Abstract
Background
Endoscopic submucosal dissection (ESD) is an effective treatment for early gastric cancer (EGC); however, its curative resection rate is low for undifferentiated-type EGC. We developed and externally validated a prediction model for curative ESD of undifferentiated-type EGC.
Methods
In this cross-sectional study, we included 448 patients who underwent ESD for undifferentiated-type EGC at 18 hospitals in Korea between 2005 and 2015 in the development cohort and 1342 patients who underwent surgery at two hospitals in the validation cohort. A prediction model was developed using the logistic regression model.
Results
Endoscopic tumor size 1–2 cm (odds ratio [OR], 2.40; 95% confidence interval [CI] 1.54–3.73), tumor size > 2 cm (OR, 14.00; 95% CI 6.81–28.77), and proximal tumor location from the lower to upper third of the stomach (OR, 1.45; 95% CI 1.03–2.04) were independent predictors of non-curative ESD. A six-score prediction model was developed by assigning points to endoscopic tumor size > 2 cm (five points), tumor size 1–2 cm (two points), upper third location (two points), and middle third location (one point). The rate of curative ESD ranged from 70.6% (score 0) to 11.6% (score 5) with an area under the receiver operating characteristic curve (AUC) of 0.720 (95% CI 0.673–0.766). The model also showed good performance in the validation cohort (AUC, 0.775; 95% CI 0.748–0.803).
Conclusions
This six-score prediction model may help in predicting curative ESD and making informed decisions about the treatment selection between ESD and surgery for undifferentiated-type EGC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endoscopic submucosal dissection (ESD) is now accepted as a standard treatment for early gastric cancer (EGC) with a very low risk of lymph node metastasis [1, 2]. The long-term survival of patients who underwent curative ESD is comparable to those who underwent surgery [3, 4]. However, ESD for EGC with an undifferentiated-type histology, including poorly differentiated adenocarcinoma, signet ring cell carcinoma, or mucinous adenocarcinoma, has been controversial because of the potential risk of lymph node metastasis [5,6,7]. Nevertheless, patients who underwent curative ESD for undifferentiated-type EGC (UD EGC) showed favorable long-term outcomes in many studies [8,9,10,11,12]. However, these studies revealed that it was more difficult to achieve curative resection for UD EGC than for differentiated-type EGC.
A systematic review reported that the curative rate of ESD was 61.4% for UD EGC [13], indicating that about 40% of patients who had undergone ESD were also subject to additional surgery. Thus, prediction of curative resection before ESD is particularly important for UD EGC in terms of appropriate treatment selection and informed decision making. However, the predictive factors for curative ESD of UD EGC remain to be elucidated. Previous studies reported that tumors > 2 cm in size, located in the upper third of the stomach, with ulceration, or with an undifferentiated-type histology were associated with an increased risk for non-curative ESD [7,8,9, 11, 12]. Some studies also developed models for predicting curative ESD based on these factors [14, 15]. Although the undifferentiated-type histology itself was identified as an independent risk factor, additional risk stratification within UD EGCs was insufficient in these studies because they mainly focused on differentiated-type cancer, which has different indications for ESD from those of undifferentiated-type cancer. Furthermore, no previous study has validated the findings in an independent cohort, resulting in limited reliability.
Therefore, we conducted the present nationwide multicenter cross-sectional study to develop a prediction model for curative ESD specifically focusing on UD EGC, and externally validated the model in an independent cohort.
Methods
Patients
This multicenter retrospective cross-sectional study involved 18 tertiary hospitals across six geographic areas in Korea. The institutional review board of each hospital approved the study protocol and waived the requirement for obtaining informed consent.
Between February 2005 and May 2015, ESD was performed for a single UD EGC at participating hospitals in a total of 1079 consecutive patients (Fig. 1). Among them, 536 patients who had been diagnosed with undifferentiated-type cancer in forceps biopsy performed before ESD were included. We excluded 88 patients who met any of following criteria: tumor size > 5 cm in diameter (n = 12), tumor with ulceration (n = 57), and history of previous gastrectomy or endoscopic resection (n = 21). Consequently, 448 patients were included in the development cohort. For the external validation of the prediction model, we reviewed 1956 patients who underwent surgical resection for a single UD EGC at two hospitals during the study period. Among them, 1800 patients met the inclusion criteria and 458 were excluded. Finally, 1342 patients comprised the validation cohort. We set the limitation to endoscopic tumor size to allow comparability between the development and validation cohorts. Although ESD is not indicated for UD EGC > 2 cm in diameter, the cutoff value was set as 5 cm instead of 2 cm because of the possibility of overestimating the tumor size in endoscopy before ESD or surgery.
