Abstract
Key message
This work contributes to the identification and understanding of CBF genes and their putative role in the mechanisms of cold tolerance in eucalypt species.
Abstract
Three new CBF genes were isolated from E. globulus-denominated EglCBF1a, c, and d, coding for proteins of 220, 229, and 196 amino acids, respectively. The sequence analysis showed that the three predicted proteins contain an AP2 DNA-binding domain and two CBF signature sequences. Phylogenetic analysis demonstrated that these proteins were highly similar to those described in E. grandis and E. gunnii. Transcript abundance analysis in three different E. globulus genotypes exposed to a cold acclimation treatment showed that these CBF genes were highly related to the acclimation process and presented the highest relative expression at freezing temperatures. EglCBF1a showed the highest expression level (1311-fold change) in the cold-tolerant genotype (R1). EglCBF1a and d genes were induced by chilling and freezing temperatures, while EglCBF1c was constitutively expressed, increasing its transcript level when plants were exposed to freezing temperatures. The constitutive overexpression of each E. globulus CBF gene in Arabidopsis plants induces the endogenous CBF regulon gene expression of Arabidopsis and enhanced its tolerance to freezing, with additional phenotypic effects including growth inhibition and delayed flowering. These results indicate that the three EglCBF genes analyzed play important roles under cold acclimation processes in E. globulus and are involved in the signaling pathway of cold stress and the freezing tolerance phenotype observed on specific genotypes of this species and in transgenic Arabidopsis lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eucalypts are among the fastest growing tree species in the world, representing about 8% of the forest plantations, with more than 20 million hectares of plantations distributed in 90 countries (FAO 2007; Iglesias-Trabado and Wilstermann 2009). In Chile, E. globulus is the main hardwood cultivated species used for pulp production, with 541,859 hectares (INFOR 2014), and is characterized by its fast growth, straightness, high wood density, and good fiber quality (Pita and Pardo 2001; Grattapaglia 2004). This species grows well on temperate regions, with temperatures between 10 and 15 °C, but it is sensitive to low temperatures, although it has been reported that it can tolerate frosts of −4.5 to −6 °C during short periods of time (Almeida et al. 1994; Tibbits et al. 2006). The most damaging effects of freezing on eucalypts take place in the establishment phase of the young trees during the late winter–early spring, especially on regions with frosts of −7 to −9 °C (Volker et al. 1994); the damage is characterized by injuries on stems, leaves, and apical buds. The final consequence of this stress is a decrease on yield or even the lost of plantations (Geldres and Schlatter 2004).
Cold is an adverse abiotic factor, with severe negative impacts on plant productivity (Ruelland et al. 2009). In temperate regions, it has been observed that the exposure of plants to low, non-freezing temperatures can increase their freezing tolerance, by triggering a genetic response that allows them to tolerate cold or freezing temperatures (Thomashow 1999), in a process known as cold acclimation (Levitt 1980; Thomashow 1990), but this ability is mainly associated to some species (Rodziewicz et al. 2014).
Several physiological and molecular changes take place during the acclimation process and are associated to changes on gene expression (Thomashow 1999; Chaves et al. 2003). There are some genes that have been reported as responsive to cold, known as COR genes (cold-regulated); some of them are the LEA (late embryogenesis abundant) proteins, LTI (low temperature induced) proteins, and dehydrins (DHNs), associated to a cold signal pathway dependent on the CBF transcription factors. This pathway is considered as a CBF regulon (Thomashow 1999; Thomashow et al. 2001), and includes essential regulatory elements in response to cold (Vinocur and Altman 2005; Chinnusamy et al. 2010).
CBF/DREB1 (C-repeat Binding Factor/Dehydration Responsive Element Binding) proteins are members of the AP2/ERF (APETALA2/Ethylene-Response Factor) protein family of transcription factors (Riechmann and Meyerowitz 1998), defined by containing a conserved 60 aminoacid region, the AP2/ERF DNA-binding domain (Jofuku et al. 1994; Ohme-Takagi and Shinshi 1995). This domain binds to the specific cis-element sequences CRT/DRE (C-Repeat/Dehydration Responsive Element) present in the promoter regions of COR genes (Yamaguchi-Shinozaki and Shinozaki 1994; Thomashow 2010). The primary feature that distinguishes the CBF proteins from others of the AP2/ERF family members corresponds to two signature sequences flanking the AP2/ERF domain (Jaglo et al. 2001). These sequences, PKKP/RAGRxKFxETRHP (abbreviated PKKPAGR) and DSAWR, are motifs located immediately up and downstream from the AP2/ERF domain, respectively, and are required for the correct activity of the protein (Canella et al. 2010). These motif sequences and some differences in amino acid residues of the AP2/ERF domain have been essential in the classification within the A1 group of DREB subfamily of AP2/ERF family transcription factors (Sakuma et al. 2002). The first CBF/DREB1 genes described were studied in Arabidopsis thaliana, with four sequences reported known as CBF1 to 4 (Stockinger et al. 1997; Liu et al. 1998; Gilmour et al. 1998; Haake et al. 2002), which are expressed under cold temperatures and water-deficit conditions (Gilmour et al. 1998; Haake et al. 2002). However, in Arabidopsis, the A1 group has six members, with other two atypical CBF homolog genes, named DDF1 and 2 being induced by high-salinity stress (Magome et al. 2004, 2008). Regarding the overexpression of CBF1 to 4 genes in Arabidopsis, an increase in the freezing tolerance of non-acclimated plants, accompanied by an increased tolerance to drought and high-salinity conditions has been reported (Jaglo-Ottosen et al. 1998; Haake et al. 2002; Gilmour et al. 2004). To date, there are many studies that have identified CBF genes in numerous herbaceous and woody plants (Zhang et al. 2004; Xiao et al. 2006; Benedict et al. 2006; Champ et al. 2007; Welling and Palva 2008), as well as its role in cold tolerance (Medina et al. 2011; Zhou et al. 2011). For eucalypts, four CBF sequences in E. gunnii have been reported (EguCBF1a-b-c-d), which are differentially induced by cold and freezing temperatures (El Kayal et al. 2006; Navarro et al. 2009). An in silico screening on the reference genome of E. grandis identified a total of 17 CBF homologous sequences (Azar et al. 2011), which were later annotated by Cao et al. (2015). In the case of E. globulus, EgCBF1 is the only CBF gene reported to date (Gamboa et al. 2007).
Recently, several studies have found that the overexpression of CBF genes improved cold and freezing tolerance in plants, including model species such as Arabidopsis and Nicotiana tabacum (Siddiqua and Nassuth 2011; Li et al. 2013; Zhou et al. 2014; Wang et al. 2014, 2015; Xue et al. 2014; Fang et al. 2015), monocotyledonous species such as rice (Xu et al. 2011; Byun et al. 2015), and dicotyledonous woody species such as eucalypts (Navarro et al. 2011), apple (Wisniewski et al. 2011, 2015), and grape (Tillett et al. 2012). Additionally, it has been reported in a transcriptome of a frost-tolerant E. globulus genotype that 12% of the differentially expressed genes correspond to transcription factors, but none of these have been identified as a CBF gene (Fernández et al. 2015). In this work, three new EglCBF genes are identified in E. globulus and their differential transcript abundances for three genotypes under cold acclimation treatments are reported. Also the overexpression of EglCBF gene in Arabidopsis is evaluated, showing a remarkable increase in cold tolerance correlated with the expression of the EglCBF genes.
