Abstract
Purpose
Epirubicin and cyclophosphamide (EC) therapy, a major chemotherapy for patients with early-stage breast cancer, has a low risk (< 10%) of febrile neutropenia (FN). However, data used in reports on the incidence rate of FN were derived primarily from non-Asian populations. In this study, we investigated the FN incidence rate using EC therapy among Japanese patients with breast cancer and evaluated the significance of prophylactic administration of granulocyte colony-stimulating factor (G-CSF).
Methods
We evaluated medical records of patients with early-stage breast cancer who had been treated with EC therapy as neoadjuvant or adjuvant therapy between November 2014 and July 2018.
Results
The incidence rate of FN was 23.9%. In patients who received G-CSF as primary prophylaxis, FN expression was completely suppressed. The incidence rate of severe leucopenia/neutropenia, emergency hospitalization, and the use of antimicrobial agents were low in patients receiving primary prophylaxis with G-CSF compared with those not receiving G-CSF (27.3% vs. 64.8%, 9.1% vs. 27.3%, and 27.3% vs. 71.6%, respectively). Furthermore, in all patients who received primary prophylaxis with G-CSF, a relative dose intensity > 85% using EC therapy was maintained.
Conclusion
The incidence of FN in EC therapy among Japanese patients was higher than expected, EC therapy appears to be a high-risk chemotherapy for FN, and prophylactic administration of G-CSF is recommended. Maintaining high therapeutic intensity is associated with a positive prognosis for patients with early breast cancer, and prophylactic administration of G-CSF is likely to be beneficial in treatment involving EC therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer chemotherapy is widely used for patients with early to advanced stage breast cancer and is a key treatment that critically affects prognosis. It is reported that many patients who have undergone chemotherapy for breast cancer have received dose reductions and/or delays in receiving chemotherapeutic agents, and consequently a reduced relative dose intensity (RDI) in relation to cancer chemotherapy [1,2,3]. Dose intensity (DI) refers to the dose of medication administered per unit time, and RDI is defined as the actual DI relative to the planned DI and is indicative of DI [4]. In early-stage breast cancer, there is a close relationship between the RDI and outcome in cancer chemotherapy, and it is considered important to maintain an RDI in neoadjuvant/adjuvant chemotherapy above 85% [5, 6].
Neutropenia is responsible for dose-limiting toxicity in most antineoplastic agents, and is also a major cause of decreasing RDI in perioperative breast cancer chemotherapy [2]. The onset of febrile neutropenia (FN) after cancer chemotherapy is a serious and potentially life-threatening condition, and incidence rates of early mortality have been reported as significantly higher in cancer patients with FN compared with patients without FN [7]. In perioperative cancer chemotherapy for patients with early-stage breast cancer, management of neutropenia and FN is important in maintaining the RDI at a high level and for survival prognosis. Granulocyte colony-stimulating factor (G-CSF) reduces the risk of neutropenia and FN by acting on neutrophil progenitor cells, promoting differentiation and proliferation of these cells, and stimulating both the release of mature neutrophils from bone marrow and neutrophil function. Currently, it is recommended that G-CSF be administered prophylactically in relation to FN according to the risk of hematologic toxicity, as recommended in American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), European Organization for Research Treatment of Cancer, European Society for Medical Oncology Cancer (EORTC), and Japanese Society of Medical Oncology (JASMO) guidelines. Prophylactic G-CSF administration should be part of chemotherapy in which there is a > 20% or greater incident rate of FN in any complication. Similarly, when the FN expression rate from chemotherapy is between 10 and 20%, preventive administration of G-CSF is recommended for patients with FN risk factors. In the JASMO guidelines, these risk factors are listed as ≥ 65 years old, and a history of cancer treatment, radiation treatment, performance status (PS) limitations, liver function disorder, renal dysfunction, recent surgical intervention, and a history of neutropenia prior to treatment. In recent years, in addition to the daily G-CSF conventionally used, a sustained G-CSF involving pegfilgrastim (a pegylated form of filgrastim) has been approved to decrease the incidence of infection due to FN, and it is considered to be more effective in patients with early-stage breast cancer [8].
