Abstract
During the last few years, many studies have directed their efforts at elucidating the molecular mechanisms that regulate plant response to cold stress using gene expression analysis. Quantitative real-time qRT-PCR has great advantages compared to traditional transcriptional detection methods due to its high sensibility, reproducibility, and specificity for the detection of low quantities of RNA. However, this technique requires the use of one or several housekeeping genes. In this work, the expression stabilities of six housekeeping genes (EF1α, ACT, α-TUB, PDF, SAND, and UBC) during the cold acclimation of E. globulus plants was analyzed. An ELIP gene that responds to photooxidative stress caused by light and cold stress was used as the target gene to identify the most suitable internal control for normalizing real-time qRT-PCR. Two additional genes involved in the ABA biosynthesis pathway (NCED) and sugar metabolism (GS) were analyzed with the most stable internal control genes in order to check the results found with the ELIP gene. The expressions of UBC, α-TUB and EF1α were the most stable across acclimation and de-acclimation treatments. The expressions of the other housekeeping genes tested varied depending upon the conditions. The relative quantification of ELIP changed according to identities and the number of reference genes used, thus demonstrating the importance of selecting an appropriate number of reference genes in order to achieve an accurate and reliable normalization of gene expression during cold acclimation in E. globulus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cold, salt and drought stress each have a major influence on plants, limiting geographical distributions and growing seasons and affecting the quality and yield of crops and forest plantations. Eucalyptus globulus Labill, just like many plants, increases its freezing resistance when exposed to low nonfreezing temperatures. Its low freezing resistance has presented a problem when attempting to expand plantations into areas where freezing temperatures often occur (Miranda and Pereira 2002). Considerable effort has thus been directed towards understanding how E. globulus responds and adapts to low temperature. Most of these studies have been based on the quantification of compatible solutes (Travert et al. 1997; Moraga et al. 2006; Costa e Silva et al. 2008), the detection of molecular markers associated with freezing resistance (Fernández et al. 2006), and expressed sequence tags (Rasmussen-Poblete et al. 2008). A large amount of EST sequencing data is available for cold stress studies (Dhanaraj et al. 2004; Joosen et al. 2006; Keller et al. 2009). In most of these collections, early light-inducible protein (ELIP) genes, which are implicated in photooxidative stress caused by light and cold stress (Houde et al. 2006; Dhanaraj et al. 2007; Soitamo et al. 2008), have been observed to be expressed during cold acclimation. From this perspective, one practical application of molecular techniques in plants is the analysis of gene expression for specific mRNA sequences by real-time qRT-PCR. In comparison to the commonly used northern blot analysis, the main advantages of real-time qRT-PCR are its higher sensitivity, specificity, and broad quantification range. The quantitative analysis of gene expression by this technique requires the use of a constitutively expressed gene as an internal control to normalize for differences in starting cDNA templates among samples. The expression levels of the reference genes should remain constant between different tissues and under different experimental conditions (Thellin et al. 1999). If these requirements are not fulfilled, normalization with these genes can lead to erroneous results (Bustin 2000). In several studies, the housekeeping genes involved in basic plant cellular processes, such as actin (Li et al. 2005), tubulin (Jeong et al. 2006), glyceraldehyde-3-phosphate dehydrogenase (Jain et al. 2006), ribosomal RNA (Nicot et al. 2005), ubiquitin C (Sekalska et al. 2006), and elongation factor 1-α (Nicot et al. 2005), have been used as internal controls for gene expression analysis and—in some cases—without testing their stability. Several reports demonstrate that the transcript levels of these genes vary considerably under different experimental conditions (Thellin et al. 1999; Czechowski et al. 2005; Jain et al. 2006; Libault et al. 2008). On the other hand, most studies recommend the use of at least two or three different reference genes as internal controls so as to avoid errors from the unstable expression of only one reference gene (Thellin et al. 1999; Vandesompele et al. 2002). Given the increasing interest in gene expression studies of woody plants and the lack of information on appropriate reference genes, six housekeeping genes commonly used in plants were selected in order to assess them as internal controls in gene expression studies during cold acclimation in E. globulus.. These genes corresponded to elongation factor 1-α (EF1α), actin (ACT), α-tubulin (α-TUB), protein phosphatase 1 A (PDF), SAND protein (SAND), ubiquitin C (UBC). ELIP was also used as a target gene. Two additional genes, 9-cis-epoxycarotenoid dioxygenase (NCED), which is involved in the ABA biosynthesis pathway, and galactinol synthase (GS), which is implicated in sugar metabolism, were tested with the most stable internal control genes in order to compare and confirm the results obtained with the ELIP gene.
