose steroids (2 to 7 mg) or mesalamine.
Most patients (418 patients; 80%) received the FEC-D chemotherapy regimen. (Fig. 1).
FN incidence
The incidence of FN in the overall cohort was 17%.
FN events mostly occurred during cycle 1 (N = 43; 47%) and cycle 4 (N = 27; 30%), corresponding to initiation of D.
Primary prophylaxis with G-CSF was used in 92 patients (18%); 64.1% of patients received pegfilgrastim.
When data were stratified by age, patients older than 70 (74.4%) were more likely to receive primary G-CSF prophylaxis than younger patients. Four of the 91 patients who received PP subsequently developed FN, compared to 87 of the 433 patients who did not receive PP.
Management of FN
FN was managed on an outpatient basis in 72% of cases. Oral dual-agent antibiotic therapy was the treatment most commonly prescribed for outpatients (N = 50; 55%). Single-agent antibiotic therapy was administered to 12 patients (13%).
Hospitalization for FN was required for 25 of the study patients (28%). Most of these patients were younger than 60 years (68%), and 75% were hospitalized in another institution.
Microbiologically proven and/or clinically documented infection was diagnosed in 32%, and bacteriemia was detected in 20% of patients. Only one patient had a urinary tract infection with multi resistant germ (Pseudomonas aeruginosa).
The median length of hospital stay was 6 days [4,5,6,7,8]. Twenty-one hospitalized patients received intravenous piperacillin/tazobactam.
Only 8 patients (7 on daily G-GSF) experienced a second episode of FN.
FN episodes required dose adaptation or a change of regimen for 22 patients (25%). No toxic deaths were observed.
Factors associated with FN
Univariate and multivariate analyses were performed on 502 patients and 85 patients with FN, respectively, because of missing data.
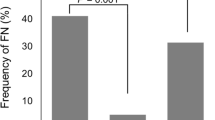
Univariate analysis did not reveal any significant difference in FN incidence as a function of age. Three (3%) of the 89 patients who received PP developed FN, compared to 82 (20%) of 413 patients who did not receive PP (p < 0.01).
The following variables were associated with FN on univariate analysis, with p < 0.2, and were therefore included in multivariate analysis: AI or inflammatory diseases, Ki67, FEC, and D (Table 2). As FEC + D was collinear with the two, the previous was not included in the multivariate analysis.
No significant difference in terms of FN rate was observed between adjuvant and neoadjuvant chemotherapy.
The addition of trastuzumab did not increase the FN incidence.
After adjustment for age and PP, AI or inflammatory diseases were associated with FN (RR = 3.08 [1.43–5.85]), whereas FEC100 was at the limit of significance (RR = 1.98 [0.96–4.8]) (Table 3).
Discussion
In this retrospective review of 524 ESBC patients treated with neoadjuvant or adjuvant chemotherapy, 17% of patients experienced FN. Most of these patients were treated by oral antibiotics. FEC chemotherapy and AI or inflammatory diseases were associated with an increased risk of FN.
FN incidence
The FN rate of 17% observed in this study is lower than the 21.9% rate reported in a review of 251 ESBC patients published in Supportive Care Cancer in 2014 [12], but these patients were older than those in our study, as the mean age of the population was 57.1 years vs 53.9 in our study. This study was also a retrospective study conducted in a single specialized center. Patients may have attended other healthcare facilities without informing the physician, leading to underestimation of the FN incidence.
Furthermore, in 2015, the French Opaline Study reported a very low rate of FN, less than 10%. [16] Similarly, in 2016, the incidence of FN was only 9% in the study by Maenpaa. [17] These results can be explained by increased use of G-CSF primary prophylaxis of 78.9 and 44% in these studies, respectively. Furthermore, 33.5 and 15.7% of patients in these two studies also received chemotherapy regimens associated with a risk of FN less than 10 vs 12.8% in our population.
Management of FN
Patients with FN were mostly treated in the outpatient clinic with oral antibiotics. According to the ESMO Clinical Practice Guidelines, the Multinational Association of Supportive Care in Cancer (MASCC) score has been validated to predict low-risk cases, in which complications are unlikely. [7] The criteria and weighting scores are listed in Table 4.
Patients with a MASCC score greater than or equal to 21 are at low risk of complications and can receive outpatient treatment when adequate follow-up is available, as exclusive outpatient management by oral antibiotics has become increasingly appealing on the grounds of the patient’s convenience, economy, and reduction in the incidence of nosocomial infections. In 2013, Kern published the first data in the JCO confirming that patients with a MASCC score indicating a low risk can generally be safely treated at home. [18] In our study, this population corresponded to 72% of all patients.