Epirubicin and cyclophosphamide (EC) therapy is a key regimen for neoadjuvant or adjuvant chemotherapy in patients with primary breast cancer. In previous studies, the incidence rate of FN in EC therapy has been reported as being as low as from 3.0 to 5.4% [9,10,11], and, in EC therapy followed by paclitaxel or docetaxel chemotherapy, the incidence rate of FN has been reported to range from 1.3 to 11.3% [12,13,14]. According to the relevant guidelines, prophylactic administration of G-CSF as part of EC therapy is not recommended. However, most reports on which this recommendation is based have involved research on non-Asian populations, and there are few reports of FN expression rates in EC therapy among Asian populations and none involving Japanese people. It has been reported that the side effects of cancer chemotherapy differ among ethnic groups, and that people of Asian ethnicity have more serious hematological toxicity than other ethnic groups [15, 16]. In this study, we investigated the incidence of FN and the appropriateness and effectiveness of prophylactic administration of G-CSF in EC therapy as a neoadjuvant or adjuvant chemotherapy among Japanese patients with early-stage breast cancer.
Materials and methods
Patients and methods
We investigated the medical records of patients with early-stage breast cancer who had been treated with EC therapy between November 2014 and July 2018 in the Department of Thoracic Endocrine Surgery and Oncology at Tokushima University Hospital. Patients who had been administered EC therapy at other hospitals and patients whose initial dose of epirubicin or cyclophosphamide had been reduced for reasons such as decreased liver and renal function were excluded. We recorded patient information including age, sex, body mass index (BMI), laboratory data, histology, clinical staging, history of treatment, use status of G-CSF and antibacterial drugs, onset of FN, and whether emergency hospitalization had occurred. Daily G-CSF was defined as involving filgrastim and lenograstim, and sustained G-CSF was defined as involving pegfilgrastim. FN was defined as present in cases where there was a single fever involving an axillary temperature of ≥ 37.5 °C (intraoral temperature of ≥ 38.0 °C) in a state in which there was an absolute neutrophil count (ANC) of < 500 cells/mm3, or when expecting a decrease in the ANC to < 500 cells/mm3 within 48 h when the ANC was < 1000 cells/mm3. Hematologic toxicities were evaluated according to the Common Terminology Criteria for Adverse Events version 4.0.
This study protocol was reviewed and approved by the Ethics Committee of Tokushima University Hospital (approval number: 2997).
Statistical analysis
Fisher’s exact test or Bowker’s test of symmetry were used for comparison between categorical data, as appropriate. Group comparisons for continuous variables were undertaken using a Mann–Whitney U test. To assess the relationship between an RDI decrease and patient characteristics and side effects, univariate analyses were performed using Fisher’s exact test and logistic regression analysis. The RDI was calculated according to the following equation:
RDI (%) = actual total dose per week (mg/m2/week)/standard planned total dose per week (mg/m2/week), where the standard planned total dose = epirubicin at 90 mg/m2 and cyclophosphamide at 600 mg/m2 over 3 weeks per one course.
We performed the statistical analyses using JMP version 14.0 (SAS Institute Inc, Cary, NC), and p values < 0.05 were considered statistically significant.
Results
Patient characteristics
During the investigation period, 103 patients with early-stage breast cancer received EC therapy as neoadjuvant or adjuvant chemotherapy. Of these, four were excluded because they had a decreased initial dose of epirubicin or cyclophosphamide due to liver dysfunction or other complicating disease. The clinical characteristics of the 99 target patients are listed in Table 1. The median patient age was 58 years [interquartile range (IQR), 48–66 years], and many patients of working age (< 65 years old) were included. Most patients (76.8%) were categorized as being in stage I or II, indicating that high long-term survival could be expected. Three to six cycles of EC therapy were planned for nearly all patients. One patient underwent surgery after two cycles of EC therapy because of rapid tumor enlargement. The blood cell count before chemotherapy administration was maintained within a normal range, and liver and renal functions were also satisfactory.
Filgrastim and lenograstim were used in therapeutic administration and pegfilgrastim was used in prophylactic administration. Pegfilgrastim was used as primary prevention for 11 patients and as secondary prevention for 14 patients. Twenty-three patients had mild side effects due to leucopenia and neutropenia, and these patients did not require G-CSF.
The effect of prophylactic administration of G-CSF on the side effects of EC therapy
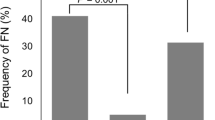
The incidence rate for FN in EC therapy was 23.9% in patients with perioperative breast cancer without prophylactic G-CSF. In contrast, no patients developed FN with a primary prophylaxis of G-CSF (Fig. 1a). Although EC therapy involves a strong myelosuppression, the onset of grade 4 leucopenia/neutropenia was significantly suppressed with prophylactic administration of G-CSF compared to non-administration (27.3% vs. 64.8%, respectively, p < 0.05) (Fig. 1b).
