Abstract
Background
Endoscopic resection (ER) is considered carefully as a curative treatment option for selected cases of undifferentiated-type early gastric cancer (UEGC). This study investigated immediate endoscopic and long-term survival outcomes of patients with UEGC treated with ER.
Methods
A review of a database of 2483 EGC consecutively enrolled patients who underwent ER between January 2004 and December 2010 identified 101 patients with UEGC who met the expanded indications. Outcomes were investigated in these patients.
Results
The rates of R0 en bloc and curative resection were 86 and 70 %, respectively. Of 30 tumors non-curatively resected, 17 were larger than 20 mm in diameter, 12 had positive resection margins, and 13 had submucosal or lymphovascular invasion on resection pathology. ER-related complications occurred in 12 patients (12 %), with all complications treated endoscopically without surgery. The median ER procedure time was 26 min [interquartile range (IQR) 20–39 min]. Only tumor location in the lower part of the stomach was significantly associated with curative ER (P = 0.038). Tumor recurrence was observed in seven patients at a median 17 months (IQR 12–47 months) after ER. During a median follow-up of 60 months (IQR 48–80 months), the 5-year overall mortality rates were 5 % in the curative and 4 % in the non-curative resection groups (P = 0.927). There were no gastric cancer-related deaths.
Conclusions
ER shows acceptable immediate endoscopic and long-term survival outcomes in selected patients with UEGC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Early gastric cancer (EGC) is defined as gastric cancer confined to the mucosa or submucosa, irrespective of regional lymph node metastasis [1]. Endoscopic resection (ER) is a primary curative treatment option for selected patients with EGC [2–4]. Currently, indications for ER include differentiated-type EGC confined to the mucosa with a diameter of <20 mm [5, 6].
Surgical gastrectomy is considered the gold-standard treatment for patients with undifferentiated-type EGC (UEGC) because the likelihood of lymph node metastasis is higher in patients with UEGC than in patients with differentiated-type EGC [7, 8]. However, previous surgical studies showed that the presence of lymph node metastasis was negligible in patients with UEGC, provided the lesion was intramucosal, smaller than 20 mm in diameter, ulcer-negative, and without lymphovascular invasion on final resection pathology (expanded indications) [9–11]. ER can be considered in these patients because of its minimal invasiveness, organ-saving results, and provision of good quality of life.
Although many studies have assessed clinical outcomes of ER in patients with differentiated-type EGC [12–15], less is known about the effects of ER in patients with UEGC. This study assessed the immediate endoscopic outcomes and long-term survival of patients with UEGC who underwent ER.
Methods
Patients
The review of a consecutively collected database of 2483 patients who underwent ER for EGC between January 2004 and December 2010 identified a total of 204 patients as having UEGC. Of these, 103 patients were excluded: 79 with tumors >20 mm in diameter, 6 with ulcerative tumors, 3 with synchronous gastric cancer, 9 who had undergone endoscopic treatment for EGC previously, and 6 who had undergone previous gastrectomy. Thus, 101 patients with UEGC who met the expanded indications endoscopically were included in this study. Tumors were evaluated prior to ER procedures by chromoendoscopy using indigo carmine, narrow band imaging, and/or circumferential mapping biopsies. Endoscopic ultrasonography was performed as needed to assess submucosal invasion of tumors. Each patient underwent contrast-enhanced computed tomography before ER to evaluate regional lymph node or distant metastases.
All patients were informed carefully about the risks and benefits of treatment with ER as well as laparoscopic or open surgical gastrectomy. Surgical gastrectomy is the standard treatment, which includes curative nodal dissection, whereas ER has the advantages of organ saving and minimal invasiveness. Written informed consent was provided by all patients for ER treatment, and this analysis was approved by the Institutional Review Board of Asan Medical Center.