Endoscopic procedure and surgery
All patients underwent upper endoscopy before ESD or surgery to evaluate the location, macroscopic appearance, size, and depth of the tumor, as well as the presence of ulceration. Chromoendoscopy with indigo carmine dye was routinely performed to define the tumor margins, and narrow-band imaging (NBI) was also used when available. If deemed necessary, demarcation biopsies taken outside the lesion to define lateral margins and endoscopic ultrasonography (EUS) to evaluate the depth of tumor invasion were conducted at the endoscopist’s discretion. Endoscopic ulcer was defined as either ulceration or ulcer scar in the lesion. Forceps biopsy specimens were taken from the lesion in all cases.
Experienced gastrointestinal endoscopists conducted the ESD procedure using a single-channel endoscope (GIF-H260; Olympus Optical, Tokyo, Japan). As previously described in a multicenter study [16], the ESD procedure was conducted in the sequential order of marking, submucosal injection, circumferential incision, and submucosal dissection. Surgical resection was performed by experienced gastric surgeons as radical gastrectomy with D1 + or more lymph node dissection following the Japanese guidelines [2].
Pathologic diagnosis
Endoscopically or surgically resected specimens were fixed in formalin, serially sectioned at 2-mm intervals for ESD specimens and at 4- to 6-mm intervals for surgical specimens, and embedded in paraffin. The histologic type, tumor size, depth of invasion, presence of ulceration and lymphovascular invasion, and horizontal and vertical resection margins were assessed according to the Japanese guidelines [2, 17]. Undifferentiated-type carcinoma was defined as poorly differentiated adenocarcinoma, signet ring cell carcinoma, or mucinous adenocarcinoma. Complete resection was defined as en bloc resection with negative horizontal and vertical margins. Curative resection was defined as complete resection with histologic tumor size ≤ 2 cm in diameter, intramucosal cancer, and absence of histologic ulceration and lymphovascular invasion [2, 17].
Statistical analysis
The patients’ clinicopathologic characteristics were compared between the development and validation cohorts using t-test or the Mann–Whitney U test for continuous variables, and the chi-square test, Fisher’s exact test, or linear-by-linear association test for categorical variables. Univariate and multivariate logistic regression analyses were conducted to identify the predictors for non-curative ESD in the development cohort. Thereafter, we developed a prediction model for curative ESD based on the adjusted logistic regression model. A risk point was assigned to each independent predictor by dividing the adjusted regression coefficient by the smallest one in the model that was rounded to the nearest integer. The risk score of each individual was the sum of the points for the patient’s risk factors. The performance of the prediction model was evaluated using the area under the curve (AUC) in receiver operating characteristic (ROC) analysis. The model was validated both internally using the bootstrapping method (2000 replications) [14] and externally in the validation cohort. We also evaluated whether the predictors for non-curative ESD were also associated with individual factors of non-curative ESD, such as pathologic tumor size > 2 cm, submucosal invasion, or lymphovascular invasion, both in the development and validation cohorts. In addition, we conducted subgroup analyses according to tumor size and depth to evaluate how the ESD outcome would change if the accuracy of evaluation for tumor size and depth before ESD was improved. All analyses were conducted using SPSS (version 21.0; SPSS Inc., Chicago, IL, USA) and STATA (version 16.0; StataCorp LP, College Station, TX, USA), and P < 0.05 was considered statistically significant.
Results
Patients
The patients’ clinicopathologic characteristics are summarized in Table 1. Patients who underwent ESD for UD EGC (development cohort) were significantly older, more likely to be male, and less likely to be a smoker than those who underwent surgery (validation cohort). The tumors in the development cohort were significantly smaller in diameter and more likely to be distally located, flat or elevated in endoscopic appearance, and poorly differentiated adenocarcinoma than those in the validation cohort.
In the development cohort, complete ESD and curative ESD were achieved in 83.7% (375/448) and 43.1% (193/448) of the patients, respectively. As expected, the complete resection rate was higher in the validation cohort because only three patients (0.2%) had a positive horizontal margin after surgery. However, the results of surgical pathology in the validation cohort fulfilled the expanded ESD criteria in 24.3% (326/1342) of the patients, which was lower than the rate in the development cohort. This was primarily because the risks of pathologic tumor size > 2 cm and submucosal invasion were higher in the validation cohort than in the development cohort.
