Abstract
Background and Aim
Although successful endoscopic resection of gastric subepithelial tumors (SETs) originating from the muscularis propria (MP) layer has been frequently reported, it requires a relatively complicated technique and has a high perforation rate. In this retrospective study, we evaluated the efficacy and safety of the snare-assisted endoscopic resection (SAER) method which is performed using a snare and insulated-tip (IT) knife via a single-channel endoscope to reduce the perforation rate.
Methods
In this study, fifty-six patients with gastric SETs originating from the MP layer treated by the SAER method at three institutions between July 2017 and December 2017 were reviewed. The procedure involved multiple steps as shown in Fig. 2. Data were obtained on demographics, SET features, histopathological diagnoses, procedure time, en bloc resection rate, R0 resection (negative margins) status, and adverse events.
Results
Endoscopic resection was successfully performed in all patients. The median overall procedure time was 43.5 min (range 26–106 min). The mean size of resected specimens was 19.73 mm (range 10–33 mm). The overall rate of en bloc resection was 96.4% (54/56). In addition, the perforation rate was 7.1% (4/56), and defects in the stomach wall were very small and easily closed using metallic clips. No postprocedural bleeding occurred in any case.
Conclusions
The SAER method is an effective, safe, less costly technique for the removal of some gastric SETs originating from the MP layer with an appropriate size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric subepithelial tumors (SETs) are often diagnosed during an upper alimentary endoscopy examination [1]. Although many SETs are benign, a large number of SETs originating from the muscularis propria (MP) layer are gastrointestinal stromal tumors (GISTs) which are considered potentially malignant [2, 3]. Endoscopic ultrasound (EUS) is increasingly being used to assess SETs. However, the accuracy of EUS alone in the diagnosis of gastric SETs is low [4, 5]. Thus, an accurate histological diagnosis can only be acquired by complete or partial removal of SETs. Therefore, endoscopic resection was suggested to provide a histological diagnosis [5, 6].
Recently, successful treatment of many SET cases with endoscopic resection has been reported. However, this procedure is associated with several serious complications, such as perforation, bleeding, and abdominal infection. Higher perforation rates (up to 50%) have been frequently reported for endoscopic resection for SETs in the MP layer [7, 8]. Although numerous useful methods have been reported to successfully close gastric perforations [8,9,10,11], postresection bleeding around the defects might not be easily avoided because there are numerous blood vessels (including arteries) that are separated outside the serosa of the stomach. Once a perforation occurs, a patient will most likely suffer hemorrhagic shock or even death if curative treatment is not performed in a timely manner. For this reason, a safe, simple, less costly procedure that could maximally reduce the gastric perforation rate is most desirable.
In this study, we report our experience regarding the efficacy and safety of snare-assisted endoscopic resection (SAER) of gastric SETs using a snare and insulated-tip (IT) knife via a single-channel endoscope with a low perforation rate.
Methods
Patients
We retrospectively analyzed 56 patients with gastric SETs originating from the MP layer in three institutions between July 2017 and December 2017. Computed tomography scans and EUS were routinely performed to evaluate the origin of the SETs before SAER. The exclusion criteria were as follows: (1) inability to provide informed consent for the procedure; (2) more than half of the body of the SET was extraluminal; (3) the width of the SET exceeded 3.0 cm; (4) the SET exhibited any high-risk EUS features, such as bleeding, ulceration, irregular border, cystic spaces, or heterogeneity; (5) inability to tolerate anesthesia with tracheal intubation; and (6) the presence of known blood coagulation disorders before the procedure.
The research was approved by the Ethics Committee of General Hospital of Southern Theater Command of PLA, the First Affiliated Hospital of Soochow University, and the Clifford Hospital in accordance with the Helsinki Declaration. All patients chose their therapeutic course voluntarily, and written informed consent was obtained from each patient.
Definitions
The procedure time was defined as the time interval between mucosal marking and wound repair. The tumor size was defined as the width diameter of the tumor. En bloc resection was defined as the resection of the entire tumor in one piece. R0 resection was defined as the removal of the entire tumor in one piece with no histopathological evidence of the tumor at the resection margins. Perforation was defined as the visualization of extramural organ or fat tissue or observation of free air on abdominal radiography. Significant bleeding was defined on the onset of clinical symptoms, such as hematemesis or melena, or based on a decrease > 20 g/L in hemoglobin level after the SAER procedure.