Endoscopic Procedure
ER was performed using a single-channel endoscope (GIF-H260; Olympus Optical, Tokyo, Japan). Patients were sedated with intravenous midazolam (0.05 mg/kg) and pethidine (50 mg), and their cardiorespiratory functions were monitored closely during the procedure. After circumferential marking of a lesion, the submucosal layer was injected with normal saline containing epinephrine (0.01 mg/ml) mixed with indigo carmine, and then the lifted mucosa was incised circumferentially. For depressed lesions or tumors larger than 10 mm in diameter, endoscopic submucosal dissection was performed using a needle knife (MTW Endoskopie, Wesel, Germany), insulated-tip knife (IT knife; MTW Endoskopie), or IT knife 2 (Olympus Optical). For elevated lesions or tumors <10 mm in diameter, endoscopic mucosal resection was performed with a snare (SD-12U-1 or SD-9U-1; Olympus Optical) after circumferential incision. The UES-30 system (Olympus Medical System) or VIO 300D (ErbeElektromedizin, Tübingen, Germany) was used as the electrosurgical unit. All visible or bleeding vessels on the artificial ulcer were coagulated using hemostatic forceps (FD-410LR; Olympus Optical). A second-look endoscopy was performed 2 days after ER, and patients were discharged if no complications had occurred. All patients were administered a proton pump inhibitor for 4 weeks.
Histopathological Examination
The resected specimen was stretched, pinned to a polystyrene plate, and totally immersed in 10 % neutral buffered formalin. After fixation, the specimen was grossly examined to identify the lesion and closest resection margin. After applying black ink to all lateral and deep resection margins, the entire specimen was sectioned into 2-mm-thick slices parallel to an imaginary line drawn from the edge of the tumor to the closest resection margin. Each sliced tissue specimen was embedded in paraffin, and 5-μm sections were cut from each paraffin block and stained with hematoxylin and eosin. All samples were independently reviewed by two experienced gastrointestinal pathologists, with any discrepancies resolved by consensus.
Pathological Definitions
R0 en bloc resection was defined as the complete removal of the entire tumor both macroscopically and microscopically without any positive resection margins. Curative resection for UEGC was based on R0 resection and was defined as the resection of intramucosal tumors without ulceration, <20 mm in diameter, and complete absence of submucosal and lymphovascular invasion on microscopic examination [9]. Macroscopic types of EGC were classified according to the Japanese Gastric Cancer Association [6], and their degree of differentiation was classified as recommended by the World Health Organization [16].
Follow-Up Schedule
Local recurrence, defined as recurrent tumor at the resection site, was assessed by regular endoscopy 3, 6, and 12 months after ER. Metachronous recurrence was defined as tumors that developed at sites other than the primary resection site during follow-up endoscopy 1 year after ER. Patients underwent abdominal computed tomography every 6 months for the first 2 years and annually thereafter to detect regional and distant recurrences.
Statistical Analysis
Baseline variables are presented as number (percentage) and median [interquartile range (IQR)]. Continuous variables were compared using Student’s t tests, and categorical variables were compared using chi-square or Fisher’s exact tests. Univariate and multivariate analyses were performed to identify factors predicting curative resection. Patient survival was calculated using the Kaplan-Meier method and compared using the log-rank test. All P values were two sided, and a P value <0.05 was considered significant. All statistical analyses were performed using SPSS version 19 (SPSS Inc., Chicago, IL).
Results
Clinical and Endoscopic Features
The median age of the study patients was 57 years (IQR 46–64 years). Of the 101 UEGCs, 54 were poorly differentiated carcinomas, 26 were signet ring cell carcinomas, and 21 were undifferentiated carcinomas mixed with poorly differentiated and signet ring cells. On gross morphology, 69 tumors were depressed and 49 were located in the lower third of the stomach. The median tumor size on endoscopy was 15 mm (IQR 15–20 mm). Most of the tumors (91 %) were resected by endoscopic submucosal dissection. Nine patients with severe underlying diseases received ER treatment; these included four with cardiovascular diseases, two with cerebral vascular accidents, one with chronic renal failure, one with liver cirrhosis, and one with chronic obstructive pulmonary disease. The clinical and endoscopic features of the patients with UEGC are shown in Table 1.
Immediate Endoscopic Outcomes
ER pathology showed that 100 tumors (99 %) were resected en bloc, and 87 (86 %) were R0 en bloc resected. ER resulted in the curative resection of 71 tumors (70 %). Of the 30 tumors non-curatively resected, 17 were larger than 20 mm in diameter and 12 had positive resection margins. In addition, submucosal or lymphovascular invasion was observed in 13 tumors.