Materials and methods
Plant materials and cold acclimation treatment in Eucalyptus
Three different E. globulus genotypes were used, previously characterized as cold resistant (R1 and R2) and cold sensitive (S1). The level of resistance/susceptibility was assessed under field conditions and collecting historical data provided by the forest company. For each genotype, thirty biological replicates (ramets) of 6-month-old plants were used, planted in Styrofoam boxes with (1:1) vermiculite and perlite substrate. Thirty ramets were distributed in three growth chambers (ten ramets of each genotype in each chamber), under a completely randomized design with controlled temperature and photoperiod. The clonal identity of each ramet was verified using microsatellite markers (data not shown).
The plants were exposed to four different treatments varying in temperature and photoperiod: non-acclimated (NA), cold acclimated before night frosts of −2°C (CABF), cold acclimated after night frosts of −2 °C (CAAF), and de-acclimated (DA) as described by Fernández et al. (2010). For each treatment, three ramets per genotype were sampled, collecting its stem and leaves between 08:00–09:00 a.m. on days 7, 14, 21, and 28, respectively. All samples were immediately frozen in liquid nitrogen and kept at −80 °C until used. Finally, a last treatment was incorporated consisting in a night frost of −6 °C (NF) on day 29, which was applied to the 18 remaining ramets per genotype, to verify the freezing tolerance of the different genotypes assayed. The ramets were kept for a recovery during 10 days under long-day photoperiod (14 h light) and 12/6 °C day/night temperature, with periodic irrigation. For each ramet, the survival and leaf damage caused by freezing temperatures were measured, and the information obtained from live and dead organs, including leaves, buds, and apical buds, were used to calculate the survival and damage, according to Fernández et al. (2012).
CBF gene sequencing and data analysis
To sequence the CBF genes of E. globulus, specific primers for the four CBF gene sequences reported in E. gunnii (El Kayal et al. 2006; Navarro et al. 2009) were designed. The genes were amplified by PCR using cDNA templates from leaves of E. globulus plants subjected at 8/4 °C day/night temperature and 10-h photoperiod for 1 week, with DNA polymerase PfuUltra II Fusion HS (Agilent Technologies) and the primers described on Supplemental data Table S1.
Sanger DNA sequencing was carried out at Macrogen (Korea). The corresponding amino acid sequences for each gene were analyzed by Geneious 6.1 software, and the putative DNA-binding domains were identified by PROSITE (http://prosite.expasy.org/). The molecular weight (MW) and theoretical isoelectric point (pI) of the deduced proteins were analyzed using the ProtParam tool (http://web.expasy.org/protparam/). The analysis of protein sequence homology was performed by multiple alignment using ClustalW with default parameters and assembled by Geneious 6.1 software. For the phylogenetic tree construction, the protein multiple alignment was performed by MEGA 6.0 software using ClustalW, and the tree was constructed using the neighbor-joining method with a bootstrap test calculated on 1000 replicates. The full-length CBF nucleotide sequences for E. globulus were deposited in Genbank.
EglCBF sequence cloning and vector construction
Each EglCBF gene was isolated and amplified from cDNA samples of E. globulus plants subjected at 8/4 °C day/night temperature, using high-fidelity PfuUltra II Fusion HS DNA Polymerase (Agilent Technologies) and specific primers for each gene (Table S1); the products were cloned into pGEM-T Easy vector (Promega). Based on this material, the open reading frame (ORF) sequence of three EglCBF genes were amplified using specific primers adding attB recombination sites for Gateway® cloning (Invitrogen) (Table S1). Each amplified product was recombined by BP reaction with the pCC1155 vector, corresponding to the pDONR221 vector with an ampicillin resistance gene, modified by Bonawitz et al. (2012). The resulting vectors were recombined for the LR reaction separately with the pMDC32 destination vector (Curtis and Grossniklaus 2003), obtaining three different expression vectors, driven by the constitutive CaMV35S promoter and identified as 35S::EglCBF1a, 35S::EglCBF1c, and 35S::EglCBF1d, respectively. Each construct was verified by sequencing, and introduced on Agrobacterium tumefaciens strain GV3101 by electroporation (Weigel and Glazebrook 2002), and used to transform Arabidopsis plants by the floral dip method (Clough and Bent 1998).
Arabidopsis transformation, growth conditions, and freezing treatment
Arabidopsis thaliana ecotype Col-0 plants were used for transformation of three CBF genes from E. globulus. Seeds collected from A. thaliana were germinated in Petri dishes containing half strength MS medium and 2.43 g/L Phytagel (Sigma), selecting the transformed plants with hygromycin B at 15 µg/ml, according to the method described by Harrison et al. (2006). At seven days, the selected plants were transplanted into pots and maintained in a growth chamber at 23 °C and 16/8 h day/night photoperiod. Growth and phenotypic development was measured in Arabidopsis transformed lines and untransformed wild-type (WT) plants, collecting information of rosette diameter and plant height of 35, 40, and 60 day-old plants.
All T0 and T1 transformed plants were selected by hygromycin B resistance and verified by PCR amplification of the gene of interest (data not shown). Transformed lines were designated as lines A, C, and D, with correlative numbering for each construct, namely 35S::EglCBF1a, 35S::EglCBF1c, and EglCBF1d. Ten T2 transformed lines and WT plants of 5 weeks old were exposed to the freezing treatment. Thirty plants per line were subjected to a temperature decrease in a Percival® LT-36VL phytotron, starting at 23 °C, with a 2 °C decrease per h until reaching −6 °C, and kept at this temperature for 3 h. Three plants per each line were sampled at 23, 4, and −6 °C and immediately stored in liquid nitrogen and then transferred to −80 °C freezer until further analysis. After this assay, 21 plants were kept at 23 °C for 7 days in order to visually estimate the survival rate by assessing the plant recovery.
RNA extraction and gene expression analysis
The total RNA was extracted from the collected plant material using the CTAB method described by Chang et al. (1993), in the case of Eucalyptus, and the protocol described by Weigel and Glazebrook (2002) for Arabidopsis samples. The RNA concentration was measured with a NanoDrop ND-1000 spectrophotometer (ThermoFisher Scientific). RNA purity of the samples was determined by its A 260/280 ratio of between 1.9 and 2.1, and an A 260/230 ratio higher than 2.0. RNA integrity was checked by electrophoresis in 2% agarose gels. For each sample, 1 µg of RNA was treated with DNaseI (Fermentas) to remove genomic DNA contamination. First-strand copy DNA (cDNA) was synthesized by reverse transcription using the High-Capacity cDNA Reverse Transcription kit (Life technologies) according to the manufacturer’s instructions.
Gene expression analysis in Eucalyptus was measured in samples under four acclimated treatments previously described and was determined by quantitative real-time PCR (qPCR) using Taqman ® probes for detection with a StepOne Plus system (Applied Biosystems). Total reaction volume was 20 µl with 10 ng cDNA template, 10 µl TaqMan Gene Expression Master Mix (Applied Biosystems) and a concentration of primers and probe of 200 nM and 250 nM, respectively. Two endogenous (housekeeping) genes were used (UBC and a-TUB), previously reported by Fernández et al. (2010). The probes and primers used were designed for each gene by Primer Express 2.0 software (Table S1). All qPCR reactions were carried out under the following conditions: 2 min at 50 °C, 10 min at 95 °C, 40 cycles of 15 s at 95 °C, and 1 min at 60 °C in 96-well optical reaction plates (Applied Biosystems). The calibrator sample corresponded to one ramet of the S1 genotype under NA treatment. For constitutive relative expression of CBF genes in E. globulus, each expression level was normalized with respect to the UBC gene, which has a single copy in the E. grandis genome.