SAER method
The SAER method was a multistep process as follows (Figs. 1 and 2): (1) the border of SETs was marked using either hemostatic forceps (FD-410LR, Olympus, Tokyo, Japan) or an electrosurgical knife (DualKnife KD-650L, Olympus, Tokyo, Japan; or O-Type HybridKnife®, ERBE, Tuebingen, German); (2) saline containing indigo carmine and epinephrine was injected into the submucosa around the tumor; (3) a circumferential incision of the overlying mucosa of the lesion was made using either a DualKnife, ITknife nano (KD-612L, Olympus, Tokyo, Japan) or O-Type HybridKnife® (thus, the head of the tumor should be visible); (4) after a snare (SD-210U-25, Olympus, Tokyo, Japan) that was tightened on the distal end of the endoscope had been delivered through the cardia of the stomach, the snare was loosened and pushed into the gastric cavity; (5) with a retractable metallic clip (ROCC-D-26-195-C, Micro-Tech, Nanjing, China) and endoscope guidance, the snare was placed around the overlying mucosa of the tumor and tightened; (6) with gentle manual traction from the snare with the help of an assistant, the exposed tumor could be easily dissected away from the MP layer with an ITknife nano or O-Type HybridKnife®; and (7) finally, the wound was carefully evaluated to determine whether there was any residual tumor or perforation before the wound was fixed with several metallic clips. The resected specimen was retrieved for histopathological diagnosis and assessment of R0 resection status.
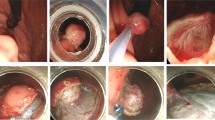
Endoscopic view of endoscopic resection of gastric SET with the SAER method. A Endoscopic view of the tumor in the corpus from the oral side. B The tumor was confirmed to originate from the MP layer by EUS. C Computed tomography view of the tumor. D Circumferential incision of the overlying mucosa of the tumor with a DualKnife. E A snare was tightened on the distal end of the endoscope. F After delivery through the cardia of the stomach by the endoscope, the snare was loosened and pushed into the gastric cavity. G The snare was placed around the overlying mucosa of the lesion with the guidance of a retractable metallic clip. H Under traction using the snare, the tumor was dissected with an IT knife nano. I The wound after resection. J The wound was fixed with several metallic clips. K The resected specimen
Schema view of endoscopic resection of gastric SET with the SAER method. A Side view of a gastric SET originating from the MP layer. B Saline containing indigo carmine and epinephrine was injected into the submucosa around the lesion. C Circumferential incision of the overlying mucosa of the tumor. D The snare was placed around the overlying mucosa of the tumor through the guidance of a retractable metallic clip and endoscope: D-1 In SET located in the greater curvature of the gastric fundus, the distal tip of the snare was placed on the oral side of the overlying mucosa. D-2 In SET located in the lesser curvature of the gastric corpus, the distal tip of the snare was placed on the anal side of the overlying mucosa. E When dragging the SET toward the intraluminal region through the snare, the tumor could be easily dissected away from the MP layer with an IT knife. F The tumor was resected without perforation. G The wound was fixed with several metallic clips
Results
The characteristics of the 56 patients included are summarized in Table 1. The mean patient age was 52 years (range 26–74 years). The mean tumor size as detected by EUS was 17.05 mm (range 8–28 mm). Tumors were located in the fundus (n = 32), corpus (n = 23), and antrum (n = 1).
Endoscopic resection was successfully performed in all patients. The median overall procedure time was 43.5 min (range 26–106 min). The mean size of the resected specimen was 19.73 mm (range 10–33 mm). The overall rate of en bloc resection was 96.4% (54/56). En bloc resection was not achieved in two cases of leiomyoma (both with lobular growth patterns). However, the residual leiomyoma was resected in a later procedure.
Postresection histopathological diagnoses of all SETs included GISTs (n = 46) and leiomyomas (n = 10). The high-power field (HPF) mitotic counts of all resected GISTs were low (< 5 mitotic figures/50 HPFs). All patients with GISTs were classified as low risk or extremely low risk according to the National Comprehensive Cancer Network (NCCN) guidelines.
Perforation occurred in four cases (7.1%); three tumors were located in the fundus and one tumor was located in the corpus. This complication occurred in two cases of GIST (both > 3 cm in length and width) and two cases of leiomyoma (both had lobular growth patterns). In all patients, defects in the stomach wall were very small and could easily be closed using metallic clips. In the remaining 52 patients, varying degrees of defects were detected in the MP layer only.
No major procedure-related significant bleeding that required a transfusion occurred. Minor bleeding during endoscopic resection was common and was successfully managed with hemostatic forceps in all cases. No delayed bleeding occurred in any case. The median duration of hospital stay after the procedure of all patients was 2.82 days (range 2–5 days).
All patients underwent follow-up endoscopy. The median time to follow-up endoscopy was 13.4 months (range 10.4–15.9 months). During the follow-up, no early- or late-onset side effects (bleeding, peritonitis, or abdominal abscess) were observed in any patient. Tumor recurrence was not detected in any patient.
Discussion
An increasing number of gastric SETs have been incidentally detected during routine upper gastrointestinal endoscopies. SETs include a wide spectrum of benign and malignant conditions such as GISTs, leiomyomas, cysts, granular-cell tumors, aberrant pancreas, fibroma, lipoma, and gastric cancer. Different from SETs located in the second or third layer, almost all SETs characterized by a hypoechoic appearance originating from the MP layer with a regular border are GISTs and leiomyomas. EUS is considered highly valuable in the evaluation of gastric SETs, especially in distinguishing invasive GISTs with irregular extraluminal borders, cystic areas, echogenic foci, and sizes greater than 30 mm. However, EUS imaging alone has low accuracy in the diagnosis of gastric SETs; the behavior of a GIST with a size less than 40 mm that contains only 1 or 2 distinguishing EUS features is difficult to predict [5, 12, 13].