ER-related complications occurred in 12 patients (12 %), including post-procedural bleeding in 11 and perforation in 1. All complications were treated endoscopically without surgery. The median ER procedure time was 26 min (IQR 20–39 min). Table 2 summarizes the immediate ER outcomes in patients with UEGC.
Clinicopathological Factors Associated with Curative Resection of UEGC
Analysis of endoscopic and pathological factors associated with curative resection (Table 3) showed that only the tumor location was significantly associated with curative ER (P = 0.038). Other factors including macroscopic type, endoscopic tumor size, histology, and resection method were not related to curative resection. On univariate and multivariate analyses, however, the tumor location was not a significant predictor for curative resection.
Tumor Recurrence and Long-Term Survival
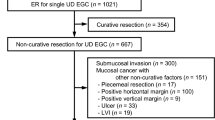
Figure 1 shows treatment flow and outcomes of ER patients with UEGC. Of the 71 patients who underwent curative resection, 65 were followed up without surgery and 6 underwent subsequent gastrectomy. During a median follow-up of 59 months (IQR 47–76 months), 3 of the 65 patients followed up without surgery experienced tumor recurrence. One patient experienced local recurrence 93 months after ER, but could not undergo gastrectomy because of severe underlying chronic obstructive pulmonary disease. He died of severe pulmonary dysfunction 8 months after local recurrence. Of the two patients who experienced metachronous recurrence, one was diagnosed 17 months after ER and treated with gastrectomy. Surgical pathology showed complete tumor resection without evidence of regional node metastasis. The second patient was diagnosed with differentiated-type EGC 13 months after ER and the lesion was treated curatively with ER. Surgical pathology of the six patients who underwent subsequent gastrectomy revealed that none had regional node metastasis.
Of the 30 non-curatively resected patients, 16 achieved R0 en bloc resection. Of these, three patients underwent subsequent gastrectomy, with their surgical specimens showing no remnant or metastatic tumors. Of the other 13 patients followed up without surgery, 4 experienced tumor recurrences at a median 60 months (IQR 49–87 months) after ER, including 1 with local and 3 with metachronous recurrence. The local recurrence was observed 47 months after ER, and the patient underwent curative gastrectomy. Surgical pathology revealed regional lymph node metastasis in 4 of 26 nodes. The three patients with metachronous recurrences presented with differentiated-type EGC and were treated endoscopically. Of these, two were curatively resected and one had a positive lateral margin that was ablated by argon plasma coagulation. Of the remaining 14 patients with non-curative resection, 12 underwent subsequent gastrectomy.
During a median follow-up of 60 months (IQR 48–80 months), 7 of the 101 patients (7 %) died (Table 4). However, none of these deaths was related with gastric cancer. The causes of death included severe infection in one patient, hepatocellular carcinoma in two, chronic obstructive pulmonary disease in one, acute complication of interventional angioplasty in one, cerebral hemorrhage in one, and drowning in one. The 5-year overall mortality rate of all patients was 5 % (5 % in patients curatively resected and 4 % in patients non-curatively resected; P = 0.927; Fig. 2).
Kaplan-Meier curve for analysis of overall mortality in the patients with undifferentiated-type early gastric cancer who underwent endoscopic resection. Five-year mortality rates were 4.8 % in all patients, 5.3 % in curatively resected patients and 3.7 % in non-curatively resected patients (P = 0.927)
Discussion
This study showed that ER achieved good immediate endoscopic and long-term survival outcomes in patients with UEGC who met the expanded indications. Of the 101 UEGC lesions, 71 (70 %) were curatively resected. ER-related complications occurred in 12 patients (12 %), all of whom were treated endoscopically without surgery. During a median follow-up of 60 months, the 5-year overall mortality rate was 5 %, and there were no gastric cancer-related deaths.
Lymph node metastasis is the most important prognostic factor for the cure of EGC using ER. The incidence of lymph node metastasis of EGC was reported to range from 3 to 20 %, and the risk of nodal metastasis was associated with tumor size, depth of tumor invasion, histological type, and the presence of lymphovascular invasion [9, 11, 17]. Previous surgical studies have attempted to define groups of patients without lymph node metastasis. A Japanese study reported that 1230 patients with intramucosal differentiated-type EGC with a diameter of <30 mm had no nodal metastases [9]. The overall risk of nodal metastases in patients with intramucosal UEGC was 4.2 %. However, nodal metastases were completely absent in the 141 patients with intramucosal UEGC, provided the lesions were <20 mm in diameter, without ulceration, and without histological evidence of lymphovascular invasion. A Korean surgical study reported that the risk of lymph node metastasis was 1.9 % (3/156) in patients with intramucosal UEGC <20 mm in diameter [11].