In transformed and WT Arabidopsis plants, the gene expression of EglCBF and endogenous Arabidopsis genes (AtCBF2, AtCBF3, and four COR genes) was determined by qPCR analysis using the specific primers listed on Supplemental data Table S2 and the Evagreen® fluorophore (Solis BioDyne). The detection was performed on a StepOne Plus system with a qPCR reaction mixture of 1X Evagreen®, 200 nM primers, and 10 ng cDNA templates, under the following conditions: 10 min at 95 °C, 40 cycles of 15 s at 95 °C, and 1 min at 60 °C in 96-well optical reaction plates. Two endogenous genes were used as controls, the elongation factor 1-alpha (Ef1-α, AT5G60390) and the protein phosphatase 2 A subunit A3 (PP2AA3, AT1G13320). To normalize the relative expression of the transgene samples, the transformed line with the lower expression was used. However, to normalize the relative expression of endogenous genes in Arabidopsis, a WT plant sample was used as a reference.
For all genes, the PCR efficiency was determined by measuring the CT to a specific threshold for a serial dilution of cDNA samples. The specificity of the amplified products was determined by the dissociation curve, with 118 cycles increasing +0.3 °C per cycle from 60 to 95 °C. The relative expression level was calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001), including three technical replicates for each of the three biological replicates.
Statistical analysis
Before data analysis, the assumptions of normality and homogeneity of variance for each variable were verified. In Eucalyptus, survival and leaf damage data were subjected to one-way analysis of variance (ANOVA) to test the effect of genotypes. For relative gene expression, data were subject to two-way ANOVA to test the effect of cold acclimation treatments and genotype. In Arabidopsis, the relative gene expression of EglCBF transgenes and endogenous genes data were subjected to one-way ANOVA to test the effect of different T2 transformed lines and WT plants. A Tukey test was applied to determine significant differences between samples.
Results
Sequencing and characterization of the three CBF genes from E. globulus
Three CBF homologous sequences were identified in E. globulus, corresponding to the paralog genes of E. gunnii. These sequences were named EglCBF1a, EglCBF1c, and EglCBF1d, and deposited on GenBank with the accession numbers KX669025, KX669026, and KX669027, respectively. In the case of EglCBF1b, there were some problems in the Taqman probe for its relative expression detection, and for this reason this gene was discarded for further analysis. The full-length sequence obtained for EglCBF1a was 736 bp, including a CDS of 660 bases corresponding to 220 amino acid residues, with a predicted MW of 24.24 kDa and an isoelectric point (pI) of 5.73. The full length obtained for EglCBF1c gene was 996 bp, including a CDS of 687 bases coding for a 229 amino acid protein, with a predicted MW of 24.98 kDa and a pI of 5.10. Finally, the full length obtained for EglCBF1d was 1285 bp, including a CDS of 588 bases corresponding to 196 amino acid residues, with a predicted MW of 21.59 kDa and a pI of 5.87.
Multiple alignment over the amino acid sequences determined the identity of the sequences as CBF transcriptional factors, with the presence of the AP2/ERF domain and two characteristic motifs of CBF proteins (Fig. 1). These motifs and domains have highly conserved amino acid residues; the comparison of the full length of the three EglCBF proteins and the domain sequences of other Eucalyptus species indicate a high degree of similarity. At the amino acid residue level, the complete sequence for EglCBF1a protein shows 77.7% similarity to EglCBF1c and 76.6% to EglCBF1d, and the EglCBF1c protein was 76.0% similar to EglCBF1d. At the domain residue level, EglCBF1a and c were 100% identical to EgrCBF6 and 1 of E. grandis sequences, respectively, and EglCBF1d was 100% identical to EguCBF1d of E. gunnii. The full-length predicted proteins have more than 94% similarity with their paralogs of E. grandis and E. gunnii.
Multiple sequence alignment of CBF proteins in Arabidopsis (AtCBF1), Citrus sinensis (CsCBF), E. globulus (EgCBF1), E. globulus (EglCBF1a-c-d), E. grandis (EgrCBF1), E. gunnii (EguCBF1a), Malus domestica (MdCBF1), Prunus persica (PpCBF1), Populus trichocarpa (PtCBF2), and Vitis vinifera (VvCBF1); the GenBank accession number is shown in parentheses; black shading indicate identical amino acid residues; asterisk on the alignment indicates the CBF signature sequences (CBFss); double underline indicates AP2/ERF domain
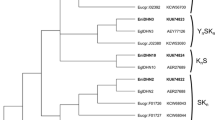
Multiple alignment of CBF protein sequences from Arabidopsis and Eucalyptus species (including E. globulus, E. grandis and E. gunnii) were used to generate the phylogenetic tree. The CBF paralogs were distributed in seven clades, five for Eucalyptus CBFs proteins, one for Arabidopsis CBFs proteins, and one for the Arabidopsis-Eucalyptus CBF (Fig. 2). EglCBF1a grouped to CBF proteins reported in E. gunnii (EguCBF1a) and in E. grandis (EgrCBF6, 8, 10, and 12). In another clade, the CBF sequences of E. grandis (EgrCBF7, 9, 11, 13 and 14) were grouped with one sequence of E. gunnii (EguCBF1b) and to the only CBF currently reported for E. globulus (EgCBF1). The EglCBF1c identified has a high similarity to EgrCBF1 and EguCBF1c, but a distant clustering to the EglCBF1d protein, which groups with the EgrCBF2 and EguCBF1d paralogs. For Arabidopsis, one clade of CBF protein was obtained (AtCBF1, 2, 3, and 4), and additionally a small clade of an atypical CBF protein (AtDDF1 and 2) was grouped with some CBF proteins of E. grandis (EgrCBF15-16), demonstrating an apparent phylogenetic relationship between these distant species.
Phylogenetic tree of CBF proteins generated by the Neighbor-Joining method using MEGA 6.0; multiple alignment full-length amino acid sequences of EglCBF1a-c-d and CBFs from Arabidopsis thaliana (AtCBF1-4 and AtDDF1-2), E. globulus (EgCBF1), E. grandis (EgrCBF1-16), and E. gunnii (EguCBF1a-b-c-d) were used. AthAP2 is a member of the AP2 family used for rooting the phylogenetic tree. Each protein has the GenBank accession number in parenthesis. A line separated each Eucalyptus clade. Bootstrap values are indicated for each branch, and low values (<50) were removed from the tree
Freezing tolerance in different cold acclimated E. globulus genotypes
The application of the night frost treatment of −6 °C allowed the assessment of freezing tolerance of the three genotypes of E. globulus studied and the determination of the survival rate and leaf damage. For survival rate, the values were 14, 35, and 10% for the R1, R2, and S1 genotypes, respectively, but considering the leaf damage, the two resistant genotypes (R1 and R2) showed less than 50% damage, while the sensitive genotype (S1) had a leaf damage of 63.5%, this difference being statistically significant (Supplementary table S3).
Expression analysis of EglCBF genes in response to cold acclimation treatments
The data obtained by gene expression analysis showed that EglCBF1a, c, and d genes showed an increased transcript accumulation in cold acclimated plants, when compared to non-acclimated plants (Fig. 3). For the three genes analyzed, the highest transcript accumulation was observed in the CAAF treatment.
