Abstract
Key message
Pollen-specific expression.
Abstract
Promoters comprise of various cis-regulatory elements which control development and physiology of plants by regulating gene expression. To understand the promoter specificity and also identification of functional cis-acting elements, progressive 5′ deletion analysis of the promoter fragments is widely used. We have evaluated the activity of regulatory elements of 5′ promoter deletion sequences of anther-specific gene OSIPP3, viz. OSIPP3-∆1 (1504 bp), OSIPP3-∆2 (968 bp), OSIPP3-∆3 (388 bp) and OSIPP3-∆4 (286 bp) through the expression of transgene GUS in rice. In silico analysis of 1504-bp sequence harboring different copy number of cis-acting regulatory elements such as POLLENLELAT52, GTGANTG10, enhancer element of LAT52 and LAT56 indicated that they were essential for high level of expression in pollen. Histochemical GUS analysis of the transgenic plants revealed that 1504- and 968-bp fragments directed GUS expression in roots and anthers, while the 388- and 286-bp fragments restricted the GUS expression to only pollen, of which 388 bp conferred strong GUS expression. Further, GUS staining analysis of different panicle development stages (P1–P6) confirmed that the GUS gene was preferentially expressed only at P6 stage (late pollen stage). The qRT-PCR analysis of GUS transcript revealed 23-fold higher expression of GUS transcript in OSIPP3-Δ1 followed by OSIPP3-Δ2 (eightfold) and OSIPP3-Δ3 (threefold) when compared to OSIPP3-Δ4. Based on our results, we proposed that among the two smaller fragments, the 388-bp upstream regulatory region could be considered as a promising candidate for pollen-specific expression of agronomically important transgenes in rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spatial and temporal expression of genes is regulated by the molecular switches called promoters. Promoters comprise of various motifs and domains (cis-regulating elements) which control development and physiology by regulating gene expression, and they are being used for genetic manipulation (Hamilton et al. 1998; Wittkopp and Kalay 2012). Several organ- or tissue-specific promoters have been cloned and characterized in different plants such as flower-specific promoter (chi-A) of petunia (Van Tunen et al. 1988), fruit-specific promoter (2A11) of tomato (Pear et al. 1989), root-specific promoter (TobRB7) of tobacco (Yamamoto et al. 1991), phloem-specific promoter (TGG1) of Arabidopsis (Husebye et al. 2002) and reproductive organs as well as vascular tissue-specific promoter of rice (OsPMCa2+ATPase) (Huda et al. 2013). Similarly, pollen-specific promoters were isolated and analyzed from various plant species (Chen et al. 2010; Hamilton et al. 2000; Rogers et al. 1992; Zou et al. 1994). Suwabe et al. (2008) demonstrated that 156 genes specifically expressed in rice anthers and based on the tissue specificity, the genes were further classified as anther-/tapetum-specific. In rice, a few promoters of anther-/pollen-specific genes have been characterized using reporter gene GUS/GFP in either heterologous system (Arabidopsis or tobacco) or homologous system (rice), for example Osg6B (Yokoi et al. 1997), RTS (Luo et al. 2006), OSIPK (Gupta et al. 2007), OsSCP1, OsSCP2, OsSCP3 (Park et al. 2006), YY2 (Kuriakose et al. 2009), OSIPA (Swapna et al. 2011), OryS1 (Azria and Bhalla 2011), OsLTP6 (Liu et al. 2013), OsbHLH, OSFBOX (Khurana et al. 2013a), OSIPP3 (Khurana et al. 2013b) and OsLSP1 to OsLSP9 (Oo et al. 2014).
The major application of anther-/pollen-specific promoter would be to use cytotoxic gene to generate cytoplasmic male sterility (CMS) for hybrid rice seed production. Recently, an anther-specific gene, OSIPP3 encoding pectin methylesterase inhibitor (PMEI) protein was identified in Oryza sativa cv. IR64 (Khurana et al. 2013b). The gene OSIPP3 expresses exclusively in the pre-pollinated spikelets of rice. Further, upstream regulatory region possessing cis-acting elements was isolated and characterized in heterologous system and it was concluded that 286-bp sequence exclusively expressed in pollen in Arabidopsis (Khurana et al. 2013b). However, expression analyses of these rice derived regulatory sequences are yet to be tested in homologous system. In this study, an attempt was made to study the activity of the anther-specific OSIPP3 promoter fragments with GUS reporter gene in Oryza sativa (L.) cv. BPT 5204. A four different 5′ promoter deletion constructs of OSIPP3 upstream regulatory sequences were used for rice transformation to determine the role of cis-regulatory element. In addition, computational analysis of OSIPP3 promoter sequence was performed to identify the pollen-specific cis-elements.