Several studies also investigated factors predictive of lymph node metastasis in patients with intramucosal UEGC [17–20]. They showed that tumor size >20 mm and the presence of lymphovascular invasion were significantly associated with the risk of lymph node metastasis. The incidence of lymph node metastasis ranged from 0.5 to 5.8 % in patients who had neither of these two risk factors, suggesting that ER could be considered for the cure of intramucosal UEGC in highly selected patients.
Immediate endoscopic outcomes of ER for the treatment of UEGC have been reported [21–26]. R0 resection was achieved in 79.3–90.7 % of cases. The rates of ER-related bleeding and perforation ranged from 1.4 to 13.8 % and 1.0 to 4.1 %, respectively, with most of these complications being treated endoscopically. In addition, the median procedure time was approximately 60 min. In our study, the R0 en bloc resection rate was 86 %, and manageable ER-related complications occurred in 12 % of cases. These data show that ER is technically feasible for UEGC lesions that meet the expanded indications.
Reportedly, curative resection rates of UEGC range from 63.9 to 82.5 %, which are relatively low compared with those of differentiated-type EGC [22, 24]. The main reasons for the comparatively low rate of non-curative resection of UEGC are positive resection margins and submucosal or lymphovascular tumor invasion on final resection pathology. UEGCs tend to show diffuse-type infiltration without exposure on the mucosal surface and progress laterally, replacing the glandular proliferative zone [27, 28]. Thus, pretreatment assessment of tumors is less accurate in patients with UEGC even when chromoendoscopy, narrow band imaging, or endoscopic ultrasonography is employed [29, 30]. Of the 101 patients in our study, 30 (30 %) underwent non-curative resection, including 17 with tumors >20 mm, 12 with positive resection margins, and 13 with submucosal or lymphovascular invasion. Analysis of endoscopic and pathological factors associated with curative resection showed that only the gastric location of tumors was a significant factor; curative resection was observed in 39/49 (80 %) tumors located in the lower third, 29/46 (63 %) in the middle third, and 3/6 (50 %) in the upper third of the stomach. The low rate of curative resection in the upper third of the stomach may be explained by the oblique view and procedural difficulty of resecting tumors located in this region. It may also be because minimally invasive ER of tumors in this region is preferred instead of total gastrectomy.
Several studies have reported long-term survival outcomes of ER treatment for UEGCs that meet the expanded indications [23–26]. Five-year overall mortality rates in curatively resected patients ranged from 3.9 to 7.0 %, with no patient dying of gastric cancer [23, 24]. In our study, the 5-year overall mortality rate was 5 % during a median follow-up of 60 months. In patients curatively resected, the 5-year mortality rate was 5 %, with no gastric cancer-related deaths. During long-term follow-up, however, two patients experienced local recurrence, one 93 months after curative ER and the other 47 months after non-curative ER. In addition, the metachronous recurrence rate was 6 %, suggesting that patients should be closely monitored for local and metachronous recurrences even after curative ER.
This study had several limitations inherent to its observational design and performance at a single tertiary center. In addition, endoscopic estimation of tumor size and invasion depth are relatively subjective and may depend on the experience of the endoscopist, which may influence immediate endoscopic outcomes. Because some patients did not undergo endoscopy or computed tomography because they were medically unfit for these procedures, recurrence rates and the effect of gastric cancer on long-term survival may have been underestimated.
In conclusion, ER showed acceptable short-term endoscopic and long-term survival outcomes for the treatment of UEGC. ER could be considered in selected patients with UEGCs that are intramucosal, <20 mm in diameter, and without lymphovascular invasion on resection pathology.
References
Carter KJ, Schaffer HA, Ritchie WP. Early gastric cancer. Ann Surg. 1984;199:604–609.
