Abstract
Key message
This work contributes in the identification and understanding of dehydrin genes and their function in the mechanisms of cold tolerance in eucalypt species.
Abstract
Dehydrins play a fundamental role in plant response and adaptation to abiotic stresses, having an important role in seed desiccation, response to abscisic acid, low temperatures, drought and salinity conditions. Eucalyptus nitens has a greater tolerance to cold than Eucalyptus globulus, which in part can be due to the role of dehydrins present in this species. This work reports the identification of four DHN genes in E. nitens and examines their response under low temperature, comparing them to those previously described in E. globulus. Transcript abundance of dehydrins increased when plants were cold acclimated, being higher in a freezing-resistant family of E. nitens than in a freezing-sensitive family. The relative levels of dhns in E. nitens were higher than the corresponding of E. globulus under the same conditions. The analysis of the promoter region for the four Enidhns showed that they contained several cold-or dehydration inducible cis elements, such as ABRE, MYC and CRT. The analyzes in the genomic sequence of dhn from E. globulus and E. nitens, together with the results of transcript abundance under cold acclimation, can be in part explained by differences found at the cis elements in the several DHN promoters studied. The most responsive gene to cold tolerance in both species was DHN2, which was used to obtain transgenic Arabidopsis thaliana containing either the coding region or the putative promoter. The EniDHN2 lines had higher transcript abundance than the A. thaliana with the EuglDHN2, although in both cases the transgenic lines had a higher survival rate to cold than the untransformed A. thaliana. In addition the EniDHN2 putative promoter drive induced higher expression levels of the gene (gus) marker than the one of E. globulus, when exposed to cold temperatures. Therefore, we hypothesize that the putative promoter of EniDHN2 and its coding region has a key role in conferring cold tolerance to this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chile is one of the main country growing eucalypts, which is widely used in forest plantations, covering a total surface of 700,000 ha, corresponding to Eucalyptus globulus (75 %) and to a less extent Eucalyptus nitens (25 %). E. globulus is the main species grown in temperate climates free of severe frosts due to its exceptional wood quality, combined with fast growth (Teulieres and Marque 2007). Of the fast-growing species E. nitens is considered suitable for planting on high-altitude sites where severe frosts and snow occur (Turnbull and Eldridge 1984). During the past years considerable effort has been directed towards understanding how E. globulus responds and adapts to low temperature due to its low freezing resistance, by different approaches, going from cold acclimation to transcriptomic analysis (Moraga et al. 2006; Costa e Silva et al. 2008; Fernández et al. 2006, 2015; Rasmussen-Poblete et al. 2008). Recently, Fernández et al. (2015) reported the identification of the main mechanisms likely to participate at cold acclimation in E. globulus, proposing that some of the differentially expressed genes responding to cold acclimation are a good source to discovering candidate genes for improving frost tolerance and potentially drought tolerance in this species such as LEA-like proteins including dehydrins. In a similar approach, Keller et al. (2013) identified more than 2500 unigenes involved in cold acclimation of E. gunnii, being some transcription factors as C-repeating factors (CBFs).
Plants are subjected to environmental variations that determine and limit their geographic distribution. Low temperature is an environmental factor limiting plant growth, affecting their development and reducing their productivity (Guzmán 2009; Amolkumar and Arun 2008). Cold resistance is one of the main mechanisms studied in plants, in some species, the phenomenon known as cold acclimation increase their cold tolerance as a response to environmental stimuli such as low non-freezing temperatures (Thomashow 1999; Fowler et al. 2005). Cold acclimation (CA) triggers a cascade of signals with physiological and biochemical changes in response to stress, providing the ability to acclimate to cold temperatures (Thomashow 1999). Gene induction in response to cold (COR) leads to the synthesis of proteins. An important group of these elements are LEA (Late Embryogenesis Abundant) proteins (Wise 2003), with a subset of proteins called dehydrins (DHNs) has been described in angiosperms (Close 1996, 1997). These proteins are involved in seed desiccation in response to low temperatures, drought, salinity and response to abscisic acid (Muñoz-Mayor et al. 2012; Layton et al. 2010; Kosová et al. 2007), and have been attributed important roles during cell dehydration, and stabilization of cell membrane contributing to stress tolerance (Close 1997). DHNs are characterized by possessing conserved domains known as K, Y and S segments (Close 1996, 1997), as well as less conserved regions rich in polar amino acids, called Φ segments (Rorat 2006). According to the presence and number of Y-, S- and K-segments, DHNs can be classified into five different subclasses: YnSKn, Kn, KnS, SKn, and Y2Kn (Close 1996). Several works suggest that the K-segment is involved in establishing hydrophobic interactions with other proteins during dehydration stress, mainly stabilizing cell membranes (Campbell and Close 1997; Danyluk et al. 1998; Koag et al. 2003). However, Perdiguero et al. (2014) recently identified dehydrin proteins with truncated K-segments and a dehydrin protein completely lacking K-segments in Pinus pinaster, being the role of these unusual dehydrins remains unrevealed.
Several studies have shown that dehydrins can protect cells against dehydration, Navarro et al. (2011) reported that the overexpression of dhn2 in transgenic lines of E. gunnii (EguCBF1a-OE) responded in a similar way than non-transformed lines previously acclimated to cold, increasing their frost tolerance. Fernández et al. (2012a, b) reported the presence of four dehydrin genes in E. globulus, namely dhn1, dhn2, dhn3 and dhn10. Relative expression analyses of these four genes in two contrasting genotypes of E. globulus (susceptible- and frost-tolerant) performed under low temperature, dehydration and at different photoperiods, showed that dhn1, dhn2 and dhn10 are mainly induced by low temperature, with higher transcript abundance in the frost-tolerant genotype than in the susceptible one, while dhn3 was mainly expressed under dehydration conditions. Further studies have demonstrated that Eugldhn2 and Eugldhn10 are mainly involved in the cold acclimation process of E. globulus (Fernández et al. 2015).
In the present study we identify genes belonging to group II LEA/dehydrin family present in E. nitens, which has been described as a more cold tolerant species. Young plants from two half-sib families of E. nitens and cuttings from one genotype of E. globulus were subjected to a cold treatment to determine the dehydrins that play a major role in CA. The dehydrin having a major role, measured as transcript abundance, were cloned and used to make transgenic Arabidopsis thaliana lines to assess cold tolerance. In parallel the putative promoter of these genes were used with a marker gene (GUS) to observe if it is cold induced and to provide evidence if the expression of genes regulated by these promoters under cold stress is tissue specific.