Relative expression analysis of three CBF genes in E. globulus plants. a EglCBF1a. b EglCBF1c. c EglCBF1d, for four treatments of cold acclimation assay NA (non-acclimated), CABF (cold acclimated before night frosts of −2 °C), CAAF (cold acclimated after night frosts of −2 °C), and DA (de-acclimated) using Taqman ® probes with internal controls UBC and α-TUB genes. Calibrator sample corresponds to one ramet of S1 genotype at NA treatment; bars indicate fold change mean n = 3; error bars represent SE; lowercase letters on top of the bars indicate statistically significant differences between treatments and genotype evaluated with Tukey test (p < 0.05)
The transcript abundance of EglCBF1a gene increased during the CABF treatment for the genotypes S1 and R2 (Fig. 3a). The highest transcript abundance was obtained in the CAAF treatment, reaching a fold change of 1311 for genotype R1, and 340 and 445 for R2 and S1, respectively. In plants exposed during 1 week to the DA treatment, the expression of this gene falls significantly with the increase of temperature (12/6 °C), reaching fold changes of 3.8, 4.4, and 3.5 in genotypes R1, R2, and S1, respectively. The transcript abundance of EglCBF1c during the CAAF treatment increased to values of 28, 18, and five-fold change in R1, R2, and S1 genotypes, respectively (Fig. 3b). For the DA treatment, EglCBF1c gene expression decreases below the levels observed for the control treatment (NA). The transcript abundance of EglCBF1d gene showed an increase in the CABF treatment in the sensitive genotype (3.4-fold) compared to the resistant genotypes (Fig. 3c). This gene presents the highest relative expression observed in the CAAF treatment, with values of 823, 590, and 180-fold for R1, R2, and S1 genotypes, respectively. These transcript accumulation levels were significantly higher in the resistant genotypes (R1-R2) when compared to the sensitive genotype (S1). As it has been observed in the previous gene assessed, EglCBF1d gene expression in DA treatment falls significantly with values of 2.6, 3.3, and 2.5-fold in R1, R2, and S1 genotypes, respectively. Additionally, the constitutive relative expression of the three EglCBF genes were calculated by normalizing each expression level, with respect to the UBC gene present into a single copy in the genome of E. grandis.
Growth and phenotypic development in transformed Arabidopsis lines
The rosette diameter and plant height on 35, 40, and 60 days old plants were measured in the WT and ten transformed lines of A. thaliana. Between 35 and 40 days, both transformed and WT plants showed rosette diameters between 4.6 (±0.2) and 7.0 (±0.7) cm, without any evident development of the inflorescence (Fig. 4a). At 60 days, the plants showed rosette sizes between 7.5 (± 0.1) and 12.2 (± 0.4) cm length, with evident induction of flowering in eight of the ten lines tested (Fig. 4a). The transformed lines with the smaller rosette diameter were A17 and C09, presenting significant differences when compared to the WT (Fig. 4b). At the same time, inflorescence development was observed in eight lines, with the exception of A17 and D32. The line A17 showed an abnormal development, with absence of the inflorescence (Fig. 4a, c, d). In the case of C09 and D32 lines, a slow floral development was observed, with well-developed stems and siliques at 80 days, while the WT have the same development at 60 days (Fig. 4d).
Growth and phenotypic development of transformed and WT plants. a plant growth at 35, 40, and 60 days after transplant to pots. b rosette diameter of different lines at 60 days. c plant height of different lines at 60 days. d lines with delayed development growth at 80 days compared to WT plant 60 days old; bars indicate fold change mean, n = 10; error bars represent SE; asterisk on top of the bars indicate significant differences between each transformed line compared to WT by Tukey test (p < 0.05)
Survival rate to freezing stress in Arabidopsis plants
WT plants of A. thaliana showed 0% survival rate when exposed to −6 °C freezing temperatures (Fig. 5). The transformed lines that showed the highest survival rates were A17 and C09, reaching 90.5%. Additionally, the transformed lines C26 and D32 showed a survival rate above 50%.
Freezing tolerance of WT and ten transformed lines that overexpress independently EglCBF1a, EglCBF1c, and EglCBF1d genes, respectively. Control 5-week-old plants growing under normal conditions at 23 °C; −6 °C 5-week-old plants under freezing treatment and then returned to normal condition for 7 day; SR survival rate calculated as recovered plants over total plants treated
Transcript abundance of EglCBF transgenes and endogenous genes in transgenic Arabidopsis lines
Several Arabidopsis thaliana transgenic lines containing the coding region for each of the three CBF transcription factors from E. globulus were generated. The T0 transformed lines for each construct were selected, with 20 lines for EglCBF1a, 30 lines for EglCBF1c, and 40 lines for EglCBF1d. All T0 lines were verified for the inserted gene integrity by PCR analysis (data not shown). On the T1 generation, four lines with low, medium, and high expression levels were selected for further analysis. In the case of EglCBF1a construct, two lines were discarded due to abnormal phenotypes at T1, which lacked flowers and seeds. The higher transcript abundance of the corresponding constructs 35S::EglCBF in T2 Arabidopsis transformed lines were found in A17, C09, and D32 lines, respectively (Fig. 6a–c).
Relative expression levels of three EglCBF1 transgenes and six endogenous genes, in ten transformed lines and WT plants. a expression of EglCBF1a in two independent overexpressing EglCBF1a lines. b expression of EglCBF1c in four independent overexpressing EglCBF1c lines. c expression of EglCBF1d in four independent overexpressing EglCBF1d lines; bars indicate fold change mean, n = 3; error bars represent SE; lowercase letters on top of the bars indicate significant differences between the respective transformed lines determined by Tukey test (p < 0.05). d expression levels of two CBF endogenous genes. e expression levels of four COR endogenous genes. The data were normalized data with the two internal control genes EF1-α and PP2AA3; asterisk on top of the bars indicate significant differences between each transformed line compared to WT determined by Tukey test (p < 0.05)
Two endogenous CBF genes (AtCBF2, AthCBF3) and four COR genes (COR15a, COR6.6, ERD10, and RAB18) of Arabidopsis were evaluated at 23 °C in the transgenic and WT plants. In the case of AtCBF2, the highest transcript levels were found in A17 and D08 lines, showing significant differences compared to the WT (Fig. 6d). For AtCBF3, the highest relative expression lines were A17, C09, C11, C26, D08, and D32, also showing significant differences when compared to WT. Of the four COR genes evaluated, the lines A17, C09, and D32 showed the highest increase on transcript accumulation compared to WT (Fig. 6e).
The effect of the temperature on the transcript abundance of COR15a, the most induced gene from all COR genes tested, was measured on three transformed Arabidopsis lines showing high frost tolerance (A17, C09 and D32), at three different levels representing control (23 °C), cold (4 °C), and frost (−6 °C) temperatures, respectively (Fig. 7). In lines A17 and C09, the transcript accumulation increased with a decrease in the temperature from 23 °C to −6 °C, with significant differences compared to the WT, while in lines D32, the transcript levels were similar at all tested temperatures but significantly different when compared to the WT on their respective treatment.
Relative expression levels of COR15a in three transformed lines and WT plants under three different temperatures: control 23 °C, cold 4 °C, and freezing − 6 °C. The data were normalized with the two internal control genes EF1-α and PP2AA3; bars indicate fold change mean, n = 3; error bars represent SE; asterisks on top of the bars indicate significant differences between each transformed line compared to WT in the respective temperature treatment, determined by Tukey test (p < 0.05)
Discussion
Three CBF homologous sequences were identified in E. globulus, containing the main signatures that characterize CBF transcriptional factors, including an AP2/ERF domain and two flanking motifs. Previous studies reported that the AP2/ERF domain is needed for the DNA-binding specificity (Sakuma et al. 2002) and that the PKKPAGR motif is a nuclear localization signal (Stockinger et al. 1997). Canella et al. (2010) have demonstrated that the AP2/ERF domain is needed for nuclear CBF protein localization, while the PKKPAGR motif is essential for the CBF-specific protein binding to CRT/DRE elements.