Rembacken BJ, Gotoda T, Fujii T, Axon AT. Endoscopic mucosal resection. Endoscopy. 2001;33:709–718.
Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11.
Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol.. 2005;23:4490–4498.
Shimada Y, JGCA (The Japan Gastric Cancer Association). Gastric cancer treatment guidelines. Jpn J Clin Oncol. 2004;34:58.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma, 2nd English edn. Gastric Cancer. 1998;1:10–24.
Yamao T, Shirao K, Ono H, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996;77:602–606.
Adachi Y, Mori M, Maehara Y, Kitano S, Sugimachi K. Prognostic factors of node-negative gastric carcinoma: univariate and multivariate analyses. J Am Coll Surg. 1997;184:373–377.
Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225.
Japanese Gastric Cancer Association. Japanese gastric cancer-treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–123.
Kwak CS, Lee HK, Cho SJ, et al. Analysis of clinicopathological factors associated with lymph node metastasis in early gastric cancer review of 2,137 cases. J Korean Cancer Assoc. 2000;32:674–681.
Yamaguchi N, Isomoto H, Fukuda E, et al. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009;80:173–181.
Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883.
Tanabe S, Ishido K, Higuchi K, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer. 2014;17:130–136.
Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485–493.
Travis WD, Brambilla E, Muller-Hermelink HK. World Health Organization Classification of tumors. Lyon: IARC; 2000.
Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–152.
Abe N, Watanabe T, Sugiyama M, et al. Endoscopic treatment or surgery for undifferentiated early gastric cancer? Am J Surg. 2004;188:181–184.
Li C, Kim S, Lai JF, et al. Risk factors for lymph node metastasis in undifferentiated early gastric cancer. Ann Surg Oncol. 2008;15:764–769.
Park YD, Chung YJ, Chung HY, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy. 2008;40:7–10.
Kim JH, Lee YC, Kim H, et al. Endoscopic resection for undifferentiated early gastric cancer. Gastrointest Endosc. 2009;69:e1–e9.
Yamamoto Y, Fujisaki J, Hirasawa T, et al. Therapeutic outcomes of endoscopic submucosal dissection of undifferentiated-type intramucosal gastric cancer without ulceration and preoperatively diagnosed as 20 millimetres or less in diameter. Dig Endosc. 2010;22:112–118.
Okada K, Fujisaki J, Yoshida T, et al. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44:122–127.
Abe S, Oda I, Suzuki H, et al. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703–707.
Kim YY, Jeon SW, Kim J, et al. Endoscopic submucosal dissection for early gastric cancer with undifferentiated histology: could we extend the criteria beyond? Surg Endosc. 2013;27:4656–4662.
Oka S, Tanaka S, Higashiyama M, et al. Clinical validity of the expanded criteria for endoscopic resection of undifferentiated-type early gastric cancer based on long-term outcomes. Surg Endosc. 2014;28:639–647.
Ninomiya Y, Yanagisawa A, Kato Y, Tomimatsu H. Unrecognizable intramucosal spread of diffuse-type mucosal gastric carcinomas of less than 20 mm in size. Endoscopy. 2000;32:604–608.
Sawada S, Fujisaki J, Yamamoto N, et al. Expansion of indications for endoscopic treatment of undifferentiated mucosal gastric cancer: analysis of intramucosal spread in resected specimens. Dig Dis Sci. 2010;55:1376–1380.
Hizawa K, Iwai K, Esaki M, Matsumoto T, Suekane H, Iida M. Is endoscopic ultrasonography indispensable in assessing the appropriateness of endoscopic resection for gastric cancer? Endoscopy. 2002;34:973–978.
Kim JH, Song KS, Youn YH, et al. Clinicopathologic factors influence accurate endosonographic assessment for early gastric cancer. Gastrointest Endosc. 2007;66:901–908.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Ji Yong Ahn and Hee Jung Park have contributed equally to this work as co-first authors.
Rights and permissions
About this article
Cite this article
Ahn, J.Y., Park, H.J., Park, Y.S. et al. Endoscopic Resection for Undifferentiated-Type Early Gastric Cancer: Immediate Endoscopic Outcomes and Long-Term Survivals. Dig Dis Sci 61, 1158–1164 (2016). https://doi.org/10.1007/s10620-015-3988-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3988-y