Materials and methods
Plant materials and cold treatments
Two half-sibs families, represented by 26-month-old rooted seedlings of E. nitens and one genotype of E. globulus (16 plants) previously characterized as frost resistant were used. All plants were transferred to polystyrene boxes covered with vermiculite to maintain the substrate humidity, placed in a cold chamber and subjected to four treatments as described by Fernández et al. (2012a). Briefly, non-acclimated (NA, control condition) plants were maintained under 14 h day length at 20/12 °C day/night temperature for 10 days; on day 10, leaves were collected and the condition of the growth chamber was changed as follows: treatment I, cold acclimated before night frosts of −2 °C (CABF), short days (10 h day) at 8/4 °C for 7 days; treatment II cold acclimated after night frosts of −2 °C (CAAF): plants were maintained at 8/4 °C where a night frost of −2 °C during four nights was applied (using a temperature decrease rate of 2 °C/h), and leaves were collected after the last frost (when the chamber reached 8 °C). For treatment III, deacclimated (DA), seedlings were exposed to long day length (14 h day length) and 12/6 °C day/night temperature for one week. A night frost of −6 °C during DA treatment was applied to determine the survival and percent of leaf damage for each genotype/family to assess their tolerance to a simulated late spring frost. Ten plants were exposed to the night frost and survival (percentage of live ramets), and leaf damage (percent of necrotic area per plant or ramet) was visually evaluated after 10 days. The growth chamber experiment was arranged as a completely randomized design. Three replicates (seedlings or ramets) were collected and stored at −80 °C until further use for qRT-PCR analysis.
Wild type and transgenic Arabidopsis thaliana ecotype Columbia were germinated on 1× Murashige and Skoog media (MS) agar plates supplemented with 2 % sucrose, pH 5.8. After 4 days of incubation at 4 °C in the dark, the seeds were transferred to the growth room at 23 °C under short-day photoperiods (12/12 day/night) and cultivated in soil for 3 weeks. To assess frost tolerance, 19 plants per line were subjected to a temperature decrease, in a phytotron (Percival LT-36VL), starting at 23 °C and reaching −6 °C, with a 2 °C decrease per hour and kept at this temperature for 3 h. Three plants per each line were collected at 23, 4 and −6 °C. After this assay, plants were kept at 23 °C for 7 days and the survival rate was estimated by visually assessing the plant recovery. When each of the tested temperatures was reached, leaves from three plants per line were collected for qRT-PCR analysis, stored at −80 °C until further use and leaves were stained for gus activity at the end of each treatment.
EniDHNs gene identification
DNA was extracted from E. nitens leaf tissue according to the protocol described by Doyle and Doyle (1987). For the identification of DHNs genes in E. nitens four pairs of primers were designed using a reference the dehydrin sequences previously described for E. globulus (Fernández et al. 2012a). The details of the primers employed for each sequence are: Enidhn1_F5′TCGTCTCATCATTTAGTGCATCGG3′ R5′GCAGCTTATCCATGATCTTGTCCA3′ (expected size 1325 bp); Enidhn2_F5′CAGCACGCCTAACTTGAATATA3′ R5′GGCGGCGGAGGAGAATAGAC3′ (1671 bp); Enidhn3 F5′CTAGTGAGGTGGGTGAGGATAGAG3′ F5′AGTCTCGGTATCTCTACTGTGTCG3′ (1473 bp) and for Enidhn10 F5′TAATCAACCGTGCTACGTTTGTCC3′ and R5′AATATAACCTGACTGCGAAACGGG3′ (552 bp). The PCR conditions were 0.9 µM primers, 1 U Taq platinum DNA polymerase (Invitrogen, USA), 200 mM dNTP mix (Invitrogen, USA), 2.5 mM MgCl2 and 2 ng of DNA in a total volume of 25 µl. The amplification process was performed following a cycle at 95 °C for 5 min, 40 cycles of 15 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C, and a final cycle of 7 min at 72 °C. PCR products for each primer pair were analyzed on a 2 % agarose gel electrophoresis and purified using the QIAquick PCR Purification Kit and under BigDye terminator cycling condition (Macrogen, Korea).
Multiple sequence alignment, cis-elements identification and phylogenic tree construction
The full-length DHNs nucleotide sequences were translated using ExPASy translate tool (http://web.expasy.org/translate/). The S-, K- and Y segments of DHNs proteins were identified using the ExPASy prosite server (http://prosite.expasy.org/). The molecular weight and isoelectric point of the four dehydrins were predicted using the ExPASy-Compute pI/MW tool (http://web.expasy.org/cgi-bin/compute_pi/pi_tool) based on their amino acid composition. The comparison of 9 or 10 DHN proteins sequences from different species per DHNs type were aligned using CLUSTAL-W version 2.1 and a phylogenetic tree was generated using PAUP version 4.0, with Neighbor-Joining method on 1000 bootstrap iterations. The putative promoter regions were analyzed for plant regulatory cis elements using the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
RNA extraction, cDNA synthesis and real time qRT-PCR
Total RNA was extracted from leaves either of E. globulus or A. thaliana using the CTAB method according to Chang et al. (1993) and quantified by UV spectrophotometry (NanoDrop ND-1000, Thermo scientific, USA). RNA integrity was visualized on a 1 % agarose gel. For cDNA synthesis, samples containing 1 µg of total RNA were pre-treated with DNAse I to remove DNA contamination, and then the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, USA) was used according to the manufacturer’s instructions.
Real-time qRT-PCR was carried out using an ABI Prism 7300 Sequence Detection System (Applied Biosystems, USA), with the following conditions: initial denaturation 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C, in 96-well optical reaction plates. Two housekeeping genes Ubiquitin C and α-Tubulin were used as described by Fernández et al. (2010). The DHNs primers employed for the relative quantification in E. nitens were: EniDHN1-F/R: 5´-AGACCAGCAGCAGCAGATGAG-3´ and 5´-CTTCTTCCTCCTCCCGCCTTG-3´; EniDHN2-F/R: 5´-CCCTGTGGAGAAGTGCGACGAG-3´ and 5´-CGGGGGCGGAGGAGAATAGAC-3´; EniDHN3-F/R: 5´-CGCATTTCCAGAACCACTACGC-3´ and 5´-ATGCTCCGCCCTGACACC-3´, and EniDHN10-F/R: 5´-CGAGAACAAGGGAGGCTTCACC-3´ and 5´-CTGTCGCTGCTGCTGCTGTC-3´. For E. globulus, primers used were those described by Fernández et al. (2012a, b). For A. thaliana, two housekeeping genes EF1a and PP2A3 were used: EF1a-F/R: 5´-TGAGCACGCTCTTCTTGCTTTCA-3´ and 5´-GGTGGTGGCATCCATCTTGTTACA-3´; PP2A3-F/R: 5´-AGGAAACTTGCGTGAGGGAGAAAG -3´ and 5´-TGGCCTGGCAGGAGAAACT-3´. The Aterd10, Atrab18 and GUS primers employed for the relative quantification in A. thaliana were: Aterd10-F/R: 5´-CGAGTGATGAAGAAGGTGAAGACG-3´ and 5´-CCCTGGTTTCTCTCCGAGTGG-3´; Atrab18-F/R5 CCGATGGGAGGAGGAGGATACG3′ and 5′ CGCCAGTTCCAAAGCCTTCA3′ and for GUS: 5´-TGGCCTGGCAGGAGAAACT3´ and 5´-CGTATCCACGCCGTATTCG´-3′. The fragment lengths varied between 79 and 173 bp and their specificity was verified by checking the melting curve (60–95 °C) after 40 cycles. Three technical replicates were analyzed for each sample. For each gene, PCR efficiency was determined by measuring the Ct to a specific threshold for a serial dilution of cDNA.