Predictors of non-curative ESD of UD EGC
In the univariate analysis conducted in the development cohort, endoscopic tumor size 1–2 cm (odds ratio [OR], 2.40; 95% confidence interval [CI] 1.54–3.73; P < 0.001) and tumor size > 2 cm (OR, 14.00; 95% CI 6.81–28.77; P < 0.001) had a significantly higher risk of non-curative ESD than tumor size < 1 cm (Table 2). Compared with a lower third tumor location, middle third (OR, 1.46; 95% CI 0.99–2.17, P = 0.057) and upper third tumor locations (OR, 2.64; 95% CI 1.16–5.99; P = 0.021) were also associated with non-curative ESD. Thus, there was an increasing trend in the risk of non-curative ESD across the longitudinal tumor location from the lower to upper third of the stomach (OR, 1.54; 95% CI 1.12–2.11; P for trend = 0.008). In the multivariate analysis, endoscopic tumor size 1–2 cm (OR, 2.28; 95% CI 1.59–3.56; P < 0.001), tumor size > 2 cm (OR, 13.59; 95% CI 6.60–27.98; P < 0.001), and proximal tumor location (OR, 1.45; 95% CI 1.03–2.04; P = 0.032) were independently associated with non-curative ESD of UD EGC.
Prediction model for curative ESD of UD EGC
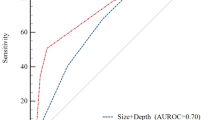
On the basis of the adjusted regression coefficient, a point was assigned to each independent predictor, as follows: endoscopic tumor size 1–2 cm, two points; upper third tumor location, two points; and middle third tumor location, one point (Table 2). We assigned a maximum of five points to endoscopic tumor size > 2 cm. The risk score for the prediction model, or the sum of the points, ranged from 0 to 5 (Table 3). As the risk score increased, the probability of curative ESD decreased from 70.6% (score 0; lower third location, tumor size ≤ 1 cm) to 11.6% (score 5; any location, tumor size > 2 cm). The expected probabilities of curative ESD according to the model were not significantly different from those observed in the Hosmer–Lemeshow goodness-of-fit test (P = 0.371). The AUC of the ROC curve in the development cohort was 0.720 (95% CI 0.673–0.766) (Fig. 2a). The model also showed slightly attenuated but still acceptable prediction performance with the 2000 internal bootstrap samples (bootstrap-corrected AUC, 0.653; 95% CI 0.600–0.709).
Receiver operating characteristic curves for the prediction model for curative endoscopic submucosal dissection in the A development cohort and B validation cohort. M cancer indicates subgroups including intramucosal gastric cancers only, size discrepancy ≤ 1 cm indicates subgroups including cases with the discrepancy between endoscopic and pathologic tumor size was 1.0 cm or smaller, and M cancer with size discrepancy ≤ 1 cm indicates subgroups where both criteria were applied. AUC Area under the curve, CI Confidence interval, M Intramucosal
External validation of the prediction model
We externally validated the prediction model for curative ESD in the independent surgical validation cohort. In the validation cohort, the probability that the surgical pathology met the curative ESD criteria linearly decreased from 62.2% in score 0 to 4.9% in score 5 (Table 3). Because the overall probability of curative ESD was lower, the probability of curative ESD at each score tier was also lower by 10% points in the validation cohort than in the development cohort. This model also showed good calibration in the validation cohort (Hosmer–Lemeshow goodness-of-fit test, P = 0.527), and the AUC of the ROC curve was 0.775 (95% CI 0.748–0.803) (Fig. 2b).
Causes of non-curative ESD according to predictors
We further explored the associations between the independent predictors and individual causes of non-curative ESD (Table 4). Larger endoscopic tumor size was associated with a higher risk of pathologic tumor size > 2 cm (P < 0.001), submucosal invasion (P = 0.039), and positive horizontal margin (P = 0.007). Meanwhile, proximal tumor location was significantly associated with higher risks of submucosal invasion (P = 0.001) and positive vertical margin (P = 0.018). In the validation cohort, larger endoscopic tumor size and proximal location were also associated with both of pathologic tumor size > 2 cm and submucosal invasion (Supplementary Table 1).