Materials and methods
Gene constructs and rice transformation
Four 5′ promoter deletion constructs of OSIPP3 [OSIPP3-Δ1 (1504 bp), OSIPP3-Δ2 (968 bp), OSIPP3-Δ3 (388 bp) and OSIPP3-Δ4 (286 bp)] were used in this study. The details of vector construction and cloning of four different deletion fragments in the binary vector pBI101 were described by Khurana et al. (2013b). The promoter constructs were kindly provided by Prof. A.K. Tyagi, NIPGR, New Delhi. For rice transformation, the promoter and GUS gene cassettes were excised from pBI101vector and subcloned into binary vector pCAMBIA3300 where bar gene was a plant selection marker. Subsequently, the binary plasmids in the backbone of pCAMBIA3300 carrying different promoter deletion fragments were mobilized into Agrobacterium strain EHA105 by tri-parental method (Lichtenstein and Draper 1985). Embryogenic calli (21 days) of indica rice cultivar BPT 5204 were transformed using Agrobacterium tumefaciens followed by co-cultivation and washing of transformed calli as described by Manimaran et al. (2013). The washed calli were maintained in selection MS basal salts (Murashige and Skoog 1962) with 2 mg/L 2,4-D, 0.5 mg/L kinetin, 500 mg/L l-proline, 500 mg/L casein hydrolysate, 30 g/L maltose, solidified with 0.3 % phytagel and supplemented with 8 mg/L phosphinothricin (Duchefa, the Netherlands) for 15 days in dark. After three cycles of selection, resistant calli were transferred to regeneration medium containing MS basal salt, 2 mg/L kinetin, 0.3 mg/L NAA, 30 g/L sucrose, 30 g/L d-sorbitol and 0.4 % phytagel. The regenerated plantlets were maintained in rooting medium (1/2 MS basal salt + 15 g/L sucrose + 0.4 % phytagel) and then transferred to hardening medium (Yoshida et al. 1976). The hardened plants were transferred to earthen pots and maintained under controlled conditions in a biosafety glass house.
Molecular confirmation and inheritance of transgene
Total genomic DNA extraction, PCRs and DNA blot analyses of transgenic plants and non-transformed control plants were followed as described previously (Manimaran et al. 2013). For confirmation of transgenic plants by PCR, primer pairs were designed from promoter and GUS region; OSIPP3 forward: 5′-AAAAGGCAACACCAAGTTTAGCC-3′ and GUS reverse: 5′-ATCCACGCCGTATTCGG-3′. For DNA blot analysis, about 10 µg of genomic DNA was digested with either Xho I (release bar gene) or BstX I (T-DNA insertion number). The coding region of bar (540 bp) and GUS (636 bp) was used as probe using ready-to-go labeling kit (GE Healthcare Ltd, UK). Probe preparation was done as per the manufacturer’s instructions. The blot was hybridized using the probe labeled with 50 µCi of α-p32 dCTP (BRIT, India). For inheritance study of transgene, T1 and T2 seeds were collected and germinated on ½ MS medium containing 4 mg/L phosphinothricin and observations were recorded on number of germinating seeds.
RNA isolation, reverse transcription and qRT-PCR
Total RNA was isolated according to the manufacturer’s instructions (NucleoSpin RNA Plant kit, Macherey–Nagel, Germany). About 100 mg each of rice tissue was used for isolation of RNA and then treated with DNase I (Qiagen, USA). RNA was quantified by Nanodrop® ND-1000 Spectrophotometer. Optical density (OD) of RNA samples with 260/280 ratio between 1.9 and 2.0 and 260/230 ratio on or above 2.0 was used for real-time PCR analysis. One microgram of total RNA was taken for first-strand cDNA synthesis using oligo d(T) primers (PrimeScript First-Strand cDNA Synthesis Kit, Takara, Japan). The cDNA after RNase treatment and normalization was mixed with 12.5 µL of 2X Maxima SYBR green/ROX qPCR master mix (Thermo Scientific, USA), 2 µM each of GUS primers (forward: 5′-AAACGGCAGAGAAGGTACTG-3′ and reverse: 5′-TAACGTATCCACGCCGTATTC) in a final volume of 25 µL. The OsActin1 primers (forward: 5′-CCGGTGGATCTTCATGCTTACCTGG-3′ and reverse: 5′-CGACGAGTCTTCTGGCGAAACTGC-3′) were used as internal control. PCR with no template control (NTC) was also performed for each primer pair. The real-time PCR was performed in Real-Time PCR system 7500 (Applied Biosystems, USA). The conditions for qRT-PCR were as follows: 2 min at 50 °C, 10 min at 95 °C, and 40 cycles of 1 s at 95 °C and 1 min at 60 °C in 96-well optical reaction plates (Applied Biosystems, USA). The amplicon specificity was verified by melt curve analysis from 60 to 95 °C after completion of 40 cycles. Each sample was replicated thrice. The comparative threshold cycle (Ct) method was used to quantify the relative expression levels in real-time PCR. ΔCt was calculated by the difference between Ct target and Ct reference. Further ΔΔCt values were calculated using the formula ΔΔCt = ΔCt of PP3Δ1 or 2 or 3 − ΔCt PP3Δ4 sample, and then fold difference was calculated from 2−ΔΔCt. OsActin1 was used for normalizing expression level of OSIPP3.