The CBF proteins of E. globulus showed high similarity to previously characterized proteins in E. gunnii (El Kayal et al. 2006; Navarro et al. 2009), and to proteins recently annotated on the E. grandis genome (Wisniewski et al. 2014; Cao et al. 2015), that are grouped on the same clade based on a phylogenetic analysis. The high similarity and conservation of sequences suggests that EglCBF1a-c-d proteins could have an important role on the transcriptional regulation in a similar manner as it has been proposed for other plants (Chinnusamy et al. 2010; Thomashow 2010).
Although several sequences to CBF homologs have been recently described in E. grandis (Cao et al. 2015), in this study, we have focused on the analysis of three EglCBF sequences, similar to the CBFs described by El Kayal et al. (2006) and Navarro et al. (2009). Nevertheless, when we screened cold expression libraries of E. globulus, we were able to find a total of 15 CBF homologous sequences to E. grandis (data not shown), but the effect of the other CBF homologs present in this species remains to be determined.
The results of transcript abundance for EglCBF1a-c-d genes (1,311, 28 and 823-fold change) in the R1 genotype during the CAAF treatment compared to the non-acclimated genotype are similar to those obtained in E. gundal, which is more cold tolerant than E. globulus, where the paralogs of EguCBF1a-c-d showed values of 1690, 91, and 436-fold change, respectively, after a 5-h exposure at 4 °C (Navarro et al. 2009). To date, the only other CBF gene reported for E. globulus is EgCBF1 (Gamboa et al. 2007). This gene is similar to EguCBF1b, considered as a gene that participates on the cold acclimation process, with a prolonged expression over time at cold temperatures when tested on acclimation experiments (El Kayal et al. 2006; Navarro et al. 2009).
In the case of EglCBF1a, there was a high transcript abundance at −2 °C (CAAF); this information is on agreement with the results for E. gunnii and its paralog, EguCBF1a, showing an early induction between 2 and 5 h after exposure of plants to 4 °C, and this expression is intensified if the experiment is conducted at short photoperiods of 8 h light/day (El Kayal et al. 2006). Navarro et al. (2009) replicated this phenomenon, observing high expression levels being reached at 2 h (1760-fold change) and 4 h (1690-fold change) after exposing the plants to a gradual change in temperature from 22 to 4 °C. The authors propose that this gene is involved in an early response to sharp changes in cold temperature, in accordance with its early expression pattern.
The expression levels of EglCBF1c gene in the three genotypes of E. globulus studied were lower than the other two CBF genes analyzed in CABF and DA treatments. In this case, the expression level falls below one-fold, suggesting that the sample used to normalize the relative quantification levels (NA condition, the calibrator sample), showed a higher basal expression compared to the EglCBF1a-d genes in the NA treatment. This was observed in quantifying constitutive transcript levels in samples under NA treatment, where EglCBF1c gene was constitutively expressed unlike the other two genes, EglCBF1a and EglCBF1d. Although several studies report that CBF genes are induced by stress conditions in plants, in addition to low temperature, drought, and high-salinity genes (Gilmour et al. 1998; Ryu et al. 2014; Fang et al. 2015), other reports indicate that there are some CBF transcription factors that are constitutively expressed in several plants species (Tang et al. 2005; Xiao et al. 2008; Peng et al. 2013). In E. gunnii, one CBF gene, EguCBF1c, has a basal expression of 2.8 copy number ng−1 cDNA, when compared with other weakly expressed genes of the same family, EguCBF1a-d, under non-stress conditions (Navarro et al. 2009). These researchers reported a putative role of EguCBF1c gene with a constitutive expression, suggesting that it may be involved in a permanent cell stress protection in response to various stimuli.
EglCBF1d presented high expression levels during the CAAF treatment, showing significant differences on resistant genotypes; this is in accordance with previous observations for the E. gunnii paralog EguCBF1d, where the expression levels increased with colder freezing temperatures of −4, −6, and −8 °C, reaching a 1367-fold change at the lowest temperature tested (Navarro et al. 2009). The proposed role for this gene is the response to frost, providing tolerance to cells with or without previous acclimation. Both eucalypt-resistant genotypes assayed presented differential relative expression levels for the three CBF genes studied (in the case of R1), or for two CBF genes (in the case of R2 genotype) on the more severe cold treatment with freezing temperatures (CAAF). The phenotypic data support this observation, since young plants of the resistant genotypes presented significantly less leaf damage when compared to the susceptible genotype, when confronted to a −6 °C frost treatment.
In order to validate the proposed function of these genes, in this work the overexpression of three E. globulus CBF genes was performed separately in A. thaliana. These EglCBF1a-c-d genes were annotated recently in the E. grandis genome and correspond to CBF-like 6–1 – 2, respectively (Cao et al. 2015). Ten transformed lines were selected for three constructs, two lines for EglCBF1a, and four lines for each EglCBF1c-d genes. Of these ten lines, only five showed a large transcript accumulation, with four of them having high survival rates to freezing treatments, suggesting that a high transcript accumulation of the EglCBF gene is correlated with an increased survival to freezing stress. The same correlation has been reported in several studies, where high survival rates were observed in transformed lines with high amounts of transcript accumulation of CBF (Siddiqua and Nassuth 2011; Wisniewski et al. 2011; Tillett et al. 2012; Li et al. 2013). Additionally, the overexpression of CBF genes from different plant species has led to increased survival rates to cold and freezing stress in Arabidopsis (Tong et al. 2009; Xue et al. 2014; Fang et al. 2015) and in other herbaceous or woody species (Navarro et al. 2011; Xu et al. 2011; Zhou et al. 2014; Byun et al. 2015). The role of these transcription factors is to regulate gene expression in response to environmental stresses, by binding to the cis-elements CRT/DRE (Stockinger et al. 1998), present in the promoter regions of cold response genes (COR). It has also been reported that a large accumulation of CBF genes induces the expression of COR genes (Jaglo-Ottosen et al. 1998; Gilmour et al. 2004; Zhou et al. 2014; Xue et al. 2014). Some broadly reported COR genes responding to cold and freezing stresses are COR6.6, COR15a, COR47, COR78, and ERD10 (Kasuga et al. 1999; Thomashow et al. 2001; Seki et al. 2001). In this work, the induction of endogenous genes in Arabidopsis was evaluated on two CBF genes (AtCBF2–3), three CBF-target genes (COR6.6-COR15a-ERD10), and one non-CBF-target gene (RAB18), an ABA-dependent pathway gene (Mäntylä et al. 1995). In the case of the endogenous CBF genes AtCBF2 and AtCBF3, two and six lines with high transcript accumulation were observed, respectively, with AtCBF3 showing the highest expression values. Interestingly, this work reports that the constitutive expression of EglCBF transgenes induces increased expression levels of the AtCBF endogenous genes in Arabidopsis, but these transcription factors do not have the regulatory sites CRT/DRE on their promoter regions, required for activation with the CBF proteins (Gilmour et al. 1998). This also has been reported by Zhou et al. (2014), where the overexpression of CbCBF from Capsella bursa-pastoris in tobacco, not only increased the transcript levels of NtERD10a–b genes for cold response, but also participated in the up-regulation of the CBF genes NtDREB1–3, providing a likely mechanism for the enhanced cold acclimation due to CbCBF. On the other hand, the phenomenon of regulation between CBF transcription factors has been reported in Arabidopsis, where AtCBF1 and AtCBF3 gene expression are negatively regulated by AtCBF2 (Novillo et al. 2004, 2007), and the effect has been recently validated by Kim et al. (2015) who reported that the relative expression of AtCBF1 and AtCBF3 genes in an Arabidopsis cbf2 mutant, defective in the AtCBF2 gene, presented higher AtCBF3 relative expression levels, while AtCBF1 expression was not affected. Additionally, they verified that AtCBF2 indirectly regulates AtCBF3 expression but does not bind to their promoter region. Moreover, other authors reported that plants of the Arabidopsis mutant erd10 exposed to cold for 24 h showed a significant loss of cold tolerance, related to the absence of induction on the CBF transcription factors, proposing that this cold signaling pathway could present a more complex level of regulation (Kim and Nam 2010).