Subgroup analyses according to tumor size and depth
We conducted three subgroup analyses. First, we included patients with intramucosal EGC only that indicated the situation where the evaluation for tumor depth was highly accurate. Although proximal location was no longer independently associated with curative ESD (Supplementary Table 2), the performance of our prediction model was slightly improved with AUC of 0.721 (95% CI 0.668–0.774) and 0.787 (95% CI 0.755–0.819) in the development and validation subgroups, respectively (Fig. 2). In addition, the curative ESD rate increased by 10% at each risk score (Supplementary Table 3). Second, we included patients in whom the discrepancy between endoscopic and pathologic tumor size was ≤ 1.0 cm that indicated the situation where the tumor size was evaluated with very high accuracy. In this analysis, tumor size and proximal location were independently associated with curative ESD, and the curative ESD rate also increased by 10% at each risk score. AUC also increased as 0.813 (95% CI 0.769–0.858) and 0.805 (95% CI 0.776–0.833) in the development and validation subgroups, respectively. Third, when we applied both criteria the curative ESD rate increased by 20% at each risk score. The AUC value was the highest in this analysis as 0.839 (95% CI 0.788–0.890) and 0.857 (0.826–0.888) in the development and validation subgroups, respectively.
We conducted another subgroup analysis by including UD EGCs with endoscopic tumor size ≤ 2 cm only. However, the multiple logistic regression analysis model and the curative ESD rate at each risk score were virtually unchanged from the main analysis that included EGCs with tumor size ≤ 5 cm except that the category of tumor size 2–5 cm was removed (Supplementary Tables 2 and 3).
Discussion
In this study, we developed a novel, simple-to-use, six-score model for predicting curative ESD of UD EGC in a nationwide multicenter ESD cohort. According to this model, the rate of curative ESD ranged from 70% for EGC ≤ 1 cm in size and located in the antrum to 10% for EGC > 2 cm in size and occurring in any location. This model also showed good prediction performance in an independent surgical cohort. Thus, the model could be used for informed decision making in the selection of the proper treatment modality between ESD and surgery for UD EGC. Additionally, we also suggested possible ways to improve the curative ESD rate. Tumors measuring > 1 cm in diameter before ESD may require meticulous evaluation for size and depth because of the high risk of non-curative ESD due to pathologic tumor size > 2 cm and submucosal invasion. Similarly, careful evaluation for depth of invasion may be helpful in proximally located tumors because of the high risk of submucosal invasion.
The implementation of a nationwide screening program for gastric cancer and advancements in endoscopic instruments have increased the detection of gastric cancer in early stages in Korea and Japan [18]. ESD has less morbidity and provides better quality of life than surgical resection and has a good long-term outcome [3]. Therefore, ESD has been increasingly adopted as a first-line treatment for indicated cases of EGC. However, this has also led to the increased burden of non-curative ESD and additional surgery, which may result in higher medical costs and rare but serious adverse events such as perforation and massive bleeding after unnecessary ESD. This has been especially problematic for UD EGC because of a high risk of non-curative ESD.
The present study included 448 patients with UD EGC, making it, to our knowledge, the largest study of its kind. We found that large tumor size and proximal location were independent predictors of non-curative ESD. These results are consistent with the findings from previous studies based on differentiated-type EGC, which showed tumor size > 2 cm, upper-body tumor location, and ulcerative tumor as risk factors [14, 15, 19,20,21]. However, with the prior results, further prediction of curative ESD within UD EGC was not possible except for cardia tumors that had lower probability of curative resection than the others. We tailored the categories for those factors, thus allowing the development of a simple score that can stratify the probability of curative ESD of UD EGC. Although UD EGC is known to have a lower curative resection rate than differentiated-type EGC, our model suggested that the curative ESD rate was as high as 70% if the cancer was ≤ 1 cm in size and located in the antrum. Meanwhile, the rate was as low as 40% for the tumor that was 1–2 cm in size and located in the lower to mid body. These lesions with different risks of non-curative ESD were evaluated as having the same risk according to the previous studies. The novel point of our study would be the ability to differentiate their risks. Another new point of our study is that the risk was presented as an estimated rate. For example, the curative ESD rate was only 20% when the lesion was 1–2 cm in size and located in the upper body, cardia, or fundus. Currently, no consensus exists on the appropriate rate of curative ESD. Some patients may consider 70% as low, whereas others may still want to undergo ESD despite knowledge of a 20% curative resection rate. Nevertheless, our model can help in the decision-making and allow selecting between ESD and surgery based on more accurate information.