GUS histochemical assay
Different tissues of rice such as leaf, root, stem, panicle (at different stages of P3–P6), spikelet (at heading stage), anther and pistil of transformed and non-transformed control plants were used for GUS staining. Different developmental stages of panicle P1–P6 were scored as per Jain et al. (2007). The histochemical assay of GUS gene expression was performed as described previously (Manimaran et al. 2013) using 5-bromo-4-chloro-3-indoxyl-β-d-glucuronide (X-gluc) (Biosynth AG, Staad, Switzerland) as a substrate. After staining, chlorophyll was cleared from the sample by 70 % ethanol treatment. Pictures were taken using a Leica EZ4D stereo microscope.
Fluorometric quantification of GUS activity
Fluorometric quantitative assay of GUS activity in transgenic rice was performed using 4-methylumbelliferyl β-d-glucuronide (MUG) as a substrate, as described by Jefferson et al. (1987). Plant tissue was homogenized in GUS extraction buffer containing 50 mM NaH2PO4 (pH 7.0), 10 mM EDTA, 0.1 % Triton X-100, 0.1 % sodium lauryl sarcosine and 10 mM β-mercaptoethanol. Protein concentration was assessed by Bradford method (Bradford 1976), using bovine serum albumin as standard. GUS assay buffer containing the substrate MUG was added to protein samples, and the reaction was incubated at 37 °C. The specific activity of GUS was expressed as nmole 4-MU formed per hour per milligram of the total protein.
Results
Cis-elements in 1.5-kb promoter region of OSIPP3
The OSIPP3 gene locus was identified in rice chromosome 5 (Locus id LOC_Os05g46530) for which the transcription start site (TSS) was mapped by Khurana et al. (2013b). Further, based on the microarray data, the OSIPP3 gene expression was reported to be in the mature pollen of rice. To find the cis-elements in the promoter sequence of OSIPP3 gene, the 1504-bp sequence was scanned using PLACE database (Higo et al. 1999). A putative TATA box was indentified at −27 position relative to TSS. Major pollen-specific cis-regulatory elements such as POLLEN1LELAT52 (AGAAA), PB Core (CCAC) and GTGANTG10 (GTGA) were identified. Apart from this pollen-specific motif, we identified enhancer elements such as LAT52 (TGTGG), LAT56 (TGTGA) and transcription activator (TAAATC) in the 1.5-kb sequence (Fig. 1). Also, root-specific motif ROOTMOTIFTAPOX1 (ATATT element) was found in three copies and as a single copy of the 1.5 kb of OSIPP3-Δ1 and 968 bp of OSIPP3-Δ2, respectively. But, this motif was not present in OSIPP3-Δ3 and OSIPP3-Δ4. Scanning of the different 5′ promoter deletion fragments in PLACE database (http://www.dna.affrc.go.jp/PLACE/signalscan.html) showed that all the four deletion regions possessed major pollen-specific elements (AGAAA), but with different copy numbers such as 4, 4, 2 and 1 in OSIPP3-Δ1, OSIPP3-Δ2, OSIPP3-Δ3 and OSIPP3-Δ4, respectively. Besides, two copies of GTGA were identified in OSIPP3-Δ1 fragment but not in the other three deletion fragments. The other major pollen-specific motif PB core CCAC was found at −78, −118 and −467 position relative to TSS, respectively. A list of relevant cis-elements, enhancer elements and their relative position from the TSS is given in Fig. 1 and Supplementary Table S1.
Graphical representation of 1.5-kb upstream regulatory region of OSIPP3 and presence of key cis-regulatory elements. Four 5′ deletion PCR amplified promoter regions possessing various cis-elements of regulatory sequences are shown. Square box represents the presence of important cis-regulatory motif on the sequence with respect to its TSS. The gene cassette (upstream regulatory sequences of OSIPP3 + GUS + nos terminator) was subcloned into the binary vector pCAMBIA3300 where bar gene is a plant selection marker
Development of transgenic rice for promoter evaluation
Agrobacterium-mediated transformation of indica rice cv. BPT 5204 was done with four deletion constructs, viz. OSIPP3-Δ1, OSIPP3-Δ2, OSIPP3-Δ3 and OSIPP3-Δ4. The cassette (OSIPP3 promoter + GUS + NOS terminator) cloned in the binary vector pB101 was excised and subcloned into the binary vector pCAMBIA3300 with bar gene as plant selection marker. Agrobacterium-mediated transformation of 21-day-old embryogenic calli was done individually with four promoter constructs. From all the four constructs, we regenerated 74, 84, 109 and 96 plants, respectively. PCR analysis of regenerated plants of all the four constructs showed a product of 606 bp of GUS gene in 24, 18, 13 and 21 plants, respectively. Further, PCR-positive plants were analyzed by DNA blot hybridization using bar gene as probe. Among the PCR-positive plants, six plants of OSIPP3-Δ1, seven plants of OSIPP3-Δ2, six plants of OSIPP3-Δ3 and five plants of OSIPP3-Δ4 showed the presence of T-DNA integration for each construct (Supplementary Fig S1A). Subsequently, selected positive plants were further confirmed as independent events (Supplementary Fig S1B). All the transgenic plants were phenotypically similar to non-transformed plants. Four different single copy T-DNA inserted transgenic lines from each construct were selected for this study. The transgenic plants of each construct were advanced to T1 generation, and the T1 seeds were segregated in a simple 3:1 Mendelian ratio (Table 1). The transgenic plants were further advanced to T2, and the seeds were grown in ½ MS supplemented with 4 mg/L of phosphinothricin and identified homozygous plants. The homozygous transgenic plants were further confirmed by PCR using OSIPP3 promoter forward primer in conjunction with a GUS reverse primers (Supplementary Fig. 1C). GUS gene expression analysis under OSIPP3 promoter was carried in the homozygous (T2) transgenic plants.