EuglDHN2 gene isolation and plant expression vectors construction
With the purpose of studying the response of EuglDHN2 and EniDHN2 genes to low temperature exposure, the coding and promoter regions were isolated using specific primers previously detailed. Gateway® Clonase Technology was used to facilitate the cloning of PCR-amplified fragments into adequate transformation vectors. Forward and reverse primers containing attB sites were designed with the Geneious Pro 6.0 software (Biomatters LTD). The PCR amplification was performed following a 95 °C cycle for 5 min, 40 cycles of 15 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C, and a final cycle of 7 min at 72 °C. Amplified fragments were then electrophoresed on 0.8 % agarose gels and then purified using a PCR purification kit (Qiagen Gel Extraction Kit).
Amplified fragments were cloned into pDONR221 vector by BP reaction and then into destination vectors by LR reaction following standard protocols. The destination vector pMDC32, for constitutive expression under the CaMV35S promoter, were used to drive the coding region of EuglDHN2, while the promoter region was cloned on the vector pMDC163 containing the β-glucuronidase gene (GUS) as reporter gene. Both cloning vectors were developed by Curtis and Grossniklaus (2003) and obtained by the Addgene service (www.adgene.org). The resulting plasmids were named 35S-EuglDHN2, 35S-EniDHN2, 5´EuglDHN2-GUS and 5´EniDHN2-GUS,
Arabidopsis thaliana transformation
The transformation of A. thaliana was carried out according to Clough and Bent (1998). Purified plasmids were electroporated to Agrobacterium tumefaciens strain GV3101 using a Biorad MicroPulser (Biorad, USA) apparatus set at 2.2 kV, recovered for 1 h at 28 °C in LB medium, and then the cells were plated and grown overnight at 28 °C in a LB medium supplemented with gentamycin, rifampicin and kanamycin at 100 mg/l each. Confirmed Agrobacterium transformants were grown on LB media supplemented with antibiotics and used for Arabidopsis transformation using the floral dip technique (Clough and Bent 1998). The seeds were collected from individual plants and plated on MS medium containing hygromycin at 15 µg/ml. Transformed seedlings were selected according to the method described by Harrison et al. 2006. Transformed seedlings were transplanted into pots containing a mixture of sterile peat moss and vermiculite in the ratio 1:1 and maintained on growth chamber at 23 °C and 16/8 photoperiod.
Molecular analysis of transgenic plants
DNA was extracted from the leaves of putative transgenic plants. PCR was carried out using sets of primers specific to each interest region. The primer pair HYGRF/R 5´-CTACACAGCCATCGGTCCAG-3 and 5´-AAAAAGCCTGAACTCACCGC-3 were used to amplify a 961 pb region of the hygromycin phosphotransferase (hph) gene while for GUS the primers used to amplify the full-length gene corresponded to GUS-F/R: 5´-CAACGTCTGCTATCAGCGCGAAGT-3´ and 5´-TATCCGGTTCGTTGGCAATACTCC-3´.
GUS staining
Histochemical GUS staining was performed according to the method described by Clough and Bent (1998). Leaves and flowers were incubated with GUS staining solution containing 0.5 M sodium phosphate buffer pH 7, 10 % Triton X-100, 100 mM potassium ferrocyanide, 100 mM potassium ferricyanide and 1 mM 5-bromo-4-chlore-3-indolyl-β-D-glucuronic acid (X-gluc). The material was incubated in the dark at 37 °C overnight. Finally the staining solution was removed with ethanol and the tissues were cleared and fixed with 70 % ethanol for further analysis.
Statistical analysis
Software SAS version 9.2 (SAS Institute Inc.,USA) was used for statistical analysis. t test for comparison of sample E. nitens resistant and susceptible families, and E. globulus resistant genotype, on each treatment and comparing each treatment with their respective control. For A. thaliana the effects of the treatments were analyzed with ANOVA and a Turkey’s test for means comparison. p value ≤ 0.05 was considered significant.
Results
Isolation and characterization of EniDHNs
A total of four dehydrins were identified in E. nitens. The isolated fragment for Enidhn1 (KU67482) corresponded to a 1325 bp fragment, containing an intron of 237 bp and a coding sequence of 539 bp corresponding to 179 amino acids with a calculated isoelectric point of 5.3 and molecular mass of 17.23 kDa which differed in 30 amino acids with the previously described EuglDHN1. The sequenced DNA segment of EniDHN2 (KU674822) is 1671 bp long with an intron of 228 bp and a coding sequence of 663 bp coding for 221 amino acids with a deduced molecular mass of 25 kDa and an isoelectric point of 5.23, having a difference of 46 amino acids with the EuglDHN2. In the case of Enidhn3 (KU674823) it was possible to isolate a single fragment of 1473 bp containing one intron of 73 bp and a coding sequence of 441 bp corresponding to 147 amino acids with a calculated isoelectric point of 8.76 and molecular mass of 16.07 kDa, differing in six amino acids. For Enidhn10 (KU674824) the sequenced DNA segment was 552 pb long with a coding sequence of 297 bp corresponding to 99 amino acids showing no difference with the EuglDHN10. The calculated isoelectric point for Enidhn10 was 6.59 with a deduced molecular mass of 10.93 kDa.
The dehydrin protein sequences identified in E. nitens were compared with existing sequences in other plant species by Clustal W. Eight dehydrin sequences showed high percentages of amino acid identity (up to 70 %) when searching in BLASTP for each EniDHNs (Supplemental material, Figure S1 and Table S1). Although an 88 % identity of EniDHN10 with a sequence of E. grandis (XP_010036245) was retrieved from NCBI, this does not correspond to a dehydrin as described in the latest version of the E. grandis genome (Phytozome).