In our study, tumor size > 1 cm estimated with endoscopy before ESD was significantly associated with the risks of pathologic tumor size > 2 cm and consequent non-curative ESD. This result is consistent with previous studies reporting tumor size > 2–3 cm as a risk factor for non-curative ESD [14, 15, 19,20,21]. A previous study demonstrated a median discrepancy of 0.5 cm between the endoscopic size and pathologic size in endoscopic resection for EGC [22]. In this study, the undifferentiated-type histology was associated with a two-fold increased risk of endoscopic underestimation of tumor size. These findings explain our results that a 0.5–1 cm underestimation of tumor size in endoscopy may not cause a considerable risk of non-curative resection for tumors ≤ 1 cm in size, but increases the risk for tumors measuring 1–2 cm. We also showed that only 10% of the cases with endoscopic tumor size > 2 cm achieved curative ESD. Because these cases are not indicated for ESD, this scenario is possible only when endoscopy overestimates the tumor size. Although chromoendoscopy is useful in the demarcation of lateral margins of EGC, its accuracy was reported to be reduced in UD EGC [23]. Accordingly, demarcation biopsies from the surrounding mucosa have been suggested for determining the lateral extent of UD EGC [24]. A recent study suggested that magnifying endoscopy with NBI may improve the accuracy of demarcation of UD EGC [25]. Therefore, for UD EGC > 1 cm in diameter estimated before ESD, routine application of magnifying endoscopy with NBI or demarcation biopsy may be recommended to reduce unnecessary ESD.
Previous studies suggested that an upper-body tumor location was associated with an increased risk of non-curative ESD [14, 15, 20, 21]. Earlier studies attributed this result to the technical difficulty in recognizing or accessing lesions during ESD in that location [15, 21]. In the present study, we further observed a trend of increasing risk of non-curative ESD from the lower to upper third of the stomach. Moreover, we showed that the primary cause of the association was the increased risk of submucosal invasion. The increasing risk of submucosal invasion with increasing proximity of tumor location was also evident in the surgical validation cohort. Although EUS may be helpful in the evaluation of invasion depth of EGC before ESD, undifferentiated-type histology is associated with the risk of underestimating depth in EUS [26]. Therefore, careful evaluation for endoscopic findings for submucosal invasion such as irregular surface, marked marginal elevation, and fold fusion, clubbing, and abrupt cutting [27] may be warranted for proximally located tumors to increase the curative ESD rate and to select the appropriate treatment for UD EGC.
In the subgroup analyses, we assumed situations where the evaluation for tumor size and depth was highly accurate. The results suggested that, with increased accuracy of evaluation for tumor size and depth, the curative ESD rate might increase up to 20%, with 10% for each factor. Our model showed better prediction performance in the subgroup analyses than in the main analysis, indicating that our model would be more useful when tumor size and depth was evaluated more accurately. Although our analysis included patients with tumor size ≤ 5 cm, not 2 cm, the results of another subgroup analysis showed that our model can be directly applied in the usual clinical practice where only UD EGC with ≤ 2 cm in size is indicated for ESD.
Our study has multiple strengths. First, a large-scale nationwide multicenter ESD cohort enabled us to obtain a reliable sample size of patients diagnosed with UD EGC before ESD. Second, we were able to validate our prediction model in an independent cohort, which was lacking in previous studies. Third, we used statistical analyses to show why tumor size and location were independent predictors of curative ESD. On the basis of the results, possible approaches to increase curative ESD rates could be suggested. However, our study also had several limitations that need to be considered. First, this study was a retrospective analysis of patients with undifferentiated-type cancer as the final pathologic diagnosis. Therefore, we did not have data on patients diagnosed with undifferentiated-type cancer in forceps biopsy before ESD but diagnosed with differentiated-type cancer in the final pathology. A previous study reported that 14% (16/113) of the patients with an undifferentiated-type histology before ESD were diagnosed with differentiated EGC after ESD [9]. Second, the data on the depth of invasion were insufficient in the surgical validation cohort. This might have resulted in the higher risk of submucosal invasion and non-curative ESD in the validation cohort. Moreover, using a surgical cohort as a validation cohort might seem inappropriate because it was not possible to evaluate the risk of non-curative ESD associated with positive vertical or horizontal margins or piecemeal resection. However, only 5.1% (13/255) of non-curative resection in the ESD cohort was purely because of these factors, and the other non-curative cases had other non-curative factors that can be evaluated in the surgical cohort such as pathologic tumor size > 2 cm or submucosal invasion. Thus, the surgical cohort could cover 95% cases of non-curative ESD. In addition, we also conducted an internal validation using the bootstrapping method. Third, we lacked endoscopy findings such as fusion of folds, mucosal nodularity, and spontaneous bleeding, which have been suggested to be predictors of curative ESD of EGC [14]. However, decisions based on these findings may be affected by subjective judgement. Nevertheless, our findings are simpler and easier to apply during endoscopy. In addition, we conducted a subgroup analysis after excluding submucosal invasive EGC to overcome this limitation and showed that our model still worked fine for intramucosal EGCs only.