Regarding the induction of CBF-target genes, part of the so-called CBF regulon (Thomashow 1999), five Arabidopsis transformed lines showed increased expression levels of the three CBF-target genes in non-acclimated plants; among them, four presented an overexpression of the EglCBF transgene and high survival rates, suggesting that the overexpression of EglCBF activates the transcript accumulation of CBF regulon genes, improving the constitutive tolerance to freezing stress. There are additional reports confirming that the overexpression of CBF genes increases the accumulation in transcripts of CBF-target genes, correlated to an enhanced freezing stress tolerance (Tong et al. 2009; Siddiqua and Nassuth 2011; Li et al. 2013; Fang et al. 2015). Additionally, we found that on five lines, the overexpression of the transgene increased the transcript accumulation of RAB18 gene, a dehydrin that is not regulated by CRT/DRE cis-elements (Wang et al. 2008). This could indicate that the constitutive expression of the EglCBF gene activates other metabolic pathways different to the CBF regulon, and in this case an ABA-dependent pathway, an effect previously reported by other authors, with an overexpression of CBF in Arabidopsis resulting in the transcription of ABA-dependent pathway genes (Fang et al. 2015; Wang et al. 2008).
Additionally, we evaluated the relative gene expression of COR15a at different temperatures: control, cold, and freezing. The results showed that the relative transcript levels increased with decreasing temperatures, in two of three lines evaluated and in the WT plants. This effect is well correlated with the cold acclimation phenomenon, where decreasing temperatures induce an increase on transcript abundance of genes required for the cold signaling pathway, and even more if these genes are expressed in control temperature (Zhuang et al. 2015). Accordingly, three EglCBF1 constitutively activated the expression of COR15a in transgenic plants and up-regulated their expression under cold stress. It is notable that the fold change of COR15a expression in transgenic plants was greater than other COR genes under normal and freezing stress conditions, suggesting that COR15a could play a prominent role in freezing tolerance of transgenic plants overexpressing EglCBF1s.
The growth and phenotype development of transformed plants in some lines had a slower growth rates than the WT, leading to a delay of flowering, and even in one line (A17) abolishing completely its presence. This phenomenon was correlated with high levels of transgene expression and a high survival to freezing treatment in these lines, suggesting that growth inhibition was an additional effect due to the high transcript abundance of EglCBF transgene, which has been observed by other authors, who point out that the overexpression of CBF genes in Arabidopsis causes dwarfism and delayed flowering (Liu et al. 1998; Gilmour et al. 2004); the same effect was found on some woody species (Navarro et al. 2011; Tillett et al. 2012). The effects of the overexpression of CBF genes was studied in more depth by Achard et al. (2008), who found that the constitutive expression of AtCBF1 in Arabidopsis induces the accumulation of DELLA proteins, which restricts growth by interfering with the gibberellin (GA) signaling pathway. Under normal growth conditions, plants produce bioactive GA, which in turn degrades DELLA proteins by the ubiquitin–proteasome pathway (McGinnis et al. 2003), but when the levels of bioactive GA decreases, DELLA proteins accumulate and inhibit growth, causing dwarfism and delayed flowering (Thomashow 2010). Achard et al. (2008) found that the overexpression of AtCBF1 induces the expression of two genes that encode for GA 2-oxidases, enzymes that catalyze the inactivation of bioactive GA. The same effect was reported by other authors, where the constitutive expression of CBF genes induces the accumulation of genes encoding enzyme GA oxidases, reducing the amount of bioactive GA, thus accumulating the DELLA proteins and producing growth inhibition in transformed plants (Tong et al. 2009; Siddiqua and Nassuth 2011; Zhou et al. 2014). Recently, Zhou et al. (2017) working in Arabidopsis suggested that CBF3 promotes the accumulation of DELLA proteins by repression of gibberellin biosynthesis and also DELLA contribute to cold induction of AtCBF genes through interaction with jasmonate signaling. One possibility for the positive regulation of the AtCBF2–3, that lacks a CBF-target cis-element on their promoters, by the overexpression of EglCBFs observed in this work, is that positive regulation between CBF and DELLAs occurs not only at low temperatures, but also there are alternative pathways of regulation for warm temperatures and cold induction. This possibility, or the interaction with other factors that does not depends on low temperatures, requires further research.
In conclusion, the three genes that code for CBF transcription factors described here for E. globulus are believed to participate actively on the cold acclimation process, and showed a strong relationship with freezing tolerance for this species. Accordingly, the coldest tolerant E. globulus, used in this study, had an increased relative expression of these genes when compared with the most sensitive one; this knowledge would be of great value for guiding future breeding programs for cold tolerance in E. globulus. Furthermore, the overexpression of the different EglCBF provides freezing tolerance in four Arabidopsis transgenic lines, by increasing the gene expression levels of cold response genes (CBF regulon), and could be useful for future genetic modification strategies in plants, but a further characterization of the effects on growth inhibition and flowering delay on transformed plants is required.
Author contribution statement
DN-C carried out all experiments of sequencing, gene expression, and characterization of transgenic plants. RL helped with the transformation of Arabidopisis and data analysis. CB was involved in the design and selection of eucalypts genotypes for the cold acclimation study.SV is the PI of this research. All authors contributed to writing and the discussion of the manuscript.
