Abstract
In our previous cDNA microarray analysis, we identified 53 mature anther-specific genes, whose function was unknown, in rice. We reanalyzed these genes from the viewpoint of the specific amino acid motif. Out of 53 genes, three genes, Os-26, Os-32, and Os-169 (renamed as OsSCP1, OsSCP2, and OsSCP3), encoded cysteine-rich motif (Cys-X3-Cys-X13-Cys-X3-Cys), indicating that they were novel small cysteine-rich proteins. From the search of specific elements in promoter regions, several pollen-specific elements were found. In order to determine whether three promoters were functional in pollen or not, the gene constructs with promoter regions fused to the β-glucuronidase gene were transformed into tobacco. Histochemical analysis showed that these promoters were active in the mature pollen grains and pollen tubes. Furthermore, OsSCP1 and OsSCP3 formed a multigene family tandemly in the rice genome. From the results, OsSCPs might have important roles in mature pollen development and pollen tube growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The male gametophyte, pollen, develops within the anther compartment of the stamen and requires cooperative functional interactions between gametophytic and sporophytic tissues. Pollen development consists of several distinct stages. A microspore mother cell undergoes meiosis to give rise to a tetrad of four microspores. After release upon the dissolution of the callose wall, each uninucleate microspore undergoes an asymmetric mitotic division to give rise to two cells with distinct fates: the vegetative cell and the generative cell. The larger vegetative cell is transcriptionally active (Bedinger 1992; McCormick 2004). Recently, a number of anther-specific cDNA clones have been identified and isolated using DNA microarray and macroarray techniques from several model plants, Lotus japonicus, Arabidopsis thaliana, and Oryza sativa (Endo et al. 2002; Amagai et al. 2003; Honys and Twell 2003; Endo et al. 2004). Although pollen-specific genes have been studied extensively in most cases, it remains unclear what roles these genes play.
The genes encoding small cysteine-rich proteins form a gene family in several plant species. The small cysteine-rich proteins are highly differentiated in their biological function and were related to several physiological phenomena, e.g. storage, protection, lipid transfer, cell–cell communication, etc. (Jose-Estanyol et al. 2004). In the case of cell–cell communication, SP11/SCR, encoding a male S determinant in Brassica self-incompatibility (SI), is a small cysteine-rich protein, and functioned as a ligand molecule for female S determinant, SRK, S receptor kinase (reviewed in Watanabe et al. 2003; Takayama and Isogai 2005). In another case, lipid transfer protein, LTP, also encoding a different type of small cysteine-rich protein, has an important role in transferring lipids in several tissues (Kader 1996). The pollen-specific gene, LAT52, also encodes a small cysteine-rich protein, which could interact with pollen-specific receptor protein kinase, LePRK2 (Twell et al. 1989; Tang et al. 2002). LAT52 is required for the pollen to germinate in vitro and achieve fertilization in vivo (Muschietti et al. 1994).
In the previous experiments, we identified over 100 anther-specific genes in rice (Endo et al. 2004). In this current study, we identified three novel pollen-specific clones, OsSCP1, OsSCP2, and OsSCP3. The deduced amino acid sequences of OsSCPs contained an open reading frame (ORF) encoding a small cysteine-rich protein. In order to determine whether the promoter regions of OsSCPs were functional in pollen or not, transgenic tobacco plants having the β-glucuronidase (GUS) gene regulated by OsSCPs promoter were made. A blue GUS signal was specifically observed in mature pollen grains and pollen tubes. Furthermore, OsSCP1 and OsSCP3 formed a multigene family in the rice genome. We discuss the function of the pollen-specific OsSCP genes.
Materials and methods
Plant material and mRNA isolation
Plants of O. sativa cv. Koshihikari were grown in a green house. Anther and pistil tissues in various stages (A1: anther of uninucleate stage; A2: anther of binucleate stage; A3: anther of trinucleate stage; P1: pistil of uninucleate stage; P2: pistil of binucleate stage; and P3: pistil of trinucleate stage) were collected for isolation mRNA as described previously (Endo et al. 2004). Leaf sheath (Ls) and leaf blade (Lb) were harvested at the heading stage. Furthermore, root (R) and shoot (S) of seedling were also collected for RNA experiment. Lemma and palea (L/P) were also harvested for mRNA isolation. Isolation of poly (A)+ RNA from each tissue was performed using a FastTrack 2.0 mRNA isolation kit (Invitrogen, San Diego, CA) as described in Watanabe et al. (2000).