OSIPP3 promoter displayed anther- and root-specific expression
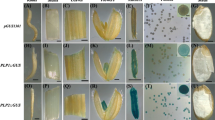
GUS histochemical analysis of vegetative and reproductive tissues of transgenic rice plants (homozygous—T2) and non-transformed plants was done. A total of 12–14 transgenic plants from each line were evaluated for GUS expression. All the homozygous transgenic plants were screened for GUS analysis both at vegetative and at reproductive tissues, and we could not find any negative GUS expression among the homozygous transgenic OSIPP3 plants. The promoter-driven GUS gene expressed preferentially in anther/pollen of all the four constructs but not in leaf, stem and pistil examined (Fig. 2a). Further, GUS activity was detected in root of OSIPP3-Δ1 and OSIPP3-Δ2 transgenic plants, but not in OSIPP3-Δ3 and OSIPP3-Δ4 roots (Fig. 2a). Subsequently, the transgenic panicles (P6 stage) of each construct were collected at 4–5 cm in length from the flag leaf base to study the expression pattern in spikelet. A strong GUS gene expression was observed in spikelets of OSIPP3-Δ1, OSIPP3-Δ2 and OSIPP3-Δ3, whereas OSIPP3-Δ4 transgenic spikelets showed less expression as compared to other three promoters (Fig. 2b). Based on the different developmental stages of panicle, it was categorized as P1–P6 as described by Jain et al. (2007). To confirm the correct stage of the GUS gene expression, transgenic flowers were collected from different panicle developmental stages, i.e., P3–P6 stages, and tested for GUS expression, while P1 and P2 stages were not tested due to very small in size. The GUS gene was preferentially expressed only in P6 stage (i.e., florets emerged from boot—late pollen stage) (Fig. 3a). The GUS gene preferentially expressed in the mature pollen of transgenic anthers, whereas no GUS expression was observed in non-transformed control anthers (Fig. 3b). Thus, the OSIPP3-Δ1 and OSIPP3-Δ2 promoter fragments conferred expression in both root and anther of transgenic plants, whereas the activity of OSIPP3-Δ3 and OSIPP3-Δ4 was restricted to pollen only.
a
GUS expression analysis of different tissues of transgenic OSIPP3 and control plants. GUS expressed only in anthers of transgenic floret but not in other tissues. b Panicle of length 4–5 cm was collected at P6 stage (flower emerged stage—late pollen stage) and checked for GUS expression. GUS expression was apparent in florets of OSIPP3-Δ1, Δ2 and Δ3, whereas OSIPP3-Δ4 showed mild expression
a Histochemical GUS analysis of different panicle stage of OSIPP3-Δ3 transgenic florets. Based on the different developmental stages of panicle, it was categorized as P1 0.5 mm, P2 0–3 cm, P3 3–10 cm, P4 10–15 cm, P5 15–22 cm, P6 22–30 cm. P1 shoot apical meristem (SAM), P2 floral transition and floral organ development, P3 meiotic stage, P4 young microspore stage P5 vacuolated pollen stage, P6 mature pollen stage (Jain et al. 2007). b GUS histochemical analysis of pollen from wild-type (WT) and transformed (T) OSIPP3-Δ3 anther
Quantification of GUS activity and its expression
GUS activity of different OSIPP3 promoter fragments was measured in transgenic plants. Four homozygous lines of each construct were used for quantification of GUS activity. For scoring the data, we used three homozygous plants from each transgenic line and mean value of these plants was taken for analyzing the expression in one transgenic line. The highest GUS activity was observed in the anthers of OSIPP3-Δ1 lines (702–1112 nmole 4MU/h/mg of protein). The fluorometric values of GUS activity in the anthers of OSIPP3-Δ2, OSIPP3-Δ3 and OSIPP3-Δ4 transgenic rice lines varied from 148 to 423, 71 to 103 and 39 to 45 nmole 4MU/h/mg of protein, respectively (Fig. 4a). Further, the GUS activity was also observed in roots of OSIPP3-Δ1 (165–258 nmole 4MU/h/mg of protein) and OSIPP3-Δ2 (58–132 nmole 