In addition, a phylogenetic tree including all EniDHNs proteins isolated and dehydrins described for other species of Eucalyptus was created (Fig. 1). Based on the phylogenetic results, the EniDHNs proteins were clustered in different groups, the EniDHN1 clustered with the Eucalyptus SKn-type, the amino acid sequence showed one S and two K-segments, for EniDHN2 the results showed a cluster with the Eucalyptus SKn-type, the amino acid sequence showed one S and two K-segments. EniDHN3 clustered with the Eucalyptus YnSKn-type protein containing three Y, one S and two K-segments, while for EniDHN10 clustered in the KS-type, the amino acid sequence showed one K and one S segment.
The analyzes of the putative promoter regions for the four EniDHNs studied, showed that the cis-elements can be classified mainly into hormone-responsive and stress-responsive elements are shown in Table 1 and Fig. 2. Among the main elements found are: for EniDHN1 three ABRE (ABA responsive elements) elements and one G-box were identified, in the case of EniDHN2 four ABRE elements and seven G-boxed were found, while in EniDHN3 three ABRE elements and five G-box elements were identified, and for EniDHN10 only one ABRE element was found. Other cis-elements responding to the ABA-independent pathway were found, in EniDHN1 and EniDHN2 four and two low temperature responsiveness element (CRT) were identified, respectively, in the same position described by Fernández et al. (2012b) for E. globulus. In EniDHN3 four MYC elements were found, these elements are related with dehydration-responsive gene promoter elements.
Location of cis-elements associated with the four dehydrins promoter region. Negative numbers indicate the position of nucleotides relative to the translation start site. Gray triangle = TCA element, white triangle = Box 4, inverted gray triangle = G-Box light responsive element, inverted black triangle = GC motif, white circle = heat stress-responsive element, black circle = regulatory element involved in circadian control, white square = CRT low temperature response motif, white trapezoid = CGTCA-motif, gray trapezoid = TGA motif, black trapezoid = CAAT-BOX enhancer region, white rectangle = ABRE motif involved in abscisic acid responsiveness, hatched rectangles = GARE-motif gibberellin-responsive element
Gene expression of EuglDHNs and EniDHNs in response to cold acclimation
Ten days after the night frost of −6 °C, the survival rate resulted in 56 % and leaf damage was 61 % for the resistant to frost (RF) E. nitens and 33 and 81 % of survival and leaf damage, respectively, for the frost susceptible (FS) E. nitens family, while for E. globulus it was 15 and 33 % of survival and leaf damage, respectively. The results obtained for E. globulus showed that all EuglDHNs increased the transcript accumulation when comparing the CAAF to the NA condition: EuglDHN1 presented a ~9-fold increase, while EuglDHN2 showed the highest transcript accumulation increasing ~60-fold, with EuglDHN10 transcripts level increased only ~8-fold (Fig. 3a). Transcript abundance of the four dehydrins described in E. nitens was assessed in both families. The results of cold treatments showed that in the case of EniDHN1, the transcript level increased ~25-fold (SF) and ~40-fold (RF) at the CAAF condition compared to the NA. For EniDHN2 both families increased their gene expression at CAAF compared to NA, with the RF showing the highest transcript accumulation of ~176-fold, while for the SF, only a ~39-fold was observed. The transcript abundance for EniDHN10 showed a ~31-fold for the RF when comparing the CAAF with the NA treatment, while for the SF a ~15-fold increase was observed (Fig. 3b, c). Since in all cases the highest transcript accumulation was detected at the CAAF condition, the transcript level for all dehydrins was compared between both E. nitens families (Fig. 4a), finding only a significant difference for EniDHN2, which had a ~141-fold change when comparing the RF to the SF. Similar to the results for E. nitens, only EuglDHN2 showed a significant difference in the transcript accumulation from NA to CAAF treatments, however, the transcript abundance for EnDHN2 was highest (Fig. 4b). EniDHN3 and EuglDHN3 were not detectable in any of the conditions tested.
Gene expression of three DHN genes in leaves of a Eucalyptus globulus b frost resistant Eucalyptus nitens and c frost susceptible Eucalyptus nitens under low temperature using two internal controls UBC and α-TUB. The asterisks on top of the bars (mean + SE) indicate statistically significant differences among NA and CAAF treatment (*p ≤ 0.05; **p ≤ 0.01, ***p ≤ 0.001)
Transcript abundance of three DHN genes measured in leaves of a two Eucalyptus nitens families, resistant (RF) and susceptible (SF) and b comparison of the transcript abundance between an Eucalyptus globulus frost resistant genotype and the frost resistance family (RF) of Eucalyptus nitens under low temperature using two internal controls UBC and α-TUB. The asterisks on top of the bars (mean + SE) indicate statistically significant differences in the CAAF treatment (**p ≤ 0.01, ***p ≤ 0.001)
The results showed that EuglDHN2 and EniDHN2 were the only genes that had a significant difference in transcript accumulation at CAAF compared to the control conditions. In the case of EniDHN2 the transcript abundance (as fold change) was three times higher than the one observed for the same dehydrin in E. globulus (Fig. 4b). Based on these results the corresponding coding sequence for dhn2 was cloned from E. globulus and E. nitens under the constitutive promoter 35SCaMV and the putative promoters of these genes were cloned and used to determine gus as a marker gene, to determine if it is induced by cold and if it is tissue specific.
Effect of EuglDHN2 and EniDHN2 in response to cold acclimation in Arabidopsis thaliana
Several transgenic lines containing either the coding region for EuglDHN2 or EniDHN2 were generated. The expression of the gene under the constitutive promoter 35SCaMV for all T0 lines was measured using qRT-PCR (data not shown). For the case of EuglDHN2 three lines each representing low, medium and high expression levels were selected for further analysis, however, two lines were discarded since they had an abnormal phenotype at T1 (dwarf or slow growth rate). For the EniDHN2 lines three low and three high expressors were selected. For further analysis EniDHN2 were selected on hygromycin B and it was observed that all lines assayed were abnormal, showing a dwarf phenotype or a very slow growth rate, despite this, they were still considered and included in the cold tolerance assay. The T1 lines were subjected to a cold treatment as previously described and the survival rate for each line was determined. In most cases a higher survival rate was observed when compared to non-transformed A. thaliana (50 %), with the lines transformed with EuglDHN2 reaching 50–100 % survival, and the lines transformed with EniDHN2 reached 10–90 %, Due to the small phenotype and the lower survival rate observed in the EniDHN2 lines, no qRT-PCR analysis was carried out, however, data was collected for the EuglDHN2 transgenic lines and sampling was carried out at 23 °C (control), 4 and −6 °C and the transcript abundance was measured in each case (Fig. 5a). No significant differences at the transcript abundance of the gene were observed when plants were submitted at different temperature, although there were significant differences between the seven EuglDHN2 T1 lines assayed. There was no correlation between the relative transcript level of the transgene and the observed survival rate. The transcript levels for the orthologous genes Aterd10 (Fig. 5b) and Atrab18, were also measured observing that in the case of Aterd10 the transcript level increased significantly with low temperatures in all lines tested, including the wt A. thaliana, while for Atrab18 no significant differences in the transcript abundance at the three temperatures analyzed could be observed for any of the lines tested (transgenic and wt). The data of these endogenous genes did not correlate with the survival rate observed. For example, L5 had a high expression level of both genes and a survival rate of 80 % at −6 °C, while L2 and L27 had a 100 % of survival rate and the expression levels of these genes were lower than the ones observed for L5 and the wt.