In conclusion, our six-score prediction model may help in predicting curative ESD and in making informed decisions in selecting between ESD and surgery for UD EGC. Tumors > 1 cm in size or with a proximal location may be further evaluated for size and depth of invasion for better prediction of curative ESD and for better selection of the appropriate treatment modality.
References
Guideline Committee of the Korean Gastric Cancer Association, Development Working Group, Review Panel (2019) Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer 19(1):1–48
Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20(1):1–19
Lee S, Choi KD, Han M, Na HK, Ahn JY, Jung KW, Lee JH, Kim DH, Song HJ, Lee GH, Yook JH, Kim BS, Jung HY (2018) Long-term outcomes of endoscopic submucosal dissection versus surgery in early gastric cancer meeting expanded indication including undifferentiated-type tumors: a criteria-based analysis. Gastric Cancer 21(3):490–499
Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, Sohn TS, Bae JM, Kim KM, Ahn JH, Carriere KC, Kim JJ, Kim S (2016) Long-term outcome of endoscopic resection vs. surgery for early gastric cancer: a non-inferiority-matched cohort study. Am J Gastroenterol 111(2):240–249
Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T (2009) Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 12(3):148–152
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y (2000) Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 3(4):219–225
Lee JH, Choi MG, Min BH, Noh JH, Sohn TS, Bae JM, Kim S (2012) Predictive factors for lymph node metastasis in patients with poorly differentiated early gastric cancer. Br J Surg 99(12):1688–1692
Oka S, Tanaka S, Higashiyama M, Numata N, Sanomura Y, Yoshida S, Arihiro K, Chayama K (2014) Clinical validity of the expanded criteria for endoscopic resection of undifferentiated-type early gastric cancer based on long-term outcomes. Surg Endosc 28(2):639–647
Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, Taniguchi H, Kushima R, Saito Y (2013) Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy 45(9):703–707
Lim JH, Kim J, Kim SG, Chung H (2019) Long-term clinical outcomes of endoscopic vs. surgical resection for early gastric cancer with undifferentiated histology. Surg Endosc 33(11):3589–3599
Park JC, Lee YK, Kim SY, Roh Y, Hahn KY, Shin SK, Lee SK, Lee YC, Kim HI, Cheong JH, Hyung WJ, Noh SH (2018) Long-term outcomes of endoscopic submucosal dissection in comparison to surgery in undifferentiated-type intramucosal gastric cancer using propensity score analysis. Surg Endosc 32(4):2046–2057
Ahn JY, Park HJ, Park YS, Lee JH, Choi KS, Jeong KW, Kim DH, Choi KD, Song HJ, Lee GH, Jung HY (2016) Endoscopic resection for undifferentiated-type early gastric cancer: immediate endoscopic outcomes and long-term survivals. Dig Dis Sci 61(4):1158–1164
Bang CS, Baik GH, Shin IS, Kim JB, Suk KT, Yoon JH, Kim YS, Kim DJ, Shin WG, Kim KH, Kim HY, Lim H, Kang HS, Kim JH, Kim JB, Jung SW, Kae SH, Jang HJ, Choi MH (2015) Endoscopic submucosal dissection for early gastric cancer with undifferentiated-type histology: a meta-analysis. World J Gastroenterol 21(19):6032–6043
Kim EH, Park JC, Song IJ, Kim YJ, Joh DH, Hahn KY, Lee YK, Kim HY, Chung H, Shin SK, Lee SK, Lee YC (2017) Prediction model for non-curative resection of endoscopic submucosal dissection in patients with early gastric cancer. Gastrointest Endosc 85(5):976–983
Hirasawa K, Kokawa A, Oka H, Yahara S, Sasaki T, Nozawa A, Morimoto M, Numata K, Taguri M, Morita S, Maeda S, Tanaka K (2011) Risk assessment chart for curability of early gastric cancer with endoscopic submucosal dissection. Gastrointest Endosc 74(6):1268–1275