No patient required readmission. Only two patients had a MASCC score of 20 because of COPD which remained stable on specific treatment. To the best of our knowledge, this is the first study to describe outpatient management without a previous hospital assessment.
In 2012, Weycker estimated that 88% of patients required inpatient care. The feasibility of outpatient care was determined at an outpatient visit with an initial IV administration of antibiotics, [19] while only 28% of patients required hospitalization in our study.
Supportive care is also a major issue. Outpatients must be taught to monitor their symptoms and contact the appropriate doctor. In our hospital, we provide oral and written information to each patient about FN. Patients are able to contact an emergency unit to report any side effects and to be correctly assessed and managed. They are also advised to consult their general practitioners, who can used guideline sheets on the side effects of anticancer drugs [20].
Factors associated with FN
In line with the literature, the data of this study suggest an excess risk of FN in patients receiving FEC100 chemotherapy with an RR of 1.98, [0.96–4.8] after adjustment for age and PP in multivariate analysis (p = 0.09). In the present study, 19% (N = 78) of patients on FEC regimen experienced FN. These results are higher than the FN rate of 8.4% reported in the pivotal phase III PACS-01 clinical trial. [8] A French study recently reported a similar low result of 4.9%. [9]
Recent studies indicate that FEC-D is associated with a higher-than-expected rate of febrile neutropenia. In 2012, a systematic review and meta-analysis of 1342 patients estimated a 31% FN rate for FEC-D without G-CSF prophylaxis. [11] In our study, 21% FN rate occurred for FEC without G-CSF vs 13% for other chemotherapy regimen. For this situation, FEC is not considered as a significant risk factor in this subpopulation (p = 0.27), but there is a probable lack of power.
The Canadian non-clinical trial literature reported a range between 22.7 and 23.4% [21, 22]. The rate was significantly lower in those patients who received PP compared to those who did not receive PP [15/235 (6.4%) vs 137/436 (31.4%); p < 0.001; risk ratio 0.20] [21].
In 2014, Assi et al. reported an incidence of 26.2% FN with FEC-D and 31% without PP. [12] In 2015, Miguel concluded that G-CSF prophylaxis should be recommended in all patients receiving FEC-D because of a high rate of 27% of FN, with and without PP. [13]
Our results, in addition to those of several published studies, provide convincing evidence to prescribe PP for FEC-D regimen.
However, the low risk of myelodysplastic syndrome and acute myeloid leukemia must be considered. This risk is probably increased by AC-containing regimens and more than 6 days of filgrastim, which is rarely the case in clinical practice [23]. In the present study, this risk was not evaluated because it usually occurs much later after chemotherapy administration.
Seven patients on daily G-CSF experienced a second episode of FN, which could be explained by the low recommended dose of filgrastim (5 vs 7 days).
As recommended by ESMO guidelines, the risk factors for FN, other than chemotherapy itself, must be assessed: elderly patients, advanced disease, history of previous FN, no antibiotic prophylaxis or G-CSF use, mucositis, poor performance status, and cardiovascular disease [7].
In our study, the only comorbidity identified in patients with breast cancer was AI or inflammatory diseases and these diseases were associated with a higher risk of FN (RR 3.08; 95% CI [1.43–5.85]; p < 0.01).
Most of these patients were not receiving any concomitant treatment as anti cytokine. Recently, it has been proved that osteoarthritis was more prevalent in patients with FN (aOR, 1.85; 95% CI, 1.07 to 3.18) [24].
This significant risk factor could be evaluated in the overall assessment of FN risk before administering chemotherapy. Other studies are necessary to confirm these risk factors.
No significant difference was observed with other comorbidities in this study, probably due to lack of power. Medical files probably contained comprehensive data.
No significant difference in FN rate was observed as a function of age greater than or less than 65 years, which is consistent with the results reported by Assi et al. [12]. However, when data were stratified by age, patients older than 70 were more likely to receive G-CSF primary prophylaxis than younger patients.
Data on “living condition” were investigated because the fact of living alone, with young children, or working (which implies being in contact with people who could be contagious at work or in public transport) could constitute risk factors for FN. However, this analysis was probably not sufficiently powered to demonstrate any significant results.
Missing data, concerning 22 (0.4%) patients, were taken into account by excluding the patients concerned from the analysis of risk factors for FN.
In conclusion, we found a 17% FN rate among ESBC patients receiving adjuvant or neoadjuvant chemotherapy.
FEC100 chemotherapy and AI or inflammatory disease were identified as independent risk factors for FN. G-CSF primary prophylaxis should be considered more carefully in patients treated with FEC or presenting an AI or inflammatory disease.