4MU/h/mg of protein) transgenic plants (Fig. 4a). However, only negligible expression was observed in roots of OSIPP3-Δ3 (1.5–3.5 nmole 4MU/h/mg of protein) and OSIPP3-Δ4 (1.1–2.4 nmole 4MU/h/mg of protein) (Fig. 4a). The level of GUS protein expression in pollen under the OSIPP3 promoter was in the following order, the maximum activity in OSIPP3-Δ1 (22X) followed by OSIPP3-Δ2 (7X) > OSIPP3-Δ3 (2X) > OSIPP3-Δ4 (OSIPP3-Δ4 was considered as 1 to calculate the fold changes). In addition, the relative expression level of GUS gene was analyzed using qPCR in OSIPP3-Δ1, OSIPP3-Δ2 and OSIPP3-Δ3 in comparison with OSIPP3-Δ4. Twenty-three-fold higher expression of GUS transcript was found in OSIPP3-Δ1 followed by eightfold in OSIPP3-Δ2 and threefold in OSIPP3-Δ3 (Fig. 4b) (OSIPP3-Δ4 was considered as 1 to calculate the fold changes). Thus, both mRNA and protein expression of GUS gene under the OSIPP3 promoter indicated the important role of the different deletion fragments.
Fluorometric GUS quantification in anther and root (a) of OSIPP3 promoter in wild-type (WT) and T2 transgenic rice plants harboring OSIPP3-Δ1, Δ2, Δ3 and Δ4 constructs. The GUS activity is expressed as nmole 4-methylumbelliferone/hr/mg protein. Each column represents the mean GUS activity from three plants of four independent transgenic lines. Standard error bars are shown. b Relative expression level of GUS mRNA/protein in anther of OSIPP3-Δ1, Δ2, Δ3 and Δ4 constructs. OSIPP3-Δ4 was considered as 1 to calculate the fold changes
Discussion
In the present investigation, we characterized four different 5′ promoter deletion sequences of OSIPP3 [OSIPP3-Δ1 (1504 bp), OSIPP3-Δ2 (968 bp), OSIPP3-Δ3 (388 bp) and OSIPP3-Δ4 (286 bp)] using GUS gene in homologous system, i.e., rice. Notably, the gene and its promoter sequence were isolated originally from indica rice cv. IR64 (Khurana et al. 2013b). Among four constructs, OSIPP3-Δ3 and OSIPP3-Δ4 promoters directed GUS expression only in anther, while OSIPP3-Δ1 and OSIPP3-Δ2 showed expression in root as well as anther. Presence of various cis-regulatory motifs obtained from in silico analysis of 1.5-kb OSIPP3 promoter further supported its organ-/tissue-specific expression. A pollen-specific cis-acting elements such as GTGA motif (−1333 and −965 bp), AGAAA (−709, −295, −185 and −64 bp) and PB core (GTGG) CCAC motifs (−78, −118 and −467 bp) were identified. The motif POLLENLELAT52, one of the two co-dependent regulatory elements (AGAAA and TCCACCATA), was responsible for pollen-specific expression of tomato LAT52 gene (Bate and Twell 1998). Similarly, GTGANTG10 (GTGA) motif was identified in pollen-specific LAT56/59 promoter of tomato (Twell et al. 1991) and in G10 promoter of tobacco (Rogers et al. 2001). In addition, enhancer cis-elements were also found to be essential for high level of expression in the pollen (Park et al. 2006; Swapna et al. 2011). In this study, we identified two enhancer sequences (TGTGG/TGTGA) of LAT 52/56 at −1350-/−966-bp position, one TGGTTA (−600 bp)—LAT quantitative element (Rogers et al. 2001; Park et al. 2006; Swapna et al. 2011) and one TAAATC (−732 bp)—a putative transcription binding site for transcription activator (Liu et al. 2013) in the 1.5-kb region. Because of the presence of these enhancer elements in OSIPP3-Δ1 sequence, very strong GUS expression was observed when compared to other three. Also, the OSIPP3-Δ1 consisted three copies of ROOTMOTIFTAPOX1 (ATATT, −1366, −1275 and −808 bp) which were responsible for root-specific expression. The motif ROOTMOTIFPOX1 was reported in the root-specific rol D promoter of Agrobacterium rhizogenes (Elmayan and Tepfer 1995). The TrAP/REn monodirectional promoter of Mung bean yellow mosaic virus (MYMV) comprised several root-specific motifs responsible for expression of GUS gene in root (Sunitha et al. 2012).