The effect of primary prophylaxis of G-CSF in epirubicin and cyclophosphamide therapy. a, b, c The incidence rate for febrile neutropenia (FN), grade 4 leucopenia/neutropenia, and emergency hospitalization in Tokushima University Hospital or other hospitals. The frequency of occurrence rate for FN in the G-CSF primary prophylaxis administration and non-administration groups was 0.0% and 23.9%, respectively. The frequency of occurrence rate for grade 4 leucopenia/neutropenia in the G-CSF primary prophylaxis administration and non-administration groups was 27.3% and 64.8%, respectively. The frequency of occurrence rate for emergency hospitalization in the G-CSF primary prophylaxis administration and non-administration groups was 9.1% and 27.3%, respectively. d The antimicrobial agent use rate. The frequency rate for antimicrobial agent use in the G-CSF primary prophylaxis administration and non-administration groups was 27.3% and 71.6%, respectively. Statistical analyses were performed using Fisher’s exact test. *p < 0.05; **p < 0.01
Antimicrobial agents were mostly used to prevent the onset of FN or for prophylactic administration in patients for whom severe myelosuppression was expected to continue for prolonged periods. Twenty-five (25.3%) patients experienced emergency hospitalization or an extended period of hospitalization, of whom 76.0% had severe myelosuppression of FN development. The frequency of antimicrobial agent use and emergency hospitalization or extended periods of hospitalization were also reduced with prophylactic administration of G-CSF (Fig. 1c, d).
Figure 2 shows the results of investigating the effects on patients using G-CSF as secondary prophylaxis (n = 14). Secondary prophylactic administration of G-CSF was effective for hematological toxicity due to EC therapy as well as for primary prophylaxis.
The effect of secondary prophylaxis of G-CSF in epirubicin and cyclophosphamide therapy. This analysis only included patients who had received G-CSF as secondary prophylaxis (n = 14). a, b The incidence rate for febrile neutropenia (FN) and grade 4 leucopenia/neutropenia. a The frequencies of occurrence rate for FN in the G-CSF secondary prophylaxis group pre-and post-administration were 49.2% and 7.1%, respectively. b The frequencies of occurrence rate for grade 4 leucopenia/neutropenia in G-CSF secondary prophylaxis pre-and post-administration were 92.9% and 28.6%, respectively. Statistical analyses were performed using Bowker’s test. *p < 0.01
The relationship between the use of G-CSF and the RDI in EC therapy
Among the 99 enrolled breast cancer patients, 47 patients (47.5%) had reduced and/or delayed EC chemotherapy. Myelosuppression was primarily responsible for dose reduction/delay, with incidence rates of 70.2% (33/47 patients). Although EC therapy is a highly emetogenic chemotherapy, dose reduction or postponement associated with vomiting, nausea, or anorexia was only observed in 7.4% (3/47) of patients.
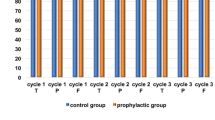
The median average RDI (ARDI) using EC therapy was 94.6% (IQR 90.7–97.7%), keeping at ≥ 85%, which is necessary for the appropriate intensity of perioperative breast cancer chemotherapy. The median RDI of epirubicin and cyclophosphamide was 94.6% (IQR 90.7–97.7%) and 97.0% (IQR 93.5–99.5%), respectively. In patients undergoing a primary prophylactic administration of G-CSF and in those not undergoing it, an RDI > 85.0% was maintained in most patients (Fig. 3a, b). The percentage of patients who were able to maintain an RDI > 85.0% was 85.7% in patients undergoing a secondary prophylactic administration of G-CSF and 78.4% in patients with only a therapeutic administration of G-CSF (Fig. 3b).