RT-PCR
RT-PCR was performed according to Endo et al. (2002). Briefly, poly (A)+ RNA was reverse-transcribed to synthesize the first strand cDNA by using the First-Strand cDNA synthesis kit (Amersham-Pharmacia, Uppsala, Sweden). Then, cDNA was used as a template for PCR amplification with a set consisting of primers specific to each gene: OsSCP1RT-F(5′-ATGGCCCAGAACAAGACCAT-3′),OsSCP1RT-R(5′-GCAACTGTCTACGCACTTCTTGTTGGTG-3′),OSCP2 RT-F(5′-ATGGCCCAAAACAAGACCAT-3′),OsSCP2 RT-R(5′-TCCTTGGCTTCCTTAGACATG-3′),OsSCP3R T-F(5′-CAGAACAAGACCATTGCGGT-3′), and OsSCP3 RT-R(5′-CGTTGTTTGAATATAAGCGACGAC-3′). PCR was performed with ExTaq DNA polymerase (TaKaRa Shuzo, Shiga, Japan) for 25 or 35 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 58°C and extension for 1 min at 72°C, followed by a final extension for 5 min. Actin primers as a control were 5′-TCCATCTTGGCATCTCTCAG-3′ (forward) and 5′-GTACCCGCATCAGGCATCTG-3′ (reverse).
5′-RACE and sequence analysis
Full-length cDNA clones of OsSCPs from the cultivar, Koshihikari, were obtained by the 5′-RACE procedure according to Watanabe et al. (1999). cDNA inserts were sequenced by the dideoxy chain-terminator method using a model 310 DNA sequencer (Applied Biosystems, Foster City, CA). The DNA sequence was analyzed with Genetyx software (Software Development, Tokyo, Japan). A homology search was performed using BLAST (Altschul et al. 1997). The motif search was performed with the TAIR web site program (http://www.arabidopsis.org/cgi-bin/patmatch/nph-patmatch.pl). The presence of a signal peptide was predicted using the PSORT program (http://psort.nibb.ac.jp).
Construction of promoter–GUS fusion
The promoter regions of OsSCP1, OsSCP2, and OsSCP3 were identified from a DNA database of genomic sequences of rice. These promoter regions were amplified by PCR using each of the following primers: OsSCP1pro-F (5′-ACTAGTTCTGTTGTTCTACCCAATATTGTTAC-3′), OsSCP1pro-R (5′-GGATCCTAGCACCGTTTCTATTGA GGGTAACTGGTGTGGAT-3′), OsSCP2pro-F (5′-ACTA GTACTGGGTCGACTTGGGCCGGGGAGGAGAGAGA- AA-3′), OsSCP2pro-R (5′-GGATCCTAGCACCGTTTAT GCTGAGCAGAATTGGT-3′), OsSCP3pro-F (5′-ACT AGTCTGCCACTGTCTACAGCGGGAAGGTTAACAAT-3′), and OsSCP3pro-R (5′-GGATCCTAGCGCTGTTTT TCTTGAGTGTAATTGGTGTGGA-3′). All PCR fragments were subcloned into the pCR2.1 plasmid vector (Invitrogen). The nucleotide sequence of these promoter fragments was confirmed by sequencing of the plasmid using the DNA sequencer (ABI 310; PE Biosystems, Foster City, CA). The binary vector, pBI–GSH, was used. This vector was derived from pBI–BG vector (Okada et al. 2000) by replacement of the Bra r 1 promoter region to multi-cloning sites. The promoter region of each OsSCP was taken as a SpeI–BamHI fragment from each plasmid and inserted into the SpeI–BamHI site of pBI–GSH. As a result, each promoter region was placed in front of the reporter gene, GUS. The constructs, each formed by promoter–GUS fusion, were named OsSCP1–GUS, OsSCP2–GUS, and OsSCP3–GUS, respectively.
Transformation of tobacco plants
The constructed OsSCP1–GUS, OsSCP2–GUS, and OsSCP3–GUS were transferred to Agrobacterium tumefaciens strain EHA101 using the freeze-thaw method (An et al. 1988). Leaf disks of Nicotiana tabacum cv. Petit Havana SR1 were transformed according to the procedure of Horsch et al. (1988). Transgenic tobacco plants were selected on the basis of hygromycin resistance (100 μg/ml). These plants were maintained under greenhouse conditions until maturity. The vegetative and floral tissues were used in GUS assay.
Histochemical GUS assay
Vegetative tissues, and anther and pollen of different developmental stages, were obtained from primary transgenic plants and wild-type plants as a control. GUS staining was performed according to Tsuchiya et al. (1994), except for addition of 20% methanol in the staining solution. Briefly, for staining, several tissues were incubated in 200 mM NaPO4, pH 7.0, 20% methanol, 0.3% Triton X-100, 12.5 mM K3Fe(CN)6, 12.5 mM K4Fe(CN)6, and 38.3 mM X-Gluc (5-bromo-4-chloro-3-indoyl-β-d-glucuronide). Plant tissues were vacuum infiltrated briefly, then incubated at 37°C overnight. After staining, chlorophyll was cleared from the sample by 75% ethanol treatment. Photos were taken using a Nikon E800 microscope system. The developmental stages of microspores and pollen grains were determined by staining nuclei with DAPI (Watanabe et al. 1991). Pollen germination was performed according to Lush et al. (1998).