References
Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P (2008) The cold-inducible CBF1 factor–dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20(8):2117–2129
Almeida M, Chaves M, Silva J (1994) Cold acclimation in eucalypt hybrids. Tree Physiol 14(7-8-9):921–932
Azar S, SanClemente H, Marque G, Dunand C, Marque C, Teulières C (2011) Bioinformatic prediction of the AP2/ERF family genes in Eucalyptus grandis: focus on the CBF family. BMC Proc 5 (Suppl 7):P165
Benedict C, Jeffrey S, Rengong M, Yongjian C, Rishikesh B, Norman P, Chad E, Tony H, Vaughan H (2006) The CBF1-dependent low temperature signalling pathway, regulon and increase in freeze tolerance are conserved in Populus spp. Plant Cell Env 29(7):1259–1272
Bonawitz N, Soltau W, Blatchley M, Powers B, Hurlock A, Seals L, Weng J-K, Stout J, Chapple C (2012) REF4 and RFR1, subunits of the transcriptional coregulatory complex mediator, are required for phenylpropanoid homeostasis in Arabidopsis. J Biol Chem 287(8):5434–5445
Byun M, Lee J, Cui L, Kang Y, Oh T, Park H, Lee H, Kim W (2015) Constitutive expression of DaCBF7, an Antarctic vascular plant Deschampsia antarctica CBF homolog, resulted in improved cold tolerance in transgenic rice plants. Plant Sci 236:61–74
Canella D, Gilmour S, Kuhn L, Thomashow M (2010) DNA binding by the Arabidopsis CBF1 transcription factor requires the PKKP/RAGRxKFxETRHP signature sequence. Biochim et Biophy Acta (BBA) Gene Regul Mech 1799(5–6):454–462
Cao PB, Azar S, SanClemente H, Mounet F, Dunand C, Marque G, Marque C, Teulières C (2015) Genome-wide analysis of the AP2/ERF family in eucalyptus grandis: an intriguing over-representation of stress-responsive DREB1/CBF genes. PLoS One 10(4):e0121041
Champ K, Febres V, Moore G (2007) The role of CBF transcriptional activators in two Citrus species (Poncirus and Citrus) with contrasting levels of freezing tolerance. Physiol Plant 129(3):529–541
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11(2):113–116
Chaves M, Maroco J, Pereira J (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30(3):239–264
Chinnusamy V, Zhu J-K, Sunkar R (2010) Gene regulation during cold stress acclimation in plants. Methods Mol Biol 639:39–55
Clough S, Bent A (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6):735–743
Curtis M, Grossniklaus U (2003) A Gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133(2):462–469
El Kayal W, Navarro M, Marque G, Keller G, Marque C, Teulières C (2006) Expression profile of CBF-like transcriptional factor genes from Eucalyptus in response to cold. J Exp Bot 57(10):2455–2469
Fang Z, Zhang X, Gao J, Wang P, Xu X, Liu Z, Shen S, Feng B (2015) A Buckwheat (Fagopyrum esculentum) DRE-Binding transcription factor gene, FeDREB1, enhances freezing and drought tolerance of transgenic Arabidopsis. Plant Mol Biol Rep 33(5):1510–1525
FAO (2007) State of the World’s Forests 2007. Electronic Publishing Policy and Support Branch, Communication Division, Rome
Fernández M, Villarroel C, Balbontín C, Valenzuela S (2010) Validation of reference genes for real-time qRT-PCR normalization during cold acclimation in Eucalyptus globulus. Trees Struct Funct 24 (6):1109–1116
Fernández M, Valenzuela S, Arora R, Chen K (2012) Isolation and characterization of three cold acclimation-responsive dehydrin genes from Eucalyptus globulus. Tree Genet Genom 8(1):149–162
Fernández M, Troncoso V, Valenzuela S (2015) Transcriptome profile in response to frost tolerance in Eucalyptus globulus. Plant Mol Biol Rep 33(5):1472–1485
Gamboa M, Rasmussen-Poblete S, Valenzuela P, Krauskopf E (2007) Isolation and characterization of a cDNA encoding a CBF transcription factor from E. globulus. Plant Physiol Biochem 45(1):1–5
Geldres E, Schlatter J (2004) Crecimiento de las plantaciones de Eucalyptus globulus sobre suelos rojo arcillosos de la provincia de Osorno, Décima Región. Bosque (Valdivia) 25:95–101
Gilmour S, Zarka D, Stockinger E, Salazar M, Houghton J, Thomashow M (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J 16(4):433–442
Gilmour S, Fowler S, Thomashow M (2004) Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol Biol 54(5):767–781
Grattapaglia D (2004) Integrating genomics into Eucalyptus breeding. Genet Mol Res 3(3):369–379
Haake V, Cook D, Riechmann J, Pineda O, Thomashow M, Zhang J (2002) Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol 130(2):639–648
Harrison S, Mott E, Parsley K, Aspinall S, Gray J, Cottage A (2006) A rapid and robust method of identifying transformed Arabidopsis thaliana seedlings following floral dip transformation. Plant. Methods 2(1):19
Iglesias-Trabado G, Wilstermann D (2009) Eucalyptus universalis. Global cultivated eucalypt forests map 2009. Version 1.0.2 In GIT Forestry Consulting’s EUCALYPTOLOGICS: Information resources on Eucalyptus cultivation worldwide. XIII World Forestry Congress, Argentina Mar 29th 2009. Retrieved from http://www.git-forestry.com
INFOR (2014) Anuario forestal. Instituto forestal: Boletín estadísico Nº144 (1):159p http://wef.infor.cl/publicaciones/publicaciones.php. Accessed 27 July 2016
Jaglo K, Kleff S, Amundsen K, Zhang X, Haake V, Zhang J, Deits T, Thomashow M (2001) Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species. Plant Physiol 127(3):910–917
Jaglo-Ottosen K, Gilmour S, Zarka D, Schabenberger O, Thomashow M (1998) Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280(5360):104–106
Jofuku K, den Boer B, Van Montagu M, Okamuro J (1994) Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6(9):1211–1225
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotech 17(3):287–291
Kim S, Nam K (2010) Physiological roles of ERD10 in abiotic stresses and seed germination of Arabidopsis. Plant Cell Rep 29(2):203–209
Kim Y, Lee M, Lee J-H, Lee H-J, Park C-M (2015) The unified ICE–CBF pathway provides a transcriptional feedback control of freezing tolerance during cold acclimation in Arabidopsis. Plant Mol Biol 89(1):187–201
Levitt J (1980) Responses of Plants to Environmental Stresses, vol 1. Academic Press, New York, NY
Li J, Wang N, Xin H, Li S (2013) Overexpression of VaCBF4, a transcription factor from Vitis amurensis, improves cold tolerance accompanying increased resistance to drought and salinity in Arabidopsis. Plant Mol Biol Rep 31(6):1518–1528
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell Online 10(8):1391–1406
Livak K, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative pcr and the 2– ∆∆Ct method. Methods 25(4):402–408
Magome H, Yamaguchi S, Hanada A, Kamiya Y, Oda K (2004) Dwarf and delayed-flowering 1, a novel Arabidopsis mutant deficient in gibberellin biosynthesis because of overexpression of a putative AP2 transcription factor. Plant J 37(5):720–729
Magome H, Yamaguchi S, Hanada A, Kamiya Y, Oda K (2008) The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J 56(4):613–626
Mäntylä E, Lång V, Palva ET (1995) Role of abscisic acid in drought-induced freezing tolerance, cold acclimation, and accumulation of LT178 and RAB18 proteins in Arabidopsis thaliana. Plant Physiol 107(1):141–148
McGinnis K, Thomas S, Soule J, Strader L, Zale J, Sun T, Steber C (2003) The Arabidopsis sleepy1 gene encodes a putative f-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15(5):1120–1130
Medina J, Catalá R, Salinas J (2011) The CBFs: Three Arabidopsis transcription factors to cold acclimate. Plant Sci 180(1):3–11
Navarro M, Marque G, Ayax C, Keller G, Borges J, Marque C, Teulières C (2009) Complementary regulation of four Eucalyptus CBF genes under various cold conditions. J Exp Bot 60(9):2713–2724