Role of the putative promoters of EuglDHN2 and EniDHN2 in response to cold acclimation in Arabidopsis thaliana
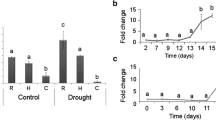
The putative promoter of EuglDHN2 and EniDHN2 was used with the gus gene to determine if it was cold induced, by transgenic lines of A. thaliana. In the case of the putative promoter of EuglDHN2 five T0 lines were assayed, which had a low germination rate when tested with hygromycin B (10–37 %), while for the T0 lines containing the putative promoter of EniDHN2 the germination rate was high (70–80 %). The A. thaliana T1 lines were tested under the same cold profile used previously and sampled at 23 °C (control), 4 and −6 °C. For the T1 lines of EuglDHN2 putative promoter three of the four lines tested, the expression of the transcript abundance of gus was significantly increased with temperature compared to the control (twofold change), while in the fourth line there was no significant differences. For the EniDHN2 putative promoter, in the five T1 lines tested, the transcript abundance of gus increased significantly with lower temperatures in all cases, at much higher levels (7–40 fold change) than those observed for the EuglDHN2 putative promoter (Fig. 6a, b). The histochemical staining of the samples, in both cases, showed that the expression was not tissue specific (data not shown).
Discussion
It has been suggested that different types of DHNs proteins are involved in responses to various growth conditions (Xu et al. 2014). Improvement of plant response to abiotic stress would enhance plant adaptation mainly because of the function of DHNs, which can protect cells from dehydration, stabilize the cell membrane, eliminate free radicals by binding metal ions or act as molecular chaperones (Close 1996). In this study four dehydrins were identified in E. nitens, corresponding to EniDHN1, EniDHN2, EniDHN3 and EniDHN10, with a potential function in the response to cold acclimation. The EniDHN1 and EniDHN2 showed the most interesting results, since they had a high transcript level at the acclimated state compared to the control (non-acclimated), being similar to the results reported by Fernández et al. (2012b) in E. globulus. Interestingly the transcript level was much higher in E. nitens than in E. globulus for both genes, taking into account that the later is more cold tolerant than E. globulus. Based on the phylogenetic tree constructed by EniDHNs and other available sequences, these dehydrins belong to the SKn-type, previous studies suggest that the synthesis of these kind of dehydrins are preferentially accumulated in plants in response to low temperatures (Allagulova et al. 2003). Fernández et al. (2012a) reported that the hydrophilic nature of SKn-type polypeptides is well suited to replace water and stabilize membranes through polar interactions during dehydration. Rorat (2006) showed that SKn-type DHNs in Solanum species accumulate in response to low temperature and are correlated with the capacity of the plants to cold acclimate and develop frost tolerance. In Eriobotrya japonica a freezing treatment resulted in up-regulation of the expression levels of two dehydrins of the SKn-type, these levels were more pronounced in the freezing-tolerant cultivar than in the sensitive cultivar (Xu et al. 2014). Additionally, Welling et al. (2004) reported that the transcript levels for SKn-type DHNs from Betula pendula decreased during autumn and started to increase in winter. EniDHN10 showed a variation in transcript levels from a non-acclimated to acclimated state being higher in the acclimated state, this dehydrin belongs to the KnS type (Rorat 2006). Fernández et al. (2012a) suggested that EuglDHN10 protein (KnS types in E. globulus) might be involved in the iron transport in phloem mediated long- distance transport. It has been shown that the lipid peroxidation as a result from reactive oxygen species (ROS) is generated in stressed plants. Xu et al. (2014) suggest that decreases in membrane fluidity, as a result of peroxidation, may be one cause of cold stress, while Thompson et al. (1987) found lipid peroxidation in plant membranes to be caused primarily by free-radical attacks. It has been frequently shown that DHNs can alleviate oxidative damage stressed plants by scavenging hydroxyl and peroxyl radicals or binding metals (Hara et al. 2004; Sun and Lin 2010). Also it was reported that the presence of KS-type dehydrins can reduce the formation of reactive oxygen species from Cu and inhibit the generation of hydrogen peroxide and hydroxyl radical Cu-ascorbate system (Hara et al. 2013). EniDHN3 was not detectable in any of the assays; previous studies suggest that the synthesis of YnSKn dehydrins is induced by drought but not by low temperatures (Allagulova et al. 2003; Fernández et al. 2012b). In wheat YSKn DHNs are mostly induced in dehydrated seedling leaves, which suggest their roles in wheat dehydration tolerance (Wang et al. 2014), in barley seedlings YSKn dehydrins are accumulated in response to ABA treatment and dehydration, but their content remains unchanged during low temperature (Choi et al. 1999).