Results
Identification and characterization of OsSCP genes
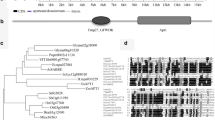
In our previous study, we isolated 89 mature anther-specific genes (Cluster RA3 genes) in rice by using cDNA microarray analysis (Endo et al. 2004). However, the function of 60% of the Cluster RA3 genes (53 genes) was unknown at the time we published the array data. Because a large number of ESTs (expressed sequence tags) and full-length cDNA clones were deposited in the public database after publishing our data (Yazaki et al. 2004), we reanalyzed these unknown function genes and their encoding proteins from the viewpoint of the specific amino acid motifs. Within 53 genes, we found that three genes, Os-26, Os-32, and Os-169 (renamed as OsSCP1, OsSCP2, and OsSCP3), had a small ORF, which was constructed from 127, 129, 129 amino acid residues, respectively, and encoded specific cysteine-rich motif (Cys–X3–Cys–X13–Cys–X3–Cys; Fig. 1). Based on the homology search of OsSCPs against the rice full-length cDNA database with the BLAST program, OsSCP1, OsSCP2, and OsSCP3 were found to be identical to full-length cDNA clones, AK064756 (chromosome 2), AK071781 (chromosome 8), and AK120999 (chromosome 2), respectively. Among three OsSCPs, the sequence identity was 35–47% at the amino acid level, and eight cysteine residues were completely conserved. Interestingly, the nucleotide difference between our cDNA clone and the full-length cDNA clone in each gene was not observed in both ORF and UTR (5′ and 3′) regions in spite of the difference of material cultivar, Koshihikari (our cDNA clone) and Nipponbare (full-length cDNA clone). Based on the comparison between cDNA and the genomic clone, the OsSCPs had no intron. By using the full-length cDNA information of OsSCPs, a BLASTP search of the OsSCPs was performed against the public database. However, OsSCPs showed no significant similarity to other known proteins. As described above, a number of small cysteine-rich proteins have been identified and characterized (Jose-Estanyol et al. 2004). When the motif search against OsSCPs was performed to find proteins, which have the same cysteine-rich motif as the OsSCPs on the web site, eight clones encoding OsSCP-like proteins having a specific cysteine motif were found only in the rice genome, not in other plant genomes (data not shown). Furthermore, we performed a signal peptide search with PSORT program. All three OsSCP proteins had N-terminal signal peptide, like other pollen-specific cysteine-rich proteins, SLR1-BP, PEC-3, and PCP-A1 (Stanchev et al. 1996; Toriyama et al. 1998; Takayama et al. 2000a). When compared the nucleotide sequences of these eight cDNA clones each other, eight cDNA clones were divided into three groups. In the first groups, the nucleotide sequence of three cDNA clones was identical to that of OsSCP1. In the second group, the nucleotide sequence of two cDNA clones was identical to that of OsSCP2. In the third group, the nucleotide sequence of three cDNA clones was identical to that of OsSCP3. Furthermore, the OsSCPs formed a small gene family in the rice genome. From these results, OsSCPs were found to be a novel type of cysteine-rich protein (Fig. 2). Thus, we carefully analyzed the OsSCPs.
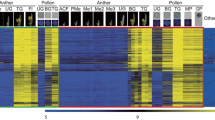
In order to confirm the expression pattern of OsSCPs, RT-PCR was performed with several rice tissues. In each gene, specific amplification was observed only in the anther at the trinucleate stage containing mature pollen grain. In other anther at the developmental stages and other tissues, no band was detected (Fig. 3). This expression pattern was coincident to that observed by cDNA microarray analysis (Endo et al. 2004).