In the OSIPP3-Δ2 promoter, approximately 500 bp from the 5′ end was deleted. The 968 bp (−860 to +108 bp) of OSIPP3-Δ2 consisted following motifs: one root motif ATATT, four copies of AGAAA, three copies of CCAC, one TGGTTA and one TAAATC. GUS histochemical assay of different tissues of OSIPP3-Δ2 showed that the reporter gene expressed in both anther and root as similar to OSIPP3-Δ1. The smaller fragments OSIPP3-Δ3 (−280 to +108 bp) possessed two copies of each AGAAA and CCAC PB core motifs, whereas OSIPP3-Δ4 (−179 to +108 bp) contained one copy of AGAAA and two copies of CCAC motifs, respectively. But these two smaller fragments did not contain any enhancer cis-elements or GTGA sequence. Further, 388-bp region showed higher GUS expression in anthers when compared to 286-bp region. It might be due to the presence of two copies of POLLENLELAT52 (AGAAA) motifs in OSIPP3-Δ3 compared to one copy of AGAAA in OSIPP3-Δ4. The analysis revealed that the 388- and 286-bp promoter region possessed both AGAAA and CCAC PB core elements responsible for pollen-specific expression that could have directed the reporter gene expression preferentially to late pollen stage. Interestingly, significant variation in expression of GUS was observed within the transgenic lines developed for a particular promoter fragment. Similar results were obtained by Khurana et al. (2013b) in Arabidopsis. This could be due to the position of integration of transgene in different locus of chromosomes.
A pollen-specific gene SBgLR in potato was isolated, and its promoter activity was characterized in tobacco plants. A smaller fragment of SBgLR promoter, i.e., −269- to −1-bp region harbored four copies of AGAAA and one GTGA element and showed high level of reporter gene expression specifically in the pollen (Lang et al. 2008). Similarly, another pollen-specific gene BAN103 was isolated from Chinese cabbage and its promoter activity was characterized in tobacco. A minimal promoter fragment of 176 bp could confer the GUS expression in pollen (Park et al. 2002). This report suggested that the minimal promoter might have got all necessary cis-regulatory elements which directed the reporter gene expression preferentially in pollen. In a similar study, promoter of rice YY2 gene coding a putative chalcone (−486 and −326 bp) showed GUS activity in anthers, while the small fragment (−186 bp) failed to show any blue staining as this region did not have tissue-specific elements. It was concluded that cis-elements responsible for pollen specificity was present in the 300-bp region of YY2 gene promoter (Kuriakose et al. 2009). The OSIPA gene promoter −617-bp region showed GUS activity in pollen because of the presence of necessary cis-element required for pollen-specific expression, whereas −199-bp smaller fragment did not show any GUS activity (Swapna et al. 2011). Above results indicate that the minimal promoter harboring pollen-specific cis-element (AGAAA) may have important role for anther-/pollen-specific expression.
In summary, the isolated OSIPP3 promoter was evaluated for its expression pattern in rice. The result obtained from this study in rice is corroborated with the results of Arabidopsis (Khurana et al. 2013b) except the expression level varied in homologous system. In this study, we supported the expression data with the identification of several cis-acting elements responsible for pollen-specific expression in different deletion fragments of OSIPP3 upstream regulatory region. In silico analysis of 1.5-kb fragment harbored pollen-specific motif, pollen-specific enhancer element, root-specific motif and other key cis-regulatory elements. The transformation studies using GUS reporter gene enabled us to confirm that the promoter conferred the gene expression in anther/pollen at P6 stage of panicle (late pollen stage) but not in early panicle stage. Quantitative expression analysis by qRT-PCR further helped in assessing more precisely the differential expression of GUS gene under different deletion fragments. When compared the expression of the two smaller fragments, OSIPP3-Δ3 (388 bp) showed strong GUS expression than OSIPP3-Δ4 (286 bp). Previous study by Khurana et al. (2013b) reported that 286 bp (OSIPP3-Δ4) was sufficient to drive a gene which expressed in pollen specifically in Arabidopsis. In this study, though OSIPP3-Δ3 (388 bp) and OSIPP3-Δ4 (286 bp) expressed exclusively in pollen, OSIPP3-Δ3 showed stronger GUS expression when compared to OSIPP3-Δ4 in rice. This might be because of the presence of two AGAAA elements in OSIPP3-Δ3. Thus, we proposed that the 388-bp fragment would be an ideal choice than 286-bp fragment to be used as pollen-specific promoter in developing male-sterile lines. It will be interesting for reproductive biology of rice to confirm the activity of the putative cis-elements that confer the pollen-specific expression by generating point mutations in the motifs contained in the 388- and 286-bp fragments and analyzing the functionality of the mutated fragments.
Author contribution statement
PM, MRR, TBR—sub-cloning, transformation, molecular characterization; PM, SKM—identified cis-acting elements; RMS—helped in data analysis. SMB—conception, designing, drafting of the manuscript.