According to the ESMO guidelines, and as confirmed in our study, patients with FN at low risk of complications should be treated as outpatients and receive a combination of oral antibiotics. This management will have a positive impact not only in terms of costs, but also on the patient’s quality of life.
References
Les cancers en France, édition2016, INCa 2017, http://www.e-cancer.fr/ressources/cancers_en_france/#page=13
Kuderer NM (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Lyman GH, Rolston (2010) How we treat febrile neutropenia in patients receiving cancer chemotherapy. J Oncol Pract 6(3):149–152
Kosaka Y (2015) Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy. Support Care Cancer 23(4):1137–1143
Smith TJ et al (2006) Update recommendations for the use of white blood cell growth factors: an evidence based clinical practice guideline. J Clinic Oncol 24(19):3187–3205
Aapro MS et al (2011) 2010 update of EORTC guidelines for the use of GCSF to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumors. Eur J Cancer 47(1):8–32
Klastersky J et al (2016) Management of febrile neutropaenia : ESMO clinical practice guidelines. Ann Oncol 27(Supplement 5):v111–v118
Roche H et al (2006) Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 trial. J Clin Oncol 24:5664–5671
Cousin S et al (2012) Febrile neutropenia incidence and hematological toxicity with the FEC100- docetaxel regimen in the treatment of early-stage breast cancer. Bull Cancer 99(7–8):75–80
Rayson D et al (2012) Incidence of febrile neutropenia during adjuvant chemotherapy for breast cancer: a prospective study. Curr Oncol 19(3):e216–e218
Younis T et al (2012) Primary G-CSF prophylaxis for adjuvant TC or FEC-D chemotherapy outside of clinical trial settings: a systemic review and meta-analysis. Support Care Cancer 20(10):2523–2530
Assi H et al (2014) Incidence of febrile neutropenia in early stage breast cancer patients receiving adjuvant FEC-D treatment. Support Care Cancer 22:3227–3234
Miguel I, Winckler P, Sousa M, Cardoso C, Moreira A, Brito M (2015) Febrile neutropenia in FEC-D regimen for early stage breast cancer: is there a place for G-CSF primary prophylaxis? Breast Dis 35(3):167–171
Hughes WT, Armstrong D, Bodey GP et al (2002) Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 34:730–751
Zou G (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706
Jacot W (2015) Granulocyte-colony stimulating factor (G-CSF) use in clinical practice in patients receiving chemotherapy for breast cancer: the Opaline Study. Bull Cancer 102(12):979–992
Maenpaa J (2016) The use of granulocyte colony stimulating factor (G-CSF) and management of chemotherapy delivery during adjuvant treatment for early-stage breast cancer: further observations from the IMPACT solid study. Breast 25:27–33
Kern WV (2013) Oral antibiotics for fever in low-risk neutropenic patients with cancer : a double-blind, randomized, multicenter trial comparing single daily moxifloxacin with twice daily ciprofloxacin plus amoxicillin/clavulanic acid combination therapy—EORTC infectious diseases group trial XV. JCO 31(9):1149–1156
Weycker D (2012) Risk and healthcare costs of chemotherapy-induced neutropenic complications in women with metastatic breast cancer. Chemotherapy 58(1):8–18
Rouge-Bugat ME (2015) Guideline sheets on the side effects of anticancer drugs are useful for general practitioners. Support Care Cancer 23(12):3473–3480
Madarnas Y (2011) Real-world experience with adjuvant FEC-D chemotherapy in four Ontario regional cancer centers. Curr Oncol 18(3):119–125
Raza S (2009) Relative dose intensity delivered to patients with early breast cancer: Canadian experience. Curr Oncol 16(6):8–12
Calip GS (2015) Myelodysplastic syndrome and acute myeloid leukemia following adjuvant chemotherapy with and without granulocyte colony-stimulating factors for breast cancer. Breast Cancer Res Treat 154:133–143
Li X, Luthra R, Morrow PK, Fisher MD, Reiner M, Barron RL, Langeberg WJ (2016) Comorbidities among patients with cancer who do and do not develop febrile neutropenia during the first chemotherapy cycle. J Oncol Pharm Pract 22(5):679–689
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
They have full control of all primary data and agree to allow the journal to review their data if requested.
Rights and permissions
About this article
Cite this article
Bacrie, J., Laurans, M., Iorio, P. et al. Febrile neutropenia in adjuvant and neoadjuvant chemotherapy for breast cancer: a retrospective study in routine clinical practice from a single institution. Support Care Cancer 26, 4097–4103 (2018). https://doi.org/10.1007/s00520-018-4280-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4280-4