Navarro M, Ayax C, Martinez Y, Laur J, El Kayal W, Marque C, Teulières C (2011) Two EguCBF1 genes overexpressed in Eucalyptus display a different impact on stress tolerance and plant development. Plant Biotechnol J 9(1):50–63
Novillo F, Alonso JM, Ecker JR, Salinas J (2004) CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc Natl Acad Sci USA 101(11):3985–3990
Novillo F, Medina J, Salinas J (2007) Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc Natl Acad Sci 104(52):21002–21007
Ohme-Takagi M, Shinshi H (1995) Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7(2):173–182
Peng Y-L, Wang Y-S, Cheng H, Sun C-C, Wu P, Wang L-Y, Fei J (2013) Characterization and expression analysis of three CBF/DREB1 transcriptional factor genes from mangrove Avicennia marina. Aquat Toxicol 140–141:68–76
Pita P, Pardos JA (2001) Growth, leaf morphology, water use and tissue water relations of Eucalyptus globulus clones in response to water deficit. Tree Physiol 21(9):599–607
Riechmann J, Meyerowitz E (1998) The AP2/EREBP family of plant transcription factors. Biol Chem 379:633–646
Rodziewicz P, Swarcewicz B, Chmielewska K, Wojakowska A, Stobiecki M (2014) Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiologiae Plantarum 36(1):1–19
Ruelland E, Vaultier M-N, Zachowski A, Hurry V (2009) Chap. 2 Cold signalling and cold acclimation in plants. In: Advances in botanical research, Vol 49. Academic Press, Cambridge, pp 35–150
Ryu J, Hong S-Y, Jo S-H, Woo J-C, Lee S, Park C-M (2014) Molecular and functional characterization of cold-responsive C-repeat binding factors from Brachypodium distachyon. BMC Plant Biol 14(1):15
Sakuma Y, Liu Q, Dubouzet J, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-Binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290(3):998–1009
Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K (2001) Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell 13(1):61–72
Siddiqua M, Nassuth A (2011) Vitis CBF1 and Vitis CBF4 differ in their effect on Arabidopsis abiotic stress tolerance, development and gene expression. Plant Cell Env 34(8):1345–1359
Stockinger E, Gilmour S, Thomashow M (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci 94(3):1035–1040
Tang M, Lü S, Jing Y, Zhou X, Sun J, Shen S (2005) Isolation and identification of a cold-inducible gene encoding a putative DRE-binding transcription factor from Festuca arundinacea. Plant Physiol Biochem 43(3):233–239
Thomashow M (1990) Molecular Genetics of Cold Acclimation in Higher Plants. In: John GS (ed) Advances in genetics, Vol 28. Academic Press, Cambridge, pp 99–131
Thomashow M (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50(1):571–599
Thomashow M (2010) Molecular basis of plant cold acclimation: Insights gained from studying the CBF cold response pathway. Plant Physiol 154(2):571–577
Thomashow M, Gilmour S, Stockinger E, Jaglo-Ottosen K, Zarka D (2001) Role of the Arabidopsis CBF transcriptional activators in cold acclimation. Physiol Plant 112(2):171–175
Tibbits W, White T, Hodge G, Borralho N (2006) Genetic variation in frost resistance of Eucalyptus globulus ssp. globulus assessed by artificial freezing in winter. Aust J Bot 54(6):521–529
Tillett R, Wheatley M, Tattersall E, Schlauch K, Cramer G, Cushman J (2012) The Vitis vinifera C-repeat binding protein 4 (VvCBF4) transcriptional factor enhances freezing tolerance in wine grape. Plant Biotechnol J 10(1):105–124
Tong Z, Hong B, Yang Y, Li Q, Ma N, Ma C, Gao J (2009) Overexpression of two chrysanthemum DgDREB1 group genes causing delayed flowering or dwarfism in Arabidopsis. Plant Mol Biol 71(1–2):115–129
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16(2):123–132
Volker P, Owen J, Borralho N (1994) Genetic variances and covariances for frost tolerance in Eucalyptus globulus and E. nitens. Silvae Genet 43(5–6):366–372
Wang Q, Guan Y, Wu Y, Chen H, Chen F, Chu C (2008) Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol Biol 67(6):589–602
Wang Z, Liu J, Guo H, He X, Wu W, Du J, Zhang Z, An X (2014) Characterization of two highly similar CBF/DREB1-like genes, PhCBF4a and PhCBF4b, in Populus hopeiensis. Plant Physiol Biochem 83:107–116
Wang L, Gao J, Qin X, Shi X, Luo L, Zhang G, Yu H, Li C, Hu M, Liu Q, Xu Y, Chen F (2015) JcCBF2 gene from Jatropha curcas improves freezing tolerance of Arabidopsis thaliana during the early stage of stress. Mol Biol Rep 42(5):937–945
Weigel D, Glazebrook J (2002) Arabidopsis: A laboratory manual. Cold Spring Harbor Laboratory Press, NY
Welling A, Palva E (2008) Involvement of CBF transcription factors in winter hardiness in birch. Plant Physiol 147(3):1199–1211
Wisniewski M, Norelli J, Bassett C, Artlip T, Macarisin D (2011) Ectopic expression of a novel peach (Prunus persica) CBF transcription factor in apple (Malus × domestica) results in short-day induced dormancy and increased cold hardiness. Planta 233(5):971–983
Wisniewski M, Nassuth A, Teulières C, Marque C, Rowland J, Cao P, Brown A (2014) Genomics of cold hardiness in woody plants. Crit Rev Plant Sci 33(2–3):92–124
Xiao H, Siddiqua M, Braybrook S, Nassuth A (2006) Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant Cell Env 29(7):1410–1421
Xiao H, Tattersall E, Siddiqua M, Cramer G, Nassuth A (2008) CBF4 is a unique member of the CBF transcription factor family of Vitis vinifera and Vitis riparia. Plant Cell Env 31(1):1–10
Xu M, Li L, Fan Y, Wan J, Wang L (2011) ZmCBF3 overexpression improves tolerance to abiotic stress in transgenic rice (Oryza sativa) without yield penalty. Plant Cell Rep 30(10):1949–1957
Xue Y, Wang Y, Peng R, Zhen J, Zhu B, Gao J, Zhao W, Han H, Yao Q (2014) Transcription factor MdCBF1 gene increases freezing stress tolerance in transgenic Arabidopsis thaliana. Biol Plant 58(3):499–506
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt Stress. Plant Cell 6(2):251–264
Zhang X, Fowler S, Cheng H, Lou Y, Rhee S, Stockinger E, Thomashow M (2004) Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J 39(6):905–919
Zhou M, Shen C, Wu L, Tang K, Lin J (2011) CBF-dependent signaling pathway: A key responder to low temperature stress in plants. Crit Rev Biotechnol 31(2):186–192
Zhou M, Xu M, Wu L, Shen C, Ma H, Lin J (2014) CbCBF from Capsella bursa-pastoris enhances cold tolerance and restrains growth in Nicotiana tabacum by antagonizing with gibberellin and affecting cell cycle signaling. Plant Mol Biol 85(3):259–275
Zhou M, Chen H, Wei D, Ma H, Lin J (2017) Arabidopsis CBF3 and DELLAs positively regulate each other in response to low temperature. Sci Rep 7:39819
Zhuang L, Yuan X, Chen Y, Xu B, Yang Z, Huang B (2015) PpCBF3 from cold-tolerant Kentucky Bluegrass involved in freezing tolerance associated with up-regulation of cold-related genes in transgenic Arabidopsis thaliana. PLoS One 10(7):e0132928
Acknowledgements
This work was funded by CORFO 12FBCT 16466 and Genómica Forestal S.A. A CONICYT Ph. D. scholarship to DN-C. Bioforest S.A. provided the E. globulus plant material used in this study. The technical assistance of Ms. Valeria Neira for sample handling and physiological data acquisition is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by F. Canovas.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Navarrete-Campos, D., Le Feuvre, R., Balocchi, C. et al. Overexpression of three novel CBF transcription factors from Eucalyptus globulus improves cold tolerance on transgenic Arabidopsis thaliana . Trees 31, 1041–1055 (2017). https://doi.org/10.1007/s00468-017-1529-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-017-1529-3