RT-PCR analysis of anther-specific cDNA clones, OsSCP1, OsSCP2, and OsSCP3. The mRNA was isolated from root of seedling (R), shoot of seedling (S), leaf sheath at the heading stage (Ls), leaf blade at the heading stage (Lb), lemma and palea (L/P), pistil of uninucleate stage (P1), pistil of binucleate stage (P2), pistil of trinucleate stage (P3), anther of uninucleate stage (A1), anther of binucleate stage (A2), and anther of trinucleate stage (A3) in rice. The gene encoding actin was used as a positive control
In order to determine the promoter region in each OsSCP, we compared the nucleotide sequence between full-length cDNA clone and genomic DNA clone of each OsSCP gene. We defined the promoter region as the nucleotide sequence between OsSCP and the nearest upstream ORF (intergenic region). The length of the putative promoter region of each OsSCP was 2033 bp, 952 bp, and 1845 bp, respectively (Fig. 4). The putative TATA box sequence was found at −74 for all OsSCP promoter sequences when the start of initiation codon (ATG) was marked as +1. Furthermore, in order to determine the spatial and temporal expression pattern of the OsSCPs, the 2033 bp promoter region (nucleotides −2034 to −1) of OsSCP1 (OsSCP1-b, see below), the 952 bp promoter region (nucleotides −953 to −1) of OsSCP2, and the 1845 bp promoter region (nucleotides −1846 to −1) of OsSCP3 (OsSCP3-a, see below) were fused with β-glucuronidase (GUS) genes (Fig. 4). For histochemical GUS staining, two to three independent tobacco transgenic plants were used in each OsSCP gene. All the transgenic lines of OsSCP1 (OsSCP1-b)–GUS, OsSCP2–GUS, and OsSCP3 (OsSCP3-a)–GUS exhibited GUS activity in pollens at a very late developmental stage, as described below (Fig. 5). No GUS activity was detected in other floral organs and vegetative organs (data not shown).
Histochemical analysis of GUS activity conferred by OsSCP1–GUS and wild-type in transgenic tobacco. Developmental stage of microspore and pollen was determined by DAPI staining (A, B, C). Representative photographs of uninucleate microspore (D), early binucleate pollen (E), late binucleate pollen (F), and germinated pollen and pollen tube (G) in transgenic plant are shown. Blue staining indicates GUS activity. In wild-type plant, GUS activity was not observed in late binucleate pollen (H) and germinated pollen and pollen tube (I). WT, wild type; VN, vegetative nucleus; GC, generative cell; M, microspore; P, pollen; GPP, GUS-positive pollen; GNP, GUS-negative pollen; GPPt, GUS-positive pollen tube; Pt, pollen tube
For the next step, we examined the GUS activity in anthers of transgenic plants according to different developmental stages. Developmental stage of microspore and pollen was determined by staining of nuclei with DAPI (Fig. 5A–C). Because transgenic plants having each OsSCP construct showed the similar GUS expression pattern, we presented the OsSCP1 construct as a representative. GUS activity was specifically observed in late binucleate pollen grain (Fig. 5F). In other words, GUS activity was not detected in uninucleate microspores or in early binucleate pollen grains (Fig. 5D and E). After growing the pollen tube in the pollen germination medium in vitro, GUS expression was also detected from the pollen tube of all transgenic plants (Fig. 5G). Segregation of GUS-positive and GUS-negative pollen grains was observed, as shown in Fig. 5C. The ratio of GUS-positive and GUS-negative pollen grains was 504:464 in transgenic plant having OsSCP1–GUS construct, indicating a segregation pattern of 1:1 (significant at the 5% level). In the non-transgenic wild-type plants, no GUS expression was detected in the pollen grain and the pollen tube (Fig. 5H and I).
Identification of pollen-specific cis-elements of the OsSCP genes
We surveyed the potential pollen-specific cis-elements, which have been already identified in other plant species, in each promoter region. The sequence motifs of the LAT52/LAT56 (TGTGG and TGTGA) enhancer element, LAT52 quantitative element (TGGTTA), LAT52 pollen-specific activation element (AGAAA), g10 late pollen gene element (GTGA), which were required for the specific expression of mature pollen (Twell et al. 1990; Eyal et al. 1995; Bate and Twell 1998; Rogers et al. 2001), were observed. In the case of LAT52/LAT56, the nucleotide sequences of the enhancer elements (TGTGG and TGTGA) were found at −1850, −1285, and −1130 for the OsSCP1 (OsSCP1-b) promoter, at −345 for the OsSCP2 promoter, and at −617 and −345 for the OsSCP3 (OsSCP3-a) promoter (Fig. 4). The sequence motifs of the LAT52 quantitative element were present at −1059 and −201 for the OsSCP1 (OsSCP1-b) promoter, at −208 for the OsSCP2 promoter, and at −201 for the OsSCP3 (OsSCP3-a) promoter (Fig. 4). In the case of LAT52, the nucleotide sequences of the pollen-specific activation element (AGAAA) were found at −1322, −694, and −326 for the OsSCP1 (OsSCP1-b) promoter, at −923, −790, −620, −548, −524, and −344 for the OsSCP2 promoter, and at −577 and −326 for the OsSCP3 (OsSCP3-a) promoter (Fig. 4). The sequence motifs of the g10 late pollen gene element (GTGA) were present at –1657, −842, and −288 for the OsSCP1 (OsSCP1-b) promoter, at −294 for the OsSCP2 promoter, and at −756 for the OsSCP3 (OsSCP3-a) promoter (Fig. 4). Furthermore, the repeated sequence (TAAGGAA) was present at position −195 and −53 for the OsSCP1 (OsSCP1-b), and −202 and −53 for the OsSCP2, though the function of this repeated sequence was unknown (Fig. 4).