The effect of secondary prophylaxis of G-CSF in epirubicin and cyclophosphamide therapy. The average relative dosing intensity (ARDI) of epirubicin and cyclophosphamide (a) and the percentage of patients with an ARDI > 85%, and (b) according to the use of G-CSF. a The median ARDI in the primary prevention, secondary prevention, therapeutic administration, and non-administration groups was 96.0%, 91.3%, 94.3%, and 97.0%, respectively. b The percentage of patients who maintained an ARDI > 85% in the primary prevention, secondary prevention, therapeutic administration, and non-administration groups was 100%, 85.7%, 78.4%, and 95.7%, respectively
Risk factors for RDI reduction
We evaluated whether there were factors contributing to the development of a lowering RDI. Developing FN, grade 4 leucopenia/neutropenia, and anemia at grade 3 or higher were found to be significant risk factors for RDI reduction in EC therapy in the univariate analysis [odds ratio (OR) 3.59, 95% confidence interval (CI) 1.31–11.00; OR 5.76, 95% CI 2.39–14.97; OR 7.46, 95% CI 1.21–143.88, respectively, Table 2]. However, in the multivariate analysis, only grade 4 leucopenia/neutropenia was identified as a risk factor for RDI reduction in EC therapy (OR 5.08, 95% CI 2.07–13.39; Table 2). There were no significant factors involved in RDI reduction in terms of the patient characteristics. Additionally, we investigated whether there were specific risk factors for severe leucopenia/neutropenia in the patient characteristics. The odds ratios of age (≥ 65 years), BMI (≥ 25), a history of chemotherapy treatment, white blood cell counts [< lower limit of normal (LLN)], and neutrophil counts (< LLN) were 2.24 (95% CI 0.88–5.73), 0.44 (95% CI 0.18–1.08), 1.33 (95% CI 0.31–5.68), 2.00 (95% CI 0.20–19.95), and 0.96 (95% CI 0.15–6.06), respectively, and no significant factor was detected.
Discussion
Cancer chemotherapy involving the use of anthracycline anticancer drugs is an indispensable treatment for many patients with early-stage breast cancer patients [17, 18]. It has been reported that the frequency of FN expression in EC therapy was from 3.0 to 5.4% among German patients, 13.8% among patients who were primarily Chinese, and 25.2% among Korean patients [9, 10, 19, 20]. The side effect profile of each chemotherapy differs among ethnic groups and it is important to evaluate safety specifically in relation to each ethnic group. To our knowledge, there has been no previous report on the frequency of FN expression in EC therapy among Japanese patients. In this study, EC therapy was observed to be a strong myelosuppressive treatment for Japanese patients. We showed that the frequencies of grade 4 leucopenia/neutropenia and FN were 65.9% and 23.9% without primary prophylactic G-CSF. Of 88 patients who did not receive primary prophylaxis with G-CSF, 65 patients (73.9%) required therapeutic administration of G-CSF. The risk of hematologic toxicity is considered high in Japanese compared to Western patients and, in this regard, the results of this study corresponded with previous reports [8, 15, 16, 21]. Pegfilgrastim is effective for suppressing the onset of FN caused through cancer chemotherapy, and it was used in 25 patients (25.3%) in this study. G-CSF support using pegfilgrastim was effective in reducing the incidence of FN and severe leucopenia/neutropenia. Broad-spectrum antimicrobial agents are used when treating FN, and fluoroquinolone antibiotics are recommended for FN prophylaxis. However, it has been reported that infections such as bacteremia are being generated as a result of widespread use of broad-spectrum antimicrobial drugs that has led to resistant bacteria [22, 23]. The frequency of antibiotic use was significantly lower in patients who received pegfilgrastim as primary prevention (27.3% vs. 71.6%). It appears that using a primary administration of G-CSF in EC therapy also inhibits the emergence and increase of drug-resistant bacteria.