Materials and methods
Plant materials and cold treatments
Two genotypes of E. globulus were obtained from a local forest company. These genotypes have shown differences in freezing resistance under field conditions; one presented a marked susceptibility to freezing temperatures (G1) while the other was resistant (G2). The company provided 6-month-old rooted plants (35 cm in height) with at least eight expanded leaves. Each genotype was represented by thirty ramets that were put in an Aislapool box covered with vermiculite to maintain the humidity of the substrate, and placed in a growth chamber with a controlled temperature and photoperiod. Temperature and photoperiod were changed as shown in Fig. 1. Five treatments were tested. Treatment 1 (nonacclimated, NA) genotypes were maintained under a 14 h day at day/night temperatures of 20/12°C. On day 7, leaves were collected and the conditions of the growth chamber were changed for treatment 2 (cold acclimated before night frosts of −2°C, CABF) to a short day length (10 h) at 8/4°C for cold acclimation. Leaves were collected on day 14. In treatment 3 (cold acclimated after night frosts of −2°C, CAAF), at 0:00 a.m. on days 19, 20, 21 and, 22 a night frost of −2°C with a decrease of 2°C per hour was applied. Leaves were collected at 9:00 a.m. on day 22. Treatment 4 (de-acclimated, DA) genotypes were subjected to de-acclimation under a long day length (14 h day) and 12/6°C day/night temperatures for 1 week. Leaves were collected on day 28. Treatment 5 (night frost of −6°C, F −6°C) corresponded to a frost of −6°C applied to DA ramets to observe the response of the genotypes to late frosts. On day 29, when the temperature reached −6°C in the cold chambers, leaves were collected. The experiment was arranged as a completely randomized design with three replications (three ramets per genotype). All leaves collected were frozen in liquid nitrogen and stored at −80°C until further use.
Total RNA extraction
RNA was extracted from leaves using the CTAB method (Chang et al. 1993), where the incubation time in the lysis buffer was modified to 20 min. The concentration of each RNA sample was measured using a NanoDrop ND-1000 spectrophotometer. Purity of RNA samples was defined by an A260/280 ratio of between 1.9 and 2.1 and an A260/230 ratio of greater than 2.0. The integrity of the RNA was checked by electrophoresis in 2% agarose gel.
Primer design
Six housekeeping genes were selected: the elongation factor 1-α (EF1α), actin (ACT), α-tubulin (α-TUB), protein phosphatase 1 A (PDF), SAND protein (SAND) and ubiquitin C (UBC) genes. The ELIP gene was chosen as the gene of interest, and two additional genes, NCED and GS, were analyzed with the most stable internal control genes. The sequences for the genes (Table 1) were retrieved from Arabidopsis thaliana and BLAST via two E. globulus cDNA libraries available in the National Center for Biotechnology Information (NCBI) (Rasmussen-Poblete et al. 2008). Primers were designed for each individual gene using the Primer Express 2.0 software (PE, Applied Biosystems, Foster City, CA, USA) using the default parameters for a real-time assay.
RT-PCR
For each sample, 1 μg of total RNA was treated with DNaseI (Fermentas Life Science, Glen Burnie, MD, USA) to remove any contaminating DNA. First-strand cDNA was synthesized by reverse transcription using a High Capacity cDNA Archive Kit (Applied Biosystems) according to the manufacturer’s instructions.
Verification of the amplified products
In order to verify the amplified products, PCRs were performed on samples with 0.3 μM of primers, 2 U of Taq DNA polymerase (Taq Platinum, Invitrogen, Carlsbad, CA, USA), 200 μM each of dNTP mix (Invitrogen), and 3.3 μl of diluted cDNA (corresponding to 10 ng as the starting amount of total RNA in the RT-PCR reaction) in a total volume of 25 μl. Amplifications were performed according to the following program: an initial step of denaturation at 95°C for 10 min, continued by 40 cycles of denaturation (30 s at 95°C), alignment (1 min at 60°C) and elongation (30 s at 72°C), with a final elongation of 7 min at 72°C. Fragment lengths were checked by electrophoresis in 2% agarose gel.