Genomic organization of the OsSCP genes
As a next step, we surveyed the genomic organization of each OsSCP gene. In the case of OsSCP2 gene, we could not find the gene duplication in the rice genome, indicating that this OsSCP2 gene was single copy in rice genome. As previously described, we found two cDNA clones of OsSCP2 in the public database. Combining these two data, these two cDNA clones were derived from single OsSCP2 gene.
In contrast, in the case of OsSCP1 and OsSCP3 genes, we identified gene duplication in the rice genome. In OsSCP1, seven OsSCP1 homologous genomic clones (OsSCP1-a to OsSCP1-g) were tandemly duplicated within the 100-kb genomic region in chromosome 2 of the rice genome. In our experiment, we used popular type of the promoter of the genomic clone, OsSCP1-b, for promoter analysis, as previously described. From the Huge plot analysis, three long duplications were also detected (Fig. 6). Interestingly, the direction of the transcription of seven duplicated genomic clones was the same. Furthermore, when we compared the promoter regions (−2033 to +1) of seven homologous sequences, they were divided into four groups (Fig. 7A). The first group was OsSCP1-a. The second group contained three genomic clones, OsSCP-b, -e, -f. In these three genomic clones, the nucleotide sequence difference up to −2033 bp was not observed. The third group contained two genomic clones, OsSCP-c, -d. In these two genomic clones, the nucleotide sequence of promoter region up to −2033 bp was completely identical. The fourth group was OsSCP1-g. In the case of the OsSCP1-a promoter sequence, the region of the high sequence similarity to other groups (99%) was restricted up to −483 bp (Fig. 7A). Furthermore, several cis-elements, which were required for pollen-specific expression as described above, were also found in each genomic clones, though the location of the elements was different among the four groups (data not shown).
In the case of OsSCP3, one duplicated homologous clone (OsSCP3-b) was located at 3.7-kb downstream of OsSCP3 (OsSCP3-a), tandemly. Although a low sequence similarity in the promoter region (−1845 to +1) was observed between OsSCP3-a and OsSCP3-b, cis-elements were found in both OsSCP3-a and OsSCP3-b (Fig. 7B).
Discussion
In this study, we identified and characterized novel pollen-specific genes encoding small cysteine-rich proteins (OsSCPs). Small cysteine-rich proteins having eight conserved cysteine residues are widely distributed inside and outside the plant kingdom (Jose-Estanyol et al. 2004). These types of proteins contain the male S determinant of Brassica self-incompatibility (SP11; Takayama et al. 2000b; Watanabe et al. 2000), pollen coat protein, which can interact with SLR1 stigma-specific protein (PCP-A1, Stanchev et al. 1996; SLR1-BP, Takayama et al. 2000a), defensin peptides, which have anti-bacterial activity (Broekaert et al. 1995; Kanzaki et al. 2002; Park et al. 2002), nodule-specific cysteine-rich protein, whose functions are related to the nodule development and defense system (NCR; Mergaert et al. 2003), lipid transfer protein, which functions to transfer several different phospholipids (Zachowski et al. 1998), and pollen-specific ligand molecule, LAT52, which can interact with pollen-specific receptor kinase, LePRK2 (Twell et al. 1989; Tang et al. 2002). When aligned with these proteins at the amino acid level, the sequence and cysteine motif are highly diverted among these cysteine-rich proteins (Fig. 2), indicating that conserved cysteine motif would be important for their specific functions in each protein.
To date, several genes encoding the pollen-expressed cysteine-rich proteins (SP11, SLR1-BP, PCP-A1, PEC-3, and LAT52) have been characterized (Takayama et al. 2000a, b; Watanabe et al. 2000; Stanchev et al. 1996; Toriyama et al. 1998; Twell et al. 1989). All of these proteins had N-terminal signal peptide, indicating that these proteins should be extracellular proteins in pollen surface. As discussed above, a part of functions has been determined, and was important for pollen development and/or pollen–stigma interaction. From these data, OsSCPs would be important for reproductive process, like other pollen-expressed cysteine-rich proteins.