Neoadjuvant/adjuvant breast cancer chemotherapy requires maintaining an RDI > 85% to be effective. Bonadonna et al. reported that patients who were able to maintain a scheduled dose > 85% had better progression-free survival and overall survival (OS) than patients with a scheduled dose < 85% among perioperative patients with breast cancer who received cyclophosphamide, methotrexate, and fluorouracil therapy [5]. Significantly, the prognosis of patients with a scheduled dose < 85% was the same as a control group who did not receive chemotherapy [5]. In a retrospective study concerning patients with early-stage breast cancer who received anthracycline-based adjuvant chemotherapy, it was reported that disease-free survival and OS among the patient group with an RDI > 85% had been significantly longer than among the patient group with an RDI < 85% [6]. Almost 50% of the patients enrolled in this study experienced dose reduction and/or delays in EC therapy. However, the ARDI in this study was maintained at a high level, with 85 patients (85.9%) able to maintain ARDI of ≥ 85%. We found the risk factors for a reduction in ARDI were severe leucopenia/neutropenia and the onset of FN. In several previous studies, various factors such as age, BMI, and laboratory data prior to chemotherapy administration have been reported as risk factors for leukocytes and neutropenia [24,25,26,27]. Patients with early-stage breast cancer are likely to have less risk of hematologic toxicity because they tend to be younger, and have undergone fewer chemotherapy treatments and less radiation therapy compared with patients with advanced cancer and other carcinomas [28,29,30]. In this study, no risk factors for hematologic toxicity such as age and bone marrow function before EC therapy administration were identified, as predicted. FN and severe hematologic toxicity were primarily responsible for lowering treatment intensity, and G-CSF was used prophylactically and therapeutically, which eventually allowed the maintenance of a high ARDI. Although EC therapy involves a high emetic risk regimen, dose reduction and delay due to nausea and vomiting were limited (3.0%). Aprepitant, dexamethasone, and palonosetron were used for all patients as antiemetic therapies, and olanzapine and metoclopramide were used according to specific case requirements. It appeared that nausea and vomiting were sufficiently manageable with these antiemetic agents.
This study was a retrospective study, concerned with the possibility of FN occurrence as a side effect, but it could not exclude other possible side effects, which is a major limitation of this study. Among the patients investigated, there may have been patients who had been hospitalized or who had received antibiotics at other hospitals. If this had been the case, then these factors were likely to have played some role. Furthermore, in terms of FN occurrence and emergency hospitalization, although we observed the efficacy of G-CSF prophylactic administration, the lack of statistical significance found may be due to limited power because of the small number of patients who received G-CSF for primary or secondary prophylaxis. However, since few patients who received G-CSF prophylactic administration developed FN and required emergency hospitalization, we consider that G-CSF prophylactic administration is effective for EC therapy.
In conclusion, we showed that the incidence rate of FN induced due to EC therapy in Japanese patients was ≥ 20%, indicating a high risk rate. In addition, we found that primary and secondary prophylactic administrations of G-CSF were effective in reducing the risk of FN. It appears from these findings that it is appropriate to perform primary prophylactic administration of G-CSF in EC therapy. In recent years, cancer chemotherapy has been increasingly administered at outpatient departments to maintain patient quality of life. Most of the cases in this study involved outpatients undergoing EC therapy. We recommend prophylactic administration of G-CSF to facilitate a safer and more effective EC therapy.
References
Weycker D, Barron R, Edelsberg J, Kartashov A, Lyman GH (2012) Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat 133:301–310