As a first attempt to isolate the promoter region putatively responsible for the cold expression of dehydrins, a computational search over the whole sequence was performed to look for previously described conserved DNA elements (Komarnytsky and Borisjuk 2003). The analysis of the 5′ upstream region of each of the dehydrins identified in E. nitens, contained most of the putative promoter, showing that the four EniDHNs had several cold or dehydration inducible cis-elements (Table 1). These elements are involved in both the ABA-dependent and ABA-independent pathways of gene regulation of dehydrins (Shinozaki and Yamaguchi-Shinozaki 2000). Within the putative promoter region of EniDHN1 it was possible to identify four CRT (C-repeat) regulatory elements while in EniDHN2 two CRT regulatory elements were found, these elements are part of an ABA-independent pathway. The high transcript levels of accumulation observed for EniDHN1 and EniDHN2 could be related to the presence of the CRT regulatory element, since this sequence is present in the promoters of many cold- induced structural genes, with several studies pointing to CBF (CRT-Binding Factor) transcription factors that interact with DRE/CRT-elements and regulate the transcription activity of dehydrin genes (Thomashow 1999; Allagulova et al. 2003; Kosová et al. 2007; Shinozaki et al. 2003; Stockinger et al. 1997). For EniDHN10 no elements involved in the ABA-independent pathways were identified, indicating that EniDHN10 is more responsive to stress by dehydration than to cold, however, in EuglDHN10 Fernández et al. (2012b) identified one LTR element (responsiveness to low temperature) in the putative promoter region. Since EniDHN10 showed a variation at the transcript level from a non-acclimated to acclimated state, being higher in acclimated state, it could be possible that this type of motif is present in the putative promoter region but was not identified within the region sequenced (224 bp). For EniDHN3 four MYC and three ABRE elements, which are mainly involved in dehydration and ABA-responsive gene expression were identified (Vornam et al. 2011). All putative promoter regions of EniDHNs studied here contained ABRE elements, concluding that the response of dehydrins to cold can be mediated by several ABA-dependent and ABA-independent mechanisms. The ABRE elements contain two sequences known as G-box and GC- motif and the presence of a single copy in the promoter region is not enough for ABA-dependent gene expression (Choi et al. 1999; Kosová et al. 2007). In barley ABA-inducible dhn1 contains a G-box and GC-motif; its removal was accompanied by the loss of promoter sensitivity to ABA (Robertson et al. 1995). When comparing the number of cis-elements present in EniDHNs and in EuglDHNs, significant differences were found between ABRE elements especially in the case of DHN2 where the number of ABRE and G-box elements in E. nitens were higher than those present in E. globulus. This is consistent with the high expression levels for this dehydrin, which is the only gene studied that showed a significant transcript accumulation in both species, at CAAF compared with the NA, and being higher the transcript abundance in E. nitens than in E. globulus. The presence of this kind of elements suggests that EniDHN3 may be more related to dehydration caused by water deficit than by cold stress, this is in agreement with the reports of EuglDHN3, an YnSK2- type DHN from E. globulus, presenting high levels of transcript in plants subject to water deficit treatment while plants subject to low temperature had a low transcript level compared to the control (Fernández et al. 2012b), and also with Shen et al. (2004), that reported the transcripts of BcDh2 (an YnSK2- type DHN from Boea crassifolia) highly accumulated when the plants were treated with drought, salinity, exogenous ABA and moderated heat shock, but showed only a slight accumulation in response to low temperature stress.
Based on these results, and since the main dehydrin responding to cold acclimation corresponded to DHN2, transgenic lines of Arabidopsis thaliana transformed with the genes EniDHN2 and EuglDHN2 under the constitutive promoter 35SCaMV that were generated. The low germination rate observed for the EuglDHN2 lines (less than 30 %) has also been observed in plants over-expressing dehydrins as in the case of A. thaliana with Aterd10 and CuCOR19 in N. tabacum (Kim and Nam 2010; Hara et al. 2003). The aberrant phenotype observed in all the EniDHN2 lines assayed has not been observed in other dhn over-expressing transgenic lines (Puhakainen et al. 2004; Houde et al. 2004; Brini et al. 2011), however, this aberrant phenotype has been observed in when other genes involved in cold stress tolerance, such as cbf are overexpressed in A. thaliana (Gilmour et al. 2004; Sharabi-Schwager et al. 2010).
The transgenic A. thaliana T1 lines obtained containing either the EuglDHN2 showed that they had a higher cold tolerance, at least in a 40 %, than the untransformed A. thaliana, when subjected to a cold tolerance assay. Similar results have been observed in Nicothiana tabacum over-expressing ZmDHN2b (Xing et al. 2011) and in Fragaria anassassa with the WCOR410 (Houde et al. 2004), therefore, suggesting that this gene can play a key role in the cold acclimation of eucalypts. Nevertheless, this is not true for the EniDHN2 lines, in which case only the high expressing lines had a higher survival rate than the wt A. thaliana, but they showed an aberrant phenotype. It is worth noting that this heterologous system, using Arabidopsis, does no necessarily represent the role that these genes will have in eucalypts. The endogenous gene Aterd10, was induced by cold temperatures in all transgenic lines tested to similar levels as the ones observed in the non-transformed plants. This gene is also an SKn having a 36 % nucleotide identity with EniDHN2 or EuglDHN2 and they respond to cold, high salinity and water stress (Nylander et al. 2001). Over-expression of Aterd10 increases cold tolerance in A. thaliana as reported by Kim and Nam 2010. In L5 the expression of this gene was significantly higher than for the rest tested, however, it did not have a role in conferring a higher cold tolerance to this line. For Atrab18, no significant difference was observed in transcript abundance due to cold in any of the lines tested (transgenic or wild type). This gene has been shown to increase under abiotic conditions such as cold, water stress or by ABA (Lång and Palva 1992).
The putative promoter of EniDHN2 was strongly induced by low temperatures (4 and −6 °C) in all lines of the transgenic A. thaliana tested, while the one from EuglDHN2 was induced to a lower extent. This is an interesting result, since the putative promoter of EniDHN2 can have a key role in a faster and stronger response to cold stress, the characteristic that makes this species to be more frost-tolerant. The differences observed at the cis elements of each promoter can as well explain the difference in the fold changes observed at the same temperature of the corresponding transgenic lines. Zhu et al. (2014) observed that the putative promoter of a SKn dehydrin in wheat, (wzyl-2) was induced by several factors involved in abiotic stress such as cold (4 °C), phytohormones (ABA, SA, MeJA) as well as PEG6000. In the case of cold temperature, the promoter was induced at early times 12 h, having a peak at 24 h. These results are similar to ones obtained in this study, where the promoter was induced at 4 °C and increased when temperatures reached −6 °C. Compared with other studies (Rodríguez et al. 2005; Nylander et al. 2001) we did not observe any transcript accumulation in a specific tissue or organ of the GUS gene under the control of the putative promoter of DHN2, rather a constitutive expression that was induced by temperature was observed. However, Nylander et al. (2001) reported a tissue specific accumulation for five different dehydrins in A. thaliana, the most similar dehydrin tested, compared to the DHN2 of eucalypts, was ERD14 (SKn-type dehydrin), in which case they found the protein in all tissues tested. Zhu et al. (2014) did not observe any staining in calli treated by cold, but they assume that this can be due to the duration of the experiment. However, gus staining is observed, at different levels, when the calli are treated with different phytohormones.
As a conclusion, the analysis in the genomic sequence of DHN genes from E. globulus, a frost sensitive tree, and E. nitens, a frost-tolerant tree, in combination with gene expression under cold acclimation, can explain the differences in this trait. The presence of different cis-acting elements identified in the putative DHN promoter regions and the results of the relative expression data demonstrate the existence of an association between the role of DHNs of both species and the process of cold acclimation, allowing to infer that they have a key role in tolerance to low temperatures in Eucalyptus. The evidence showed that EniDHN2 putative promoter acted as a strong promoter induced by cold temperatures, therefore, this could have a key role in conferring frost tolerance to this species.
Author contribution statement
PA and MO contributed equally to the results of the sequencing of dhn sequences in E. nitens and qRT-PCR data. JS and FN were responsible of making the transgenic lines. DN contributed with the phylogenic data, RL with the analysis of transgenic plants, MF with analysis of cis elements and SV is the PI.