Gene duplication was observed in several genes in plants (Blanc et al. 2000). In the case of the plant disease resistance gene (R gene), genetically linked multigene families were clustered into a single locus. This R gene cluster should have contributed to the generation of the new R gene, because the nucleotide sequence diversity was observed among the duplicated homologous genes (Song et al. 1997; Dixon et al. 1998). The Arabidopsis genome contained gene families homologous to the pollen coat proteins (LCRs) and male S determinant of Brassica SI (SCRLs). Both gene families encoded different types of small cysteine-rich protein, and a part of the gene family formed a gene cluster (Vanoosthuyse et al. 2001). In the gene cluster, each clone was highly diverted. Although there was no experimental data showing that these clustered genes were inherited together as a unit (haplotype), these genes might have had different functions, respectively. In the Brassica SI, the duplication of the SP11 was also observed. In this duplication, the nucleotide sequence of two SP11 genes was completely identical. However, the deletion of the promoter region was found in one SP11 gene, indicating that this clone should have been a pseudogene (Shiba et al. 2004). In this study, in OsSCP1 and OsSCP3, gene duplication was observed (Fig. 6). Interestingly, the nucleotide sequences of ORF in the duplicated clones were completely conserved except for one clone in OsSCP1. Furthermore, pollen-specific cis-elements were also observed in each clone, indicating that these six duplicated clones of OsSCP1 would have been redundant, though the precise expression pattern was determined in only the representative clone, OsSCP1-b. From the RT-PCR analysis, OsSCPs were specifically expressed in the mature anther containing trinucleate pollen grains (Fig. 3). In addition to these data, the nucleotide sequence of OsSCPs in two different varieties, Koshihikari and Nipponbare was completely conserved, though several nucleotide variations in other genomic regions were observed in these two varieties (Shirasawa et al. 2004). Combining the results of cis-elements and RT-PCR of OsSCPs, OsSCPs, which were identified and characterized in this experiment, would have been mature anther-specific genes. This high redundancy and high sequence conservation of OsSCPs indicate the importance of this gene in pollen maturation, pollen germination, and pollen tube growth.
In our previous study, 111 mature anther-specific genes were identified in model legume, Lotus japonicus (Endo et al. 2002). Out of 111 mature anther-specific genes, one interesting gene encoding receptor-like protein kinase was found. On the basis of our preliminary data, this gene was specifically expressed in mature pollen grain in L. japonicus (H. Masuko, M. Endo, and M. Watanabe, unpublished data). To date, two types of the pollen-specific receptor kinases (PRK1 and LePRK2) have been isolated and characterized (Mu et al. 1994; Muschietti et al. 1998). The receptor domain of LePRK2 could interact with pollen-specific small cysteine-rich protein, LAT52 (Tang et al. 2002). This pollen-specific ligand and receptor should be important for pollen germination and pollen tube elongation (Tang et al. 2002). In the rice genome, 2210 candidate genes encoding receptor-like kinases have been identified (Shiu et al. 2004). However, the functions of the receptor-like kinases have been discovered in only a few genes (Song et al. 1995; Nonomura et al. 2003; Suzaki et al. 2004). If the OsSCPs which were identified in this study were functioned as a ligand, the discovery of the orphan receptor would be important for understanding the pollination and fertilization in future.
Abbreviations
- GUS:
-
β-glucuronidase
- OsSCPs:
-
Oryza sativa small cysteine-rich proteins
- RT-PCR:
-
reverse transcription – polymerase chain reaction
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25:3389–3402
Amagai M, Ariizumi T, Endo M, Hatakeyama K, Kuwata C, Shibata D, Toriyama K, Watanabe M (2003) Identification of reproductive organ-specific genes of cruciferous model plant, Arabidopsis thaliana, by using a combination of Arabidopsis macroarray and mRNA derived from Brassica olearacea. Sex Plant Reprod 15:213–220
An G, Ebert PR, Mitra A, Ha SB (1988) Binary vectors. In: Gelvin SB, Schilpeoort RA (eds) Plant molecular biology manual. Kluwer, Dordrecht, pp 1–19
Bate N, Twell D (1998) Functional architecture of a late pollen promoter: pollen-specific transcription is developmentally regulated by multiple stage-specific and co-dependent activator elements. Plant Mol Biol 37:859–869