Link BK, Budd GT, Scott S, Dickman E, Paul D, Lawless G, Lee MW, Fridman M, Ford J, Carter WB (2001) Delivering adjuvant chemotherapy to women with early-stage breast carcinoma: current patterns of care. Cancer 92:1354–1367
Lyman GH, Dale DC, Crawford J (2003) Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol 21(24):4524–4531
Epelbaum R, Haim N, Ben-Shahar M, Ron Y, Cohen Y (1988) Dose-intensity analysis for CHOP chemotherapy in diffuse aggressive large cell lymphoma. Isr J Med Sci 24:533–538
Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C (1995) Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med 332:901–906
Chirivella I, Bermejo B, Insa A, Perez-Fidalgo A, Magro A, Rosello S, Garcia-Garre E, Martin P, Bosch A, Lluch A (2009) Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat 114:479–484
Lyman GH, Michels SL, Reynolds MW, Barron R, Tomic KS, Yu J (2010) Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 116:5555–5563
Kosaka Y, Rai Y, Masuda N, Takano T, Saeki T, Nakamura S, Shimazaki R, Ito Y, Tokuda Y, Tamura K (2015) Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy. Support Care Cancer 23:1137–1143
Blohmer JU, Schmid P, Hilfrich J, Friese K, Kleine-Tebbe A, Koelbl H, Sommer H, Morack G, Wischnewsky MB, Lichtenegger W, Kuemmel S (2010) Epirubicin and cyclophosphamide versus epirubicin and docetaxel as first-line therapy for women with metastatic breast cancer: final results of a randomised phase III trial. Ann Oncol 21:1430–1435
Untch M, Muscholl M, Tjulandin S, Jonat W, Meerpohl HG, Lichinitser M, Manikhas AG, Coumbos A, Kreienberg R, du Bois A, Harbeck N, Jackisch C, Muller V, Pauschinger M, Thomssen C, Lehle M, Catalani O, Luck HJ (2010) First-line trastuzumab plus epirubicin and cyclophosphamide therapy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: cardiac safety and efficacy data from the Herceptin, Cyclophosphamide, and Epirubicin (HERCULES) trial. J Clin Oncol 28:1473–1480
Eckhoff L, Nielsen M, Moeller S, Knoop A (2011) TAXTOX—a retrospective study regarding the side effects of docetaxel given as part of the adjuvant treatment to patients with primary breast cancer in Denmark from 2007 to 2009. Acta Oncol 50:1075–1082
Margolin S, Bengtsson NO, Carlsson L, Edlund P, Hellstrom M, Karlsson P, Lidbrink E, Linderholm B, Lindman H, Malmstrom P, Pettersson Skold D, Soderberg M, Villman K, Bergh J (2011) A randomised feasibility/phase II study (SBG 2004-1) with dose-dense/tailored epirubicin, cyclophosphamide (EC) followed by docetaxel (T) or fixed dosed dose-dense EC/T versus T, doxorubicin and C (TAC) in node-positive breast cancer. Acta Oncol 50:35–41
Schonherr A, Aivazova-Fuchs V, Annecke K, Juckstock J, Hepp P, Andergassen U, Augustin D, Simon W, Wischnik A, Mohrmann S, Salmen J, Zwingers T, Kiechle M, Harbeck N, Friese K, Janni W, Rack B (2012) Toxicity analysis in the ADEBAR trial: sequential anthracycline–taxane therapy compared with FEC120 for the adjuvant treatment of high-risk breast cancer. Breast Care (Basel) 7:289–295
Martin M, Ruiz Simon A, Ruiz Borrego M, Ribelles N, Rodriguez-Lescure A, Munoz-Mateu M, Gonzalez S, Margeli Vila M, Barnadas A, Ramos M, Del Barco Berron S, Jara C, Calvo L, Martinez-Janez N, Mendiola Fernandez C, Rodriguez CA, Martinez de Duenas E, Andres R, Plazaola A, de la Haba-Rodriguez J, Lopez-Vega JM, Adrover E, Ballesteros AI, Santaballa A, Sanchez-Rovira P, Baena-Canada JM, Casas M, del Carmen Camara M, Carrasco EM, Lluch A (2015) Epirubicin plus cyclophosphamide followed by docetaxel versus epirubicin plus docetaxel followed by capecitabine as adjuvant therapy for node-positive early breast cancer: results from the GEICAM/2003-10 study. J Clin Oncol 33:3788–3795
Gandara DR, Kawaguchi T, Crowley J, Moon J, Furuse K, Kawahara M, Teramukai S, Ohe Y, Kubota K, Williamson SK, Gautschi O, Lenz HJ, McLeod HL, Lara PN Jr, Coltman CA Jr, Fukuoka M, Saijo N, Fukushima M, Mack PC (2009) Japanese–US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol 27:3540–3546
Hasegawa Y, Kawaguchi T, Kubo A, Ando M, Shiraishi J, Isa S, Tsuji T, Tsujino K, Ou SH, Nakagawa K, Takada M (2011) Ethnic difference in hematological toxicity in patients with non-small cell lung cancer treated with chemotherapy: a pooled analysis on Asian versus non-Asian in phase II and III clinical trials. J Thorac Oncol 6:1881–1888