References
Allagulova CR, Gimalov FR, Shakirova FM, Vakhitov VA (2003) The plant dehydrins: structure and putative functions. Biochemistry 68:945–951
Amolkumar S, Arun S (2008) Signal transduction during cold stress in plant. Physiology and Molecular Biology of Plant 14:69–79
Brini F, Yamamoto A, Jlaiel L, Takeda S, Hobo T, Dinh HQ, Hattori T, Masmoudi K, Hanin M (2011) Pleiotropic effects of the wheat dehydrin DHN-5 on stress responses in Arabidopsis. Plant Cell Physiol 52:676–688
Campbell SA, Close TJ (1997) Dehydrins: genes, proteins, and associations with phenotypic traits. New Phytol 137:61–74. doi:10.1046/j.1469-8137.1997.00831.x
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Report 11(2):113–116
Choi DW, Zhu B, Close TJ (1999) The barley (Hordeum vulgare L.) dehydrin multigene family: sequences, allele types, chromosome assignments, and expression characteristics of 11 Dhn genes of cv Dicktoo. Theor Appl Genet 98:1234–12478
Close TJ (1996) Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiologia Plant 97:795–803
Close TJ (1997) Dehydrins: a commonality in the response of plants to dehydration and low temperature. Plant Physiol 100:291–296
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Costa e Silva F, Shvaleva A, Broetto F, Ortunño MF, Rodrigues ML, Almeida MH, Chaves MM, Pereira JS (2008) Acclimation to shortterm low temperatures in two Eucalyptus globulus clones with contrasting drought resistance. Tree Physiol 29:77–86
Curtis M, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133:462–469
Danyluk J, Perron A, Houde M, Limin A, Fowler B, Benhamou N et al (1998) Accumulation of an acidic dehydrin in the vicinity of the plasma membrane during cold acclimation of wheat. Plant Cell Online 10:623–638. doi:10.1105/tpc.10.4.623
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure from small quantities of fresh leaf tissues. Phytochem Bull 19:11–15
Fernández M, Valenzuela S, Balocchi C (2006) RAPD and freezing resistance in Eucalyptus globulus. Electron J Biotech 9:303–309
Fernández M, Valenzuela S, Arora R, Chen K (2012a) Isolation and characterization of three cold acclimation-responsive dehydrin genes from Eucalyptus globulus. Tree Genetic Genomes 8:149–162
Fernández M, Valenzuela S, Barraza H, Latorre J, Neira V (2012b) Photoperiod, temperature and water deficit differentially regulate the expression of four dehydrin genes from Eucalyptus globulus. Trees 26:1483–1493
Fernández M, Villarroel C, Balbontín C, Valenzuela S (2010) Validation of reference genes for real-time qRT-PCR normalization during cold acclimation in Eucalyptus globulus. Trees Struct Funct 24(6):1109–1116
Fernández M, Troncoso V, Valenzuela S (2015) Transcriptome Profile in Response to Frost Tolerance in Eucalyptus globulus. Plant Mol Biol Report 33(5):1472–1485
Fowler S, Cook D, Thomashow MF (2005) The CBF cold response pathwat. In: Jenks MA, Hasegawa PM (eds) Plant Abiotic Stress. Blackwell publishing, Oxford, pp 71–99
Gilmour SJ, Fowler SG, Thomashow MF (2004) Arabidopsis transcriptional activators CBF1, CBF2 and CBF3 have matching functional activities. Plant Mol Biol 54:767–781
Guzmán M (2009) Análisis funcional y estructural del promotor del gen CBF-1 de Eucalyptus globulus en la respuesta a estrés por frío. Universidad de Chile, Santiago
Hara M, Terashima S, Fukaya T, Kuboi T (2003) Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta 217:290–298
Hara M, Fujinaga M, Kuboi T (2004) Radical scavenging activity and oxidative modification of citrus dehydrin. Plant Physiol Biochem 42:657–662
Hara M, Kondo M, Kato T (2013) A KS-type dehydrin and its related domains reduce Cu-promoted radical generation and the histidine residues contribute to the radical-reducing activities. J Exp Bot 64:1615–1624
Harrison S, Mott E, Parsley K, Aspinall S, Gray J, Cottage A (2006) A rapid and robust method of identifying transformed Arabidopsis thaliana seedlings following floral dip transformation. Plant Methods 2:19
Houde M, Dallaire S, N’Dong D, Sarhan F (2004) Overexpression of the acidic dehydrin WCOR410 improves freezing tolerance in transgenic strawberry leaves. Plant Biotechnol J 2:381–387
Keller G, Bang Cao P, San Clemente H, Kayal WE, Marque C, Teulières C (2013) Transcript profiling combined with functional annotation of 2662 ESTs provides a molecular picture of Eucalyptus gunnii cold acclimation. Trees 27:1713–1735
Kim SY, Nam KH (2010) Physiological roles of ERD10 in abiotic stresses and seed germination of Arabidopsis. Plant Cell Rep 29:203–209
Koag MC, Fenton RD, Wilkens S, Close TJ (2003) The binding of Maize DHN1 to lipid vesicles. Gain of structure and lipid specificity. Plant Physiol 131:309–316
Komarnytsky S, Borisjuk N (2003) Functional analysis of promoter elements in plants. In: Setlow JK (ed) Genetic engineering: principles and methods, vol 25. Springer US, Boston, MA, pp 113–141. doi:10.1007/978-1-4615-0073-5_6
Kosová K, Vítámvás P, Prásil IT (2007) The role of dehydrins in plant response to cold. Biol Plant 51:601–617
Lång V, Palva ET (1992) The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 20(5):951–962
Layton B, Boyd M, Bitonti B, Norman M, Dollahon R, Balsamo R (2010) Dehydration- induced expression of a 31-kDa dehydrin in Polypodium polypodioides (Polypodiaceae) may enable large, reversible deformation of cell walls. Am J Bot 97:535–544
Lois R, Dietrich A, Hahlbrock K, Schulz W (1989) Aphenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J 8:1641–1648
Maruyama K, Todaka D, Mizoi J, Yoshida T, Kidokoro S, Matsukura S, Takasaki H, Sakurai T, Yamamoto YY, Yoshiwara K, Kojima M, Sakakibara H, Shinozaki K, Yamaguchi-shinozaki K (2012) Identification of Cis-Acting Promoter Elements in Cold- and Dehydration-Induced Transcriptional Pathways in Arabidopsis, Rice, and Soybean. DNA Res 19:37–49
Moraga P, Escobar R, Valenzuela S (2006) Resistance to freezing in three Eucalyptus globulus Labill subspecies. Electronic J of Biotech 9:310–314