Real-time qRT-PCR
The PCR mixture contained 1 μl of diluted cDNA (corresponding to 3 ng as a starting amount of RNA), 10 μl of SYBR Green PCR Master Mix (Applied Biosystems), and 0.9 μM of each gene-specific primer in a final volume of 20 μl. PCRs with no template controls were also assessed for each primer pair. The real-time qRT-PCRs were done using the ABI Prism 7300 Sequence Detection System and software (Applied Biosystems). All of the real-time qRT-PCRs were carried out under the following conditions: 10 min at 95°C, 40 cycles of 15 s at 95°C, and 1 min at 60°C in 96-well optical reaction plates (Applied Biosystems). The specificity of the amplicons was verified by checking the melting curve (60–95°C) after 40 cycles. Three biological replicates were used for each sample, and three technical replicates were analyzed for each biological replicate. For each gene, the PCR efficiency was determined by measuring the CT to a specific threshold for a serial dilution of bulk cDNA. All PCRs displayed efficiencies of between 90 and 100%.
Analysis of gene expression stability
To analyze the gene expression stability, geNORM v.3.4 software (Vandesompele et al. 2002) was used. The Microsoft Excel file containing the raw expression values for the six genes in five treatments for both genotypes (10 cDNA pools) that was imported into geNORM is given in the Electronic supplementary material (Table S1).
Results
Identification of gene sequences
Primers were designed for the orthologs of the six housekeeping genes and the target genes representing different functional classes in blue gum eucalypt. The gene names, accession numbers, primer sequences and amplicon lengths are shown in Table 1. The similarity between A. thaliana sequences and the eucalypt EST of EF1α was 64% on 642 nucleotides, ACT 81% on 683 nucleotides, α-TUB 69% on 705 nucleotides, PDF 79% on 573 nucleotides, SAND 80% on 678 nucleotides, UBC 85% on 449 nucleotides, NCED 45% on 973 nucleotides, and GS 68% on 635 nucleotides. The stabilities of these six housekeeping genes were assessed by real-time qRT-PCR in a set of two E. globulus genotypes exposed to five treatments of cold acclimation and de-acclimation. Agarose gel electrophoresis (Fig. 2A) and melting-curve analysis (Fig. 2B) confirmed that all six primer pairs and the target gene amplified a single PCR product of the desired size.
Amplicon length verification. a Agarose gel (2%) showing amplification of a specific PCR product of the expected size for each housekeeping gene tested in the study. b Dissociation curves for four housekeeping genes that were used to normalize ELIP gene expression. Each peak was derived using three technical replicates of cDNA pools and a no-template control (NTC)
Variation of reference genes
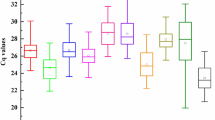
The transcript abundances of all reference genes were similar; the average CT value found ranged from 21 to 28 (Fig. 3). The mean CT value (average of three technical replicates) for all RNA and each gene was used to measure the expression stability (Electronic supplementary material Table S1). The average expression stability value (M) of the PDF housekeeping gene was the highest (1.333), while those for UBC and α-TUB (0.415) were the lowest (Fig. 4a). To determine the utility of including more than two reference genes for normalization, the pairwise variation V n/n+1 was calculated. For two genes, the cut-off value suggested by Vandesompele et al. (2002) is 0.15. For the two most stable genes, UBC and α-TUB, the V value was 0.25 (Fig. 4b), thus requiring the inclusion of a third reference gene (EF1α).