Abbreviations
- d:
-
Days
- MS:
-
Murashige and Skoog medium
- AS:
-
Acetosyringone
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- NAA:
-
Naphthalene acetic acid
- BAP:
-
6-Benzylaminopurine
- GUS:
-
β-Glucuronidase
- PPT:
-
Phosphinothricin phosphotransferase
- MUG:
-
4-Methylumbelliferyl β-d-glucuronide
- qRT-PCR:
-
Quantitative real-time PCR
- TSS:
-
Transcription start site
- UTR:
-
Untranslated region
References
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15:63–78
Azria D, Bhalla PL (2011) Agrobacterium-mediated transformation of Australian rice varieties and promoter analysis of major pollen allergen gene, Ory s 1. Plant Cell Rep 30:1673–1681
Bate N, Twell D (1998) Functional architecture of a late pollen promoter: pollen-specific transcription is developmentally regulated by multiple stage-specific and co-dependent activator elements. Plant Mol Biol 37:859–869
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen L, Tu Z, Hussain J, Cong L, Yan Y, Jin L et al (2010) Isolation and heterologous transformation analysis of a pollen-specific promoter from wheat (Triticum aestivum L.). Mol Biol Rep 37:737–744
Elmayan T, Tepfer M (1995) Evaluation in tobacco of the organ specificity and strength of the rolD promoter, domain A of the 35S promoter and the 35S2 promoter. Transgenic Res 4:388–396
Grace ML, Chandrasekharan MB, Hall TC, Crowe AJ (2004) Sequence and spacing of TATA box elements are critical for accurate initiation from the beta-phaseolin promoter. J Biol Chem 279:8102–8110
Gupta V, Khurana R, Tyagi AK (2007) Promoters of two anther-specific genes confer organ-specific gene expression in a stage-specific manner in transgenic systems. Plant Cell Rep 26:1919–1931
Hamilton DA, Schwarz YH, Mascarenhas JP (1998) A monocot pollen-specific promoter contains separable pollen-specific and quantitative elements. Plant Mol Biol 38:663–669
Hamilton DA, Schwarz YH, Rueda J, Mascarenhas JP (2000) Comparison of transient and stable expression by a pollen-specific promoter: the transformation results do not always agree. Sex Plant Reprod 12:292–295
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27:297–300
Huda KMK, Banu MSA, Pathi KM, Tuteja N (2013) Reproductive organ and vascular specific promoter of the rice plasma membrane Ca2+ ATPase mediates environmental stress responses in plants. PLoS One 8:e57803
Husebye H, Chadchawan S, Winge P, Thangstad OP, Bones AM (2002) Guard cell- and phloem idioblast-specific expression of thioglucoside glucohydrolase 1 (myrosinase) in Arabidopsis. Plant Physiol 128:1180–1188
Jain M, Nijhawan A, Arora R, Agarwal P, Ray S, Sharma P et al (2007) F-box proteins in rice: genome-wide analysis, classification and spatial and temporal gene expression during panicle and seed development and regulation by light and abiotic stress. Plant Physiol 143:1467–1483
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Khurana R, Kapoor S, Tyagi AK (2013a) Spatial and temporal activity of upstream regulatory regions of rice anther-specific genes in transgenic rice and Arabidopsis. Transgenic Res 22:31–46
Khurana R, Kathuria H, Mukhopadhyay A, Kapoor S, Tyagi AK (2013b) A 286 bp upstream regulatory region of a rice anther-specific gene, OSIPP3, confers pollen-specific expression in Arabidopsis. Biotechnol Lett 35:455–462
Kuriakose B, Arun V, Gnanamanickam SS, Thomas G (2009) Tissue-specific expression in transgenic rice and Arabidopsis thaliana plants of GUS gene driven by the 50 regulatory sequences of an anther specific rice gene YY2. Plant Sci 177:390–397
Lang Z, Zhou P, Yu J, Ao G, Zhao Q (2008) Functional characterization of the pollen-specific SBgLR promoter from potato (Solanum tuberosum L.). Planta 227:387–396
Lichtenstein C, Draper J (1985) Genetic engineering of plants. In: Glover DM (ed) DNA cloning a practical approach. IRL Press, Washington, DC, pp 67–118
Liu X, Shangguan Y, Zhu J, Lu Y, Han B (2013) The rice OsLTP6 gene promoter directs anther-specific expression by a combination of positive and negative regulatory elements. Planta 238:845–857
Luo H, Lee JY, Hu Q, Vasilchik KS, Eitas TK, Lickwar C et al (2006) RTS, a rice anther-specific gene is required for male fertility and its promoter sequence directs tissue-specific gene expression in different plant species. Plant Mol Biol 62:397–408
Manimaran P, Ravikumar G, Raghurami Reddy M, Jain S, Bhaskar Rao T, Mangrauthia SK et al (2013) Infection of early and young callus tissues of indica rice BPT 5204 enhances regeneration and transformation efficiency. Rice Sci 20:415–426