Blum JL, Flynn PJ, Yothers G, Asmar L, Geyer CE Jr, Jacobs SA, Robert NJ, Hopkins JO, O’Shaughnessy JA, Dang CT, Gomez HL, Fehrenbacher L, Vukelja SJ, Lyss AP, Paul D, Brufsky AM, Jeong JH, Colangelo LH, Swain SM, Mamounas EP, Jones SE, Wolmark N (2017) Anthracyclines in early breast cancer: the ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol 35:2647–2655
Watanabe T, Kuranami M, Inoue K, Masuda N, Aogi K, Ohno S, Iwata H, Mukai H, Uemura Y, Ohashi Y (2017) Comparison of an AC-taxane versus AC-free regimen and paclitaxel versus docetaxel in patients with lymph node-positive breast cancer: final results of the National Surgical Adjuvant Study of Breast Cancer 02 trial, a randomized comparative phase 3 study. Cancer 123:759–768
Kim CG, Sohn J, Chon H, Kim JH, Heo SJ, Cho H, Kim IJ, Kim SI, Park S, Park HS, Kim GM (2016) Incidence of febrile neutropenia in Korean female breast cancer patients receiving preoperative or postoperative doxorubicin/cyclophosphamide followed by docetaxel chemotherapy. J Breast Cancer 19:76–82
Chan A, Chen C, Chiang J, Tan SH, Ng R (2012) Incidence of febrile neutropenia among early-stage breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer 20:1525–1532
Jones SE, Savin MA, Holmes FA, O’Shaughnessy JA, Blum JL, Vukelja S, McIntyre KJ, Pippen JE, Bordelon JH, Kirby R, Sandbach J, Hyman WJ, Khandelwal P, Negron AG, Richards DA, Anthony SP, Mennel RG, Boehm KA, Meyer WG, Asmar L (2006) Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 24:5381–5387
Kimura M, Araoka H, Yoshida A, Yamamoto H, Abe M, Okamoto Y, Yuasa M, Kaji D, Kageyama K, Nishida A, Ishiwata K, Takagi S, Yamamoto G, Asano-Mori Y, Uchida N, Hishinuma A, Izutsu K, Wake A, Taniguchi S, Yoneyama A (2016) Breakthrough viridans streptococcal bacteremia in allogeneic hematopoietic stem cell transplant recipients receiving levofloxacin prophylaxis in a Japanese hospital. BMC Infect Dis 16:372
Busca A, Cavecchia I, Locatelli F, D’Ardia S, De Rosa FG, Marmont F, Ciccone G, Baldi I, Serra R, Gaido E, Falda M (2012) Blood stream infections after allogeneic stem cell transplantation: a single-center experience with the use of levofloxacin prophylaxis. Transpl Infect Dis 14:40–48
Jenkins P, Freeman S (2009) Pretreatment haematological laboratory values predict for excessive myelosuppression in patients receiving adjuvant FEC chemotherapy for breast cancer. Ann Oncol 20:34–40
Tang NL, Liao CD, Wang X, Mo FK, Chan VT, Ng R, Pang E, Suen JJ, Woo J, Yeo W (2013) Role of pharmacogenetics on adjuvant chemotherapy-induced neutropenia in Chinese breast cancer patients. J Cancer Res Clin Oncol 139:419–427
Aslani A, Smith RC, Allen BJ, Pavlakis N, Levi JA (2000) The predictive value of body protein for chemotherapy-induced toxicity. Cancer 88:796–803
Rabinowitz AP, Weiner NJ, Tronic BS, Fridman M, Liberman RF, Delgado DJ (2006) Severe neutropenia in CHOP occurs most frequently in cycle 1: a predictive model. Leuk Lymphoma 47:853–858
Sakurada T, Kakiuchi S, Tajima S, Horinouchi Y, Okada N, Nishisako H, Nakamura T, Teraoka K, Kawazoe K, Yanagawa H, Nishioka Y, Minakuchi K, Ishizawa K (2015) Characteristics of and risk factors for interstitial lung disease induced by chemotherapy for lung cancer. Ann Pharmacother 49:398–404
Sakurada T, Kakiuchi S, Tajima S, Horinouchi Y, Konaka K, Okada N, Nishisako H, Nakamura T, Teraoka K, Kawazoe K, Yanagawa H, Nishioka Y, Ishizawa K (2015) Pemetrexed-induced rash may be prevented by supplementary corticosteroids. Biol Pharm Bull 38:1752–1756
Okada N, Hanafusa T, Sakurada T, Teraoka K, Kujime T, Abe M, Shinohara Y, Kawazoe K, Minakuchi K (2014) Risk factors for early-onset peripheral neuropathy caused by vincristine in patients with a first administration of R-CHOP or R-CHOP-like chemotherapy. J Clin Med Res 6:252–260
Acknowledgements
The author(s) received no grants or funding for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakurada, T., Bando, S., Zamami, Y. et al. Prophylactic administration of granulocyte colony-stimulating factor in epirubicin and cyclophosphamide chemotherapy for Japanese breast cancer patients: a retrospective study. Cancer Chemother Pharmacol 84, 1107–1114 (2019). https://doi.org/10.1007/s00280-019-03948-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03948-6