Bedinger P (1992) The remarkable biology of pollen. Plant Cell 4:879–887
Blanc G, Barakat A, Guyot R, Cooke R, Delseny M (2000) Extensive duplication and reshuffling in the Arabidopsis genome. Plant Cell 12:1093–1101
Broekaert WF, Terras FR, Cammue BP, Osborn RW (1995) Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol 104:1353–1358
Dixon MS, Hatzixanthis K, Jones DA, Harrison K, Jones JDG (1998) The tomato Cf-5 disease resistance gene and six homologs show pronounced allelic variation in leucine-rich repeat copy number. Plant Cell 10:1915–1925
Endo M, Matsubara H, Kokubun T, Masuko H, Takahata Y, Tsuchiya T, Fukuda H, Demura T, Watanabe M (2002) The advantages of cDNA microarray as an effective tool for identification of reproductive organ-specific genes in a model legume, Lotus japonicus. FEBS Lett 514:229–237
Endo M, Tsuchiya T, Saito H, Matsubara H, Hakozaki H, Masuko H, Kamada M, Higashitani A, Takahashi H, Fukuda H, Demura T, Watanabe M (2004) Identification and molecular characterization of novel anther-specific genes in Oryza sativa L. by using cDNA microarray. Genes Genet Syst 79:213–226
Eyal Y, Curie C, McCormick S (1995) Pollen specificity elements reside in 30 bp of the proximal promoters of two pollen-expressed genes. Plant Cell 7:373–384
Honys D, Twell D (2003) Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol 132:640–652
Horsch RB, Fry J, Hoffmann N, Neidermeyer J, Rogers SG, Fraley RT (1988) Leaf disc transformation. In: Gelvin SB, Schilperoort RA (eds) Plant molecular biology manual. Kluwer, Dordrecht, pp A5/1–A5/9
Jose-Estanyol M, Gomis-Ruth FX, Puigdomenech P (2004) The eight-cysteine motif, a versatile structure in plant proteins. Plant Physiol Biochem 42:355–365
Kader J-C (1996) Lipid-transfer proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 47:627–654
Kanzaki H, Nirasawa S, Saito H, Ito M, Nishihara M, Terauchi R, Nakamura I (2002) Overexpression of the wasabi defensin gene confers enhanced resistance to blast fungus (Magnaporthe grisea) in transgenic rice. Theor Appl Genet 105:809–814
Lush WM, Grieser F, Wolters-Arts M (1998) Directional guidance of Nicotiana alata pollen tubes in vitro and on the stigma. Plant Physiol 118:733–741
McCormick S (2004) Control of male gametophyte development. Plant Cell 16:S142–S153
Mergaert P, Nikovics K, Kelemen Z, Maunoury N, Vaubert D, Kondorosi A, Kondorosi E (2003) A novel family in Medicago truncatula consisting of more than 300 nodule-specific genes coding for small, secreted polypeptides with conserved cysteine motifs. Plant Physiol 132:161–173
Mu JH, Lee HS, Kao Th (1994) Characterization of a pollen-expressed receptor-like kinase gene of Petunia inflata and the activity of its encoded kinase. Plant Cell 6:709–721
Muschietti J, Dircks L, Vancanneyt G, McCormick S (1994) LAT52 protein is essential for tomato pollen development: pollen expressing antisense LAT52 RNA hydrates and germinates abnormally and cannot achieve fertilization. Plant J 6:321–338
Muschietti J, Eyal Y, McCormick S (1998) Pollen tube localization implies a role in pollen–pistil interactions for the tomato receptor-like protein kinase LePRK1 and LePRK2. Plant Cell 10:319–330
Nonomura K, Miyoshi K, Eiguchi M, Suzuki T, Miyao A, Hirochika H, Kurata N (2003) The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell 15:1728–1739
Okada T, Sasaki Y, Ohta R, Onozuka N, Toriyama K (2000) Expression of Bra r 1 gene in transgenic tobacco and Bra r 1 promoter activity in pollen of various plant species. Plant Cell Physiol 41:757–766
Park HC, Kang YH, Chun HJ, Koo JC, Cheong YH, Kim CY, Kim MC, Chung WS, Kim JC, Yoo JH, Koo YD, Koo SC, Lim CO, Lee SY, Cho MJ (2002) Characterization of a stamen-specific cDNA encoding a novel plant defensin in Chinese cabbage. Plant Mol Biol 50:59–69
Rogers HJ, Bate N, Combe J, Sullivan J, Sweetman J, Swan C, Lonsdale DM, Twell D (2001) Functional analysis of cis-regulatory elements within the promoter of the tobacco late pollen gene g10. Plant Mol Biol 45:577–585
Shiba H, Park JI, Suzuki G, Matsushita M, Nou IS, Isogai A, Takayama S, Watanabe M (2004) Duplicated SP11 genes produce alternative transcripts in the S 15 haplotype of Brassica oleracea. Genes Genet Syst 79:87–93
Shirasawa K, Monna L, Kishitani S, Nishio T (2004) Single nucleotide polymorphisms in randomly selected genes among japonica rice (Oryza satava L.) varieties identified by PCR-RF-SSCP. DNA Res 11:275–283
Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH (2004) Comparative analysis of the receptor-like kinase family in Arabidopsis and Rice. Plant Cell 16:1220–1234
Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, Gardner J, Wang B, Zhai WX, Zhu LH, Fauquet C, Ronald P (1995) A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 270:1804–1806
Song WY, Pi LY, Wang GL, Gardner J, Holsten T, Ronald PC (1997) Evolution of the rice Xa21 disease resistance gene family. Plant Cell 9:1279–1287
Stanchev BS, Doughty J, Scutt CP, Dickinson H, Croy RR (1996) Cloning of PCP1, a member of a family of pollen coat protein (PCP) genes from Brassica oleracea encoding novel cysteine-rich proteins involved in pollen–stigma interactions. Plant J 10:303–313
Suzaki T, Sato M, Ashikari M, Miyoshi M, Nagato Y (2004) The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development 131:5649–5657
Takayama S, Isogai A (2005) Self-incompatibility in plants. Annu Rev Plant Biol 56:467–489
Takayama S, Shiba H, Iwano M, Asano K, Hara M, Che F-S, Watanabe M, Hinata K, Isogai A (2000a) Isolation and characterization of pollen coat proteins of Brassica campestris that interact with S-locus related glycoprotein 1 involved in pollen–stigma adhesion. Proc Natl Acad Sci USA 97:3765–3770
Takayama S, Shiba H, Iwano M, Shimosato H, Che F-S, Kai N, Watanabe M, Suzuki G, Hinata K, Isogai A (2000b) The pollen determinant of self-incompatibility in Brassica campestris. Proc Natl Acad Sci USA 97:1920–1925
Tang W, Ezcurra I, Muschietti J, McCormick S (2002) A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell 14:227–2287
Toriyama K, Hanaoka K, Okada T, Watanabe M (1998) Molecular cloning of a cDNA encoding a pollen extracellular protein as a potential source of a pollen allergen in Brassica rapa. FEBS Lett 424:234–238
Tsuchiya T, Toriyama K, Ejiri S, Hinata K (1994) Molecular characterization of rice genes specifically expressed in the anther tapetum. Plant Mol Biol 26:1737–1746
Twell D, Wing R, Yamaguchi J, McCormick S (1989) Isolation and expression of an anther-specific gene from tomato. Mol Gen Genet 217:240–245
Twell D, Yamaguchi J, McCormick S (1990) Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development 109:705–713
Vanoosthuyse V, Miege C, Dumas C, Cock JM (2001) Two large Arabidopsis thaliana gene families are homologous to the Brassica gene superfamily that encodes pollen coat proteins and the male component of the self-incompatibility response. Plant Mol Biol 16:17–34
Watanabe M, Shiozawa H, Isogai A, Suzuki A, Takeuchi T, Hinata K (1991) Existence of S-glycoprotein-like proteins in anthers of self-incompatible species of Brassica. Plant Cell Physiol 32:1039–1047
Watanabe M, Suzuki G, Toriyama K, Takayama S, Isogai A, Hinata K (1999) Two anther-specific expressed genes downstream of SLG 9: identification of a novel S-linked gene specifically expressed in anthers at the uninucleate stage of Brassica campestris (syn. rapa). Sex Plant Reprod 12:127–134
Watanabe M, Ito A, Takada Y, Ninomiya C, Kakizaki T, Takahata Y, Hatakeyama K, Hinata K, Suzuki G, Takasaki T, Satta Y, Shiba H, Takayama S, Isogai A (2000) Highly divergent sequences of the pollen self-incompatibility (S) gene in class-I S haplotypes of Brassica campestris (syn. rapa) L. FEBS Lett 473:139–144
Watanabe M, Takayama S, Isogai A, Hinata K (2003) Recent progresses on self-incompatibility research in Brassica species. Breed Sci 53:199–208
Yazaki J, Kojima K, Suzuki K, Kishimoto N, Kikuchi S (2004) The Rice PIPELINE: a unification tool for plant functional genomics. Nucl Acids Res 32:D383–D387
Zachowski A, Guerbette F, Grosbios M, Jolliot-Croquin A, Kader JC (1998) Characterization of acyl-binding by a plant lipid-transfer protein. Eur J Biochem 257:443–448
Acknowledgements
This work was supported in part by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Rice Genome Project MA-2211), and Grants-in-Aid for the 21 Century Center of Excellence Program from the Japan Society for Promotion of Science (JSPS) to MW. The authors are grateful to Ayako Chiba, Yukiko Ohyama, and Hiroyuki Ishikawa (Iwate University) for technical assistance. The authors also thank Ryo Tsuwamoto and Tomohiro Kakizaki (Iwate University) for providing pBI-GSH binary vector and for their helpful discussions. ME, HH, and YT are the recipients of a Research Fellowship of the JSPS for Young Scientists
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Toriyama
Rights and permissions
About this article
Cite this article
Park, JI., Hakozaki, H., Endo, M. et al. Molecular characterization of mature pollen-specific genes encoding novel small cysteine-rich proteins in rice (Oryza sativa L.). Plant Cell Rep 25, 466–474 (2006). https://doi.org/10.1007/s00299-005-0077-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-005-0077-2