Muñoz-Mayor A, Pineda B, García-Abellán J, Antón T, García-Sogo B, Sanchez-Bel P, Flores F, Atarés A, Angosto T, Pintor-Toro J, Moreno V, Bolarin M (2012) Overexpression of dehydrin tas14 gene improves the osmotic stress imposed by drought and salinity in tomato. J Plant Physiol 169:459–468
Nash J, Luehrsen KR, Walbot V (1990) Bronze-2 gene of maize: reconstruction of wild-type allele and analysis of transcription and splicing. Plant Cell 2:1039–1049
Navarro M, Ayax C, Martinez Y, Laur J, Kayal W, Marque C, Teulières C (2011) Two EguCBF1 genes overexpressed in Eucalyptus display a different impact on stress tolerance and plant development. Plant Biotechnol J 9:50–63
Nylander M, Svensson J, Palva T, Welin BV (2001) Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana. Plant Mol Biol 45:263–279
Pastuglia M, Rody D, Dumas C, Cock JM (1997) Rapid induction by wounding and bacterial infection of an S gene family receptor-like kinase gene Brasica oleracea. Plant Cell 9:49–60
Perdiguero P, Collada C, Soto A (2014) Novel dehydrins lacking complete K-segments in Pinaceae, the exception rather than the rule. Front. Plant Sci. 5:682. doi:10.3389/fpls.2014.00682
Pichersky E, Bernatzky R, Tanksley SD, Breidenbach RB, Kausch AP, Cashmore AR (1985) Molecular characterization and genetic mapping of two clusters of genes encoding chlorophyll a/b-biding proteins in Lycopersicon esculentum (tomato). Gene 40:247–258
Puhakainen T, Hess MW, Mäkelä P, Svensson J, Heino P, Palva T (2004) Overexpression of multiple dehydrin genes enhances tolerance to freezing stress in Arabidopsis. Plant Mol Biol 54:743–753
Qin F, Sakuma Y, Li J, Liu Q, Li YQ, Shinozaki K, Yamaguchi-Shinozaki K (2004) Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol 45:1042–1052
Rasmussen-Poblete S, Valdes J, Gamboa MC, Valenzuela PDT, Krauskopf E (2008) Generation and analysis of an Eucalyptus globulus cDNA library constructed from seedlings subjected to low temperature conditions. E J Biotechnol 11(2):14
Robertson M, Cuming AC, Chandler PM (1995) Sequence analysis and hormonal regulation of a dehydrin promoter from barley, Hordeum vulgare. Physiol Plant 94:470–478
Rodríguez EM, Svensson JT, Malatrasi EM, Choi DW, Close ETJ (2005) Barley Dhn13 encodes a KS-type dehydrin with constitutive and stress responsive expression. Theor Appl Genet 110:852–858
Rorat T (2006) Plant dehydrins tissue location, structure and function. Cell Mol Biol Lett 11:536–556
Rouster J, Leah R, Mundy J, Cameron-Mills V (1997) Identification of a methyl jasmonate-responsive region in the promoter of a lipoxygenase 1 gene expressed in barley grain. Plant J 11:513–523
Sakai T, Takahashi Y, Nagata T (1996) Analysis of the promoter of the auxin-inducible gene, parC, of tobacco. Plant Cell Physiol 23:906–913
Sharabi-Schwager M, Lers A, Samach A, Guy CL, Porat R (2010) Overexpression of the CBF2 transcriptional activator in Arabidopsis delays leaf senescence and extends plant longevity. J Exp Bot 61:261–273
Shen Y, Tang MJ, Hu YL, Lin ZP (2004) Isolation and characterization of a dehydrin-like gene from drought-tolerant Boea crassifolia. Plant Sci 166:1167–1175
Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol 3:217–223
Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2003) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6:410–417
Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci U S A 94:1035–1040
Straub P, Shen Q, Ho D (1994) Structure and promoter analysis of an ABA-and stress-regulated barley gene, HVA1. Plant Mol Biol 26:617–630
Sun X, Lin HH (2010) Role of plant dehydrins in antioxidation mechanisms. Biologia 65:755–759
Teulieres C, Marque C (2007) Eucalyptus. In: Pua EC, Davey MR (eds) Biotechnology in agriculture and forestry 60, Transgenic Crops V. Springer, New York, pp 387–402
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev Plant Physiol Plant Mol Biol 50:571–599
Thompson JE, Legge RL, Barber RF (1987) The role of free radicals in senescence and wounding. New Phytol 105:317–344
Turnbull JW, Eldridge KG (1984) The natural environment of Eucalyptus as the basis for selecting frost-resistant species. In: Proceedings, IUFRO Colloque international sur les Eucalyptus résistants au froid, Bordeaux, Paris, pp 43–62
Viret JF, Mabrouk Y, Bogorad L (1994) Transcriptional photoregulation of cell-type-preferred expression of maize rbcS-m3: 3' and 5' sequences are involved. Proc Natl Acad Sci U S A 91(18):8577–8581
Vornam B, Gailing O, Derory J, Plomion C, Kremer A, Finkeldey R (2011) Characterization and natural variation of a dehydrin gene in Quercus petraea (Matt.) Liebl. Plant Biol 13:881–887
Wang Y, Xu H, Zhu H, Tao Y, Zhang G, Zhang L, Zhang C, Zhang Z, Ma Z (2014) Classification and expression diversification of wheat dehydrin genes. Plant Sci 214:113–120
Welling A, Rinne P, Viherä-Aarnio A, Kontunen-Soppela S, Heino P, Tapio Palva E (2004) Photoperiod and temperature differentially regulate the expression of two dehydrin genes during overwintering of birch (Betula pubescens). J Exp Bot 55:507–516
Wise M (2003) Leaping to conclusions: a computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics 4:52
Xing X, Liu Y, Kong X, Liu Y, Li D (2011) Overexpression of a maize dehydrin gene, ZmDHN2b, in tobacco enhances tolerance to low temperature. Plant Growth Regul 65:109–118
Xu H, Yang Y, Xie L, Li X, Feng C (2014) Involvement of multiple types of dehydrins in the freezing response in Loquat (Eriobotrya japonica). PLoS One 9:87575
Zhu W, Zhang D, Lu X, Zhang L, Yu Z, Lv H, Zhang H (2014) Characterisation of an SKn-type dhydin promoter from wheat and its responsiveness to various abiotic and biotic stresses. Plant Mol Biol Rep 32:664–678
Acknowledgments
This research was supported by the project FONDECYT 1130780 from CONICYT and Genómica Forestal SA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by I. Porth.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aguayo, P., Sanhueza, J., Noriega, F. et al. Overexpression of an SKn-dehydrin gene from Eucalyptus globulus and Eucalyptus nitens enhances tolerance to freezing stress in Arabidopsis . Trees 30, 1785–1797 (2016). https://doi.org/10.1007/s00468-016-1410-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-016-1410-9