Average expression stability values of the control genes, obtained by geNorm analysis. a Expression stabilities and rankings of the housekeeping genes as calculated by geNORM in all 10 cDNA samples, per gene. b Pairwise variation (V), which was used to determine the optimal number of control genes for accurate normalization. Asterisk indicates the optimal number of genes for normalization
ELIP gene expression
The expression level of the ELIP gene was quantified according to the instructions for geNORM (Vandesompele et al. 2002) using three reference genes as internal controls (UBC, α-TUB and EF1α). ELIP expression was the highest in genotype G1, it increased 11.4-fold after 1 week of acclimation (CABF) compared with NA ramets, and it remained constant at CAAF (Fig. 5a). The expression level at DA was 2.7-fold higher compared with the NA plants. During F −6°C, the expression of ELIP decreased, reaching similar expression levels to those in the NA plants. For genotype G2, the expression level increased 3-fold at CABF and 11-fold at CAAF compared with the NA plants (Fig. 5a). For DA and F −6°C, the expression of ELIP decreased 3-fold compared to NA ramets. To confirm and compare the results from the quantification of ELIP gene expression with those from an appropriate internal control gene, ELIP expression was normalized using the two most stable genes, UBC and α-TUB, as well as the UBC, α-TUB, EF1α genes individually. Additionally, the reference gene with the lowest expression stability (PDF) was analyzed. UBC and α-TUB together (Fig. 5b) led to similar results for both genotypes to those obtained using the three housekeeping genes. Only genotype G1 at CABF showed an increase in the expression level to 14.1-fold compared to NA ramets. Using α-TUB as a unique internal control, for genotype G1, the ELIP expressions at CABF and CAAF increased 19.8- and 9.8-fold compared to to that in NA, and maintained a similar expression level for other treatments (Fig. 5d). Expression for genotype G2 at CABF increased 5.8-fold compared to NA ramets. When using EF1α as a unique internal control, the expression levels for G1 and G2 at CABF (Fig. 5e) were lower compared with those observed when normalization was performed using three reference genes. When the least stable housekeeping gene (PDF) was employed, the ELIP expression pattern varied considerably (Fig. 3f); the expression level at CAAF for replicate 1 was much higher (80-fold) than that for the control treatment in both genotypes.
Relative quantification of ELIP expression using a unique internal control or the two or three most stable housekeeping genes as defined by geNORM analysis. a Internal control UBC α-TUB, EF1α. b Internal control: UBC and α-TUB. c Internal control UBC. d Internal control α-TUB. e Internal control EF1α. f Internal control PDF. R1 ramet 1, R2 ramet 2, R3 ramet 3
NCED and GS gene expression
The expression levels of the NCED and GS genes were quantified according to the instructions for geNORM (Vandesompele et al. 2002) using three reference genes as internal controls (UBC, α-TUB, EF1α) and the two most stable genes (UBC and α-TUB). Just as for the normalization of the ELIP gene, the relative expressions of NCED and GS obtained using the three internal controls (Fig. 6a, c) were similar (for both genotypes) to those found using the two housekeeping genes (Fig. 6b, d). However, in contrast to the results for the ELIP gene, the relative expressions of NCED and GS decreased after acclimation. In G1, NCED gene expression was higher than in G2 and decreased 3.9-fold at CABF and 13.9-fold at CAAF compared to NA ramets (Fig. 6a). For genotype G2, NCED gene expression was lower, but exhibited the same rate of decline in all treatments (Fig. 6a). The relative expression of GS for G1 decreased 3.8-fold at CABF and 4.3-fold at CAAF compared to NA ramets (Fig. 6c). The expression level at DA was 1.5-fold lower than for the NA plants but decreased 2.7-fold at F −6°C compared to NA. For genotype G2, the expression level decreased 2.4-fold at CABF, 4-fold at CAAF, and 2-fold at DA compared with the NA plants, but in contrast to G1, the relative expression at F −6°C reached almost the same level as in the NA plants (Fig. 6c).