Yang HJ, Kim SG, Lim JH, Choi JM, Oh S, Park JY, Han SJ, Kim J, Chung H, Jung HC (2018) Novel risk stratification for metachronous recurrence after curative endoscopic submucosal dissection for early gastric cancer. Gastrointest Endosc 87(2):419-428 e413
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14(2):101–112
Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, Jung KW, Lee CW, Choi IJ, Park EC, Lee D (2017) Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology 152(6):1319-1328 e1317
Ohara Y, Toshikuni N, Matsueda K, Mouri H, Yamamoto H (2016) The superficial elevated and depressed lesion type is an independent factor associated with non-curative endoscopic submucosal dissection for early gastric cancer. Surg Endosc 30(11):4880–4888
Kim YI, Kim HS, Kook MC, Cho SJ, Lee JY, Kim CG, Ryu KW, Kim YW, Choi IJ (2016) Discrepancy between clinical and final pathological evaluation findings in early gastric cancer patients treated with endoscopic submucosal dissection. J Gastric Cancer 16(1):34–42
Ohnita K, Isomoto H, Yamaguchi N, Fukuda E, Nakamura T, Nishiyama H, Mizuta Y, Akiyama M, Nakao K, Kohno S, Shikuwa S (2009) Factors related to the curability of early gastric cancer with endoscopic submucosal dissection. Surg Endosc 23(12):2713–2719
Shim CN, Song MK, Kang DR, Chung HS, Park JC, Lee H, Shin SK, Lee SK, Lee YC (2014) Size discrepancy between endoscopic size and pathologic size is not negligible in endoscopic resection for early gastric cancer. Surg Endosc 28(7):2199–2207
Lee BE, Kim GH, Park DY, Kim DH, Jeon TY, Park SB, You HS, Ryu DY, Kim DU, Song GA (2010) Acetic acid-indigo carmine chromoendoscopy for delineating early gastric cancers: its usefulness according to histological type. BMC Gastroenterol 10:97
Yao K, Nagahama T, Matsui T, Iwashita A (2013) Detection and characterization of early gastric cancer for curative endoscopic submucosal dissection. Dig Endosc 25(Suppl 1):44–54
Horiuchi Y, Fujisaki J, Yamamoto N, Shimizu T, Miyamoto Y, Tomida H, Omae M, Ishiyama A, Yoshio T, Hirasawa T, Yamamoto Y, Tsuchida T, Igarashi M, Takahashi H (2016) Accuracy of diagnostic demarcation of undifferentiated-type early gastric cancers for magnifying endoscopy with narrow-band imaging: endoscopic submucosal dissection cases. Gastric Cancer 19(2):515–523
Kuroki K, Oka S, Tanaka S, Yorita N, Hata K, Kotachi T, Boda T, Arihiro K, Chayama K (2020) Clinical significance of endoscopic ultrasonography in diagnosing invasion depth of early gastric cancer prior to endoscopic submucosal dissection. Gastric Cancer. https://doi.org/10.1007/s10120-020-01100-5
Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS (2011) Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc 73(5):917–927
Funding
This work was supported by the Korean College of Helicobacter and Upper Gastrointestinal Research (Woon Geon Shin in 2017 and Hyo-Joon Yang in 2018) and the National Research Foundation of Korea (2020R1G1A1010927).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Hyo-Joon Yang, Moon Kyung Joo, Jae Myung Park, Ji Yong Ahn, Jae-Young Jang, Joo Hyun Lim, Su Youn Nam, Jie-Hyun Kim, Byung-Hoon Min, Wan-Sik Lee, Bong Eun Lee, Woon Geon Shin, Hang Lak Lee, Tae-Geun Gweon, Moo In Park, Jeongmin Choi, Chung Hyun Tae, Young-Il Kim, Keun Won Ryu and Il Ju Choi have no conflicts of interest or financial ties to disclose.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, HJ., Joo, M.K., Park, J.M. et al. Prediction model for curative endoscopic submucosal dissection of undifferentiated-type early gastric cancer. Surg Endosc 36, 1414–1423 (2022). https://doi.org/10.1007/s00464-021-08426-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08426-w