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Oo MM, Bae HK, Nguyen TD, Moon S, Oh SA, Kim JH et al (2014) Evaluation of rice promoters conferring pollen-specific expression in a heterologous system, Arabidopsis. Plant Reprod 27:47–58
Park BS, Park YD, Kim HU, Jin YM, Kim HI (2002) BAN103, A pollen-preferential gene, from Chinese cabbage and its promoter activity. Mol Cells 14:150–157
Park JI, Hakozaki H, Endo M, Takada Y, Ito H, Uchida M et al (2006) Molecular characterization of mature pollen-specific genes encoding novel small cysteine-rich proteins in rice (Oryza sativa L.). Plant Cell Rep 25:466–474
Pear JR, Ridge N, Rasmussen R, Rose RE, Houck CM (1989) Isolation and characterization of a fruit-specific cDNA and the corresponding genomic clone from tomato. Plant Mol Biol 13:639–651
Rogers HJ, Harvey A, Lonsdale DM (1992) Isolation and characterization of a tobacco gene with homology to pectate lyase which is specifically expressed during microsporogenesis. Plant Mol Biol 20:493–502
Rogers HJ, Bate N, Combe J, Sullivan J, Sweetman J, Swan C et al (2001) Functional analysis of cisregulatory elements within the promoter of the tobacco late pollen gene g10. Plant Mol Biol 45:577–585
Sakai H, Aoyama T, Oka A (2000) Arabidopsis ARR1 and ARR2 response regulators operate as transcriptional activators. Plant J 24:703–711
Shirsat A, Wilford N, Croy R, Boulter D (1989) Sequences responsible for the tissue specific promoter activity of a pea legumin gene in tobacco. Mol Gen Genet 215:326–331
Simpson SD, Nakashima K, Narusaka Y, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J 33:259–270
Sunitha S, Mahajan N, Veluthambi K (2012) The TrAP/REn monodirectional promoter of Mungbean yellow mosaic geminivirus (MYMV) displays root-specific expression in transgenic tobacco. Plant Cell Tissue Organ Cult 109:535–545
Suwabe K, Suzuki G, Takahashi H, Shiono K, Endo M, Yano K et al (2008) Separated transcriptomes of male gametophyte and tapetum in rice: validity of a laser microdissection (LM) microarray. Plant Cell Physiol 49:1407–1416
Swapna L, Khurana R, Kumar SV, Tyagi AK, Rao KV (2011) Pollen-specific expression of Oryza sativa indica pollen allergen gene (OSIPA) promoter in rice and Arabidopsis transgenic systems. Mol Biotechnol 48:49–59
Twell D, Yamaguchi J, Wing RA, Ushiba J, McCormick S (1991) Promoter analysis of genes that are coordinately expressed during pollen development reveals pollen-specific enhancer sequences and shared regulatory elements. Genes Dev 5:496–507
Van Tunen AJ, Koes RE, Spelt CE, Van der Krol AR, Stuitje AR, Mol JN (1988) Cloning of the two chalcone flavanone isomerase genes from Petunia hybrida: co-ordinate, light-regulated and differential expression of flavonoid genes. EMBO J 7:1257–1263
Wittkopp PJ, Kalay G (2012) Cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence. Nat Rev 13:59–69
Yamamoto YT, Taylor CG, Acedo GN, Cheng CL, Conkling MA (1991) Characterization of cis-acting sequences regulating root-specific gene expression in tobacco. Plant Cell 3:371–382
Yanagisawa S, Schmidt RJ (1999) Diversity and similarity among recognition sequences of Dof transcription factors. Plant J 17:209–214
Yokoi S, Tsuchiya T, Toriyama K, Hinata K (1997) Tapetum-specific expression of the Osg6B promoter-β-glucuronidase gene in transgenic rice. Plant Cell Rep 16:363–367
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice, 3rd edn. Int Rice Res Inst, Manila
Zou JT, Zhan XY, Wu HM, Wang H, Cheung HY (1994) Characterization of rice pollen-specific gene and its expression. Am J Bot 81:522–561
Acknowledgments
We gratefully acknowledge Prof. A.K. Tyagi, Director, NIPGR, New Delhi, and Dr. Reema Khurana, University of Delhi South Campus, New Delhi, for providing the binary vectors: OSIPP3-Δ1, Δ2, Δ3 and Δ4. The authors thank the Department of Biotechnology (DBT), Government of India, for financial support through the Grant F.No.BT/AB/FG-II (Ph-II)/2009.
Conflict of interest
No conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Twell.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manimaran, P., Raghurami Reddy, M., Bhaskar Rao, T. et al. Identification of cis-elements and evaluation of upstream regulatory region of a rice anther-specific gene, OSIPP3, conferring pollen-specific expression in Oryza sativa (L.) ssp. indica . Plant Reprod 28, 133–142 (2015). https://doi.org/10.1007/s00497-015-0264-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-015-0264-4