Relative quantification of NCED and GS expression using the three and the two most stable housekeeping genes as defined by geNORM analysis. a Normalization of NCED with UBC, α-TUB, and EF1α used as internal controls. b Normalization of NCED with UBC and α-TUB used as internal controls. c Normalization of GS with UBC, α-TUB, and EF1α used as internal controls. d Normalization of GS with UBC and α-TUB used as internal controls. R1 ramet 1, R2 ramet 2, R3 ramet 3
Discussion and conclusions
Eucalypts are a good model for studying cold acclimation, since there is no interference with endodormancy or leaf senescence mechanisms that occur under the same environmental conditions for deciduous woody plants (Keller et al. 2009). Molecular tools such as real-time qRT-PCR have become important in the analysis of gene expression. Given the increasing interest in gene expression studies in woody plants and, specifically, the current lack of information about appropriate internal control genes for normalization, in this study a set of genes that can be used as a constitutive reference gene for E. globulus were identified and experimentally validated. Data normalization to obtain reliable results from real-time qRT-PCR analysis and accurate normalization of gene expression against a control gene represent a critical step in gene expression analysis. Normalizing with an unsuitable housekeeping gene strongly impacts on the expression pattern of the target gene (Czechowski et al. 2005). One disadvantage of gene validation is that considerable effort and cost is required to perform an analysis. For studies of gene expression in rice, more than two genes were required for normalization (Jain et al. 2006). The use of several housekeeping genes (at least two) was recommended by Thellin et al. (1999) in order to compare gene expression levels to housekeeping gene transcripts used as internal standards. According to Vandesompele et al. (2002), the purpose of normalization is to remove sampling differences (such as in RNA quantity and quality) in order to avoid gene-specific variation. They provided evidence that a conventional normalization strategy based on a single gene led to erroneous normalization. Following this assumption, the expression stabilities of six housekeeping genes commonly used as internal controls for the analysis of ELIP gene expression were analyzed in this study. When the two most stable housekeeping genes were used, the V values were higher than the value suggested for geNORM analysis (0.25), making it necessary to include a third gene to normalize the expression of the ELIP gene (Fig. 5). Therefore, for five treatments, the three genes with the most stable expression levels were found to be optimal for reliable normalization with a pairwise variation cut-off value of 0.15. It is important to mention that the pairwise value when using three housekeeping genes was at the limit of the cut-off V value (0.15). To confirm and compare the results of the quantification of ELIP gene expression with those for an appropriate internal control gene, normalization using the two most stable reference genes, the three most stable genes individually (UBC, α-TUB, EF1α), and the least stable gene (PDF) was carried out. The quantification of ELIP expression using UBC and α-TUB together (Fig. 5b) did not significantly change the quantification of the expression level compared to normalization with three reference genes. To check these results, we analyzed two additional target genes (NCED and GS). In contrast with the ELIP gene, NCED and GS decreased their gene expressions with acclimation (Fig. 6). However, it was possible to confirm that normalizing with two internal controls produces similar results to normalizing with three internal controls for NCED, GS, and ELIP. When the assay was normalized with UBC or α-TUB as a single housekeeping gene (Fig. 5c, d) the ELIP expression pattern varied, specifically in genotype G1. When the least stable reference gene (PDF) was used, the ELIP expression pattern was overestimated and varied considerably (Fig. 5f), due to the relative instability of the expression level for PDF. In blueberry, Dhanaraj et al. (2007) reported a 9-fold increase in the expression of ELIP during cold acclimation compared to NA field plants. Peng et al. (2008) reported an approximate 5- to 8-fold overexpression in acclimated rhododendron compared to the level in NA plants. In this study, the expression level increased 11.4-fold at CABF compared to NA in the susceptible genotype G1. The resistant genotype G2 showed a 3-fold increase in expression level at CABF, which rose to 11-fold at CAAF (Fig. 5a). These results may suggest that G2 needs the stimulus of subfreezing temperatures to increase its ELIP expression. Zarter et al. (2006) suggest that Elips may play a role in facilitating sustained ΔpH-independent energy dissipation in evergreen plants during long-term acclimation to conditions of extreme stress by interacting with the pool of zeaxanthin. Evidently, detailed study will be necessary to clarify the role of Elips in cold acclimation; however, the importance of evaluating reference genes for the analysis of gene expression of any target gene is clear. In this work, three reference genes were required as internal controls, thus demonstrating the need to evaluate the stability and the appropriate number of genes to use as internal controls under all of the conditions where the expression of a target gene will be tested.
References
Bustin SA (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 2:169–193
Chang S, Puryear J, Cairney J (1993) A simple method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Costa e Silva F, Shvaleva A, Broetto F, Ortuño MF, Rodrigues ML, Almeida MH, Chaves MM, Pereira JS (2008) Acclimation to short-term low temperatures in two Eucalyptus globulus clones with contrasting drought resistance. Tree Physiol 29:77–86
Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139:5–17
Dhanaraj AL, Slovin JP, Rowland LJ (2004) Analysis of gene expression associated with cold acclimation in blueberry floral buds using expressed sequence tags. Plant Sci 166:863–872
Dhanaraj AL, Alkharouf NW, Beard HS, Chouikha IB, Matthews BF, Wei H, Arora R, Rowland LJ (2007) Major differences observed in transcript profiles of blueberry during cold acclimation under field and cold room conditions. Planta 225:735–751
Fernández M, Valenzuela S, Balocchi C (2006) RAPD and freezing resistance in Eucalyptus globulus. Electron J Biotechnol 9:303–309
Houde M, Belcaid M, Ouellet F, Danyluk J, Monroy AF, Dryanova A, Gulick P, Bergeron A, Laroche A, Links MG, MacCarthy L, Crosby WL, Sarhan F (2006) Wheat EST resources for functional genomics of abiotic stress. BMC Genomics 7:149
Jain M, Nijhawan A, Tyagi AK, Khurana JP (2006) Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun 345:646–665
Jeong YM, Mun JH, Lee I, Woo JC, Hong CB, Kim SG (2006) Distinct roles of the first introns on the expression of Arabidopsis profiling gene family members. Plant Physiol 140:196–209
Joosen RVL, Lammers M, Balk PA, Brønnum P, Konings MC, Perks M, Stattin E, Van Wordragen MF, Van der Geest AL (2006) Correlating gene expression programs to physiological parameters and environmental conditions during cold acclimation of pine (Pinus sylvestris). Tree Physiol 26:1297–1313
Keller G, Marchal T, SanClemente H, Navarro M, Ladouce N, Wincker P, Couloux A, Teulières C, Marque C (2009) Development and functional annotation of an 11,303-EST collection from Eucalyptus for studies of cold stress. Tree Genet Genomes 5:317–327
Li L, Xu J, Xu ZH, Xue HW (2005) Brassinosteroids stimulate plant tropisms through modulation of polar auxin transport in Brassica and Arabidopsis. Plant Cell 17:2738–2753
Libault M, Thibivilliers S, Radwan O, Clough SJ, Stacey G (2008) Identification of four soybean reference genes for gene expression normalization. Plant Genome 1:44–54
Miranda I, Pereira H (2002) Variation of pulpwood quality with provenances and site in Eucalyptus globulus. Ann For Sci 59:283–291
Moraga P, Escobar R, Valenzuela S (2006) Resistance to freezing in three Eucalyptus globulus Labill subspecies. Electron J Biotechnol 9:310–314
Nicot M, Hausman JF, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56(421):2907–2914
Peng Y, Lin W, Wei H, Krebs SL, Arora R (2008) Phylogenetic analysis and seasonal cold acclimation-associated expression of early light-induced protein genes of Rhododendron catawbiense. Physiol Plantarum 132:44–52
Rasmussen-Poblete S, Valdés J, Gamboa M, Valenzuela P, Krauskopf E (2008) Generation and analysis of an Eucalyptus globulus cDNA library constructed from seedlings subjected to low temperature conditions. Electronic J Biotech 11:2
Sekalska B, Ciechanowicz A, Dolegowska B, Naruszewicz M (2006) Optimized RT-PCR method for assaying expression of monocyte chemotactic protein type 1 (MCP-1) in rabbit aorta. Biochem Genet 44:133–143
Soitamo AJ, Piippo M, Allahverdiyeva Y, Battchikova N, Aro E (2008) Light has a specific role in modulating Arabidopsis gene expression at low temperature. BMC Plant Biol 8:13
Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, Grisar T, Igout A, Heinen E (1999) Housekeeping genes as internal standards: use and limits. J Biotechnol 75:291–295
Travert S, Valeria L, Fourasté I, Boudet AM, Teuliéres C (1997) Enrichment in specific soluble sugars of two Eucalyptus cell suspension cultures by various treatments enhances their frost tolerance via a non colligative mechanism. Plant Physiol 114:1433–1442
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Zarter CR, Adams WW III, Ebbert V, Adamska I, Jansson S, Demmig-Adams B (2006) Winter acclimation of PsbS and related proteins in the evergreen Arctostaphylos uva-ursi as influenced by altitude and light environment. Plant Cell Environ 29:869–878
Acknowledgments
This research was supported by Genomica Forestal S.A. and a scholarship from Conicyt to MF. Gratitude is expressed to Valeria Neira for technical assistance. We thank Dr. Leon Bravo for moving and establishing the growth chamber assay.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. M. Cánovas.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fernández, M., Villarroel, C., Balbontín, C. et al. Validation of reference genes for real-time qRT-PCR normalization during cold acclimation in Eucalyptus globulus . Trees 24, 1109–1116 (2010). https://doi.org/10.1007/s00468-010-0483-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-010-0483-0