The most common gastric SETs originating from the MP layer are GISTs, and GISTs are considered potentially malignant. Therefore, obtaining a tissue diagnosis is necessary to evaluate the malignant potential of SETs. The reported success rates of accurate classification with EUS-guided biopsies are variable. However, according to the most previous studies, histological tissue sampling cannot be reliably achieved by endoscopic or endosonographic puncture techniques, including EUS-guided fine needle aspiration and trucut biopsy [1, 13,14,15,16,17,18]. Therefore, diagnostic and therapeutic endoscopic resection of gastric SETs may be the best approach to guide treatment protocols.
In recent years, many successful methods that focus on endoscopic resection of gastric SETs have been reported. However, perforation rates remained high for endoscopic resection of SETs in the MP layer [7, 8, 19]. Therefore, a variety of techniques were adopted to repair the perforations in these cases to avoid conversion from endoscopic resection to surgery [6, 10, 11, 19]. Although minimal postresection abdominal bleeding was reported, a lower perforation rate will obviously lead to a lower abdominal bleeding rate.
In the present study, the SAER method was associated with a relatively low perforation rate. Furthermore, high rates of en bloc resection and R0 resection were achieved, and no tumor recurrence occurred during the short-term follow-up. Compared with other endoscopic resection methods, the SAER method has several advantages. First, the dissection plane can be easily visualized through snare traction, which provides more accurate identification of the cutting line and less inadvertent cutting of submucosal vessels and the MP layer that can result in bleeding and perforation. Second, less “push cut” is needed in the dissection procedure. “Push cut” is typically adopted in conventional endoscopic resection methods to expose the border of gastric SETs originating from the MP layer. However, perforation or even damage to adjacent organs frequently occurs under these circumstances. In the SAER method, SETs are dragged toward the intraluminal region, reducing the likelihood of perforation and damage in adjacent organs. Third, compared with the traction method using dental floss and a hemoclip, the SAER method can provide tighter traction force on gastric SETs with a snare to ensure successful dissection. In addition, a snare tightened on a tumor can prevent dissected gastric SETs from falling into the peritoneal cavity when perforation occurs during endoscopic resection. Finally, the traction materials utilized in the SAER method, such as a snare and a retractable metallic clip, are commonly available and inexpensive. Placement of the snare on the overlying mucosa of SETs was easily achieved. Furthermore, the retractable metallic clip could also be used to fix the wound.
Nonetheless, this method has several potential limitations. First, this method might not be suitable for gastric SETs located in the gastric cardia. When dissecting SETs located in the gastric cardia, a small space in the gastric cardia limits the performance of this method. Second, the direction of the traction can be provided on the oral side only. However, in most cases, this traction direction is sufficient for resection of SETs located in the fundus and the corpus (98.2% in total in this study). We easily accomplished the dissection procedure with this method for only one tumor located in the antrum. Third, perforation cannot be completely avoided in all cases. In this study, the total perforation rate was 7.14%; perforations occurred in GISTs > 3 cm in length and width and in leiomyomas with lobular growth patterns. Dissection of gastric SETs with a wide base will cause a wide defect in the stomach wall, likely with a greater perforation rate. However, further investigation is needed to explore the risk factors associated with the perforation rate when using this method.
This study has several limitations. First, this study was retrospective in nature. Next, the number of subjects enrolled was relatively small. Furthermore, a multicenter randomized controlled trial comparing endoscopic resection of gastric SETs originating from the MP layer with and without the SAER method is necessary.
In conclusion, the SAER method we applied to the endoscopic resection procedure for gastric SETs originating from the MP layer was safe, effective, inexpensive, and ensured high curability and a low perforation rate. Therefore, the use of the SAER method may be preferable as part of the standard procedure for the removal of some gastric SETs originating from the MP layer with an appropriate size.
Abbreviations
- SAER:
-
Snare-assisted endoscopic resection
- SETs:
-
Subepithelial tumors
- MP:
-
Muscularis propria
- IT:
-
Insulated-tip
- GISTs:
-
Gastrointestinal stromal tumors
- EUS:
-
Endoscopic ultrasound
- HPF:
-
High-power field
- NCCN:
-
National Comprehensive Cancer Network
References
Hedenbro JL, Ekelund M, Wetterberg P (1991) Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc 5:20–23
Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, Motomura Y, Honda K, Watanabe M, Nagaie T (2007) Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol 13:2077–2082
Miettinen M, Sobin LH, Lasota J (2005) Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 29:52–68
Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB (2005) A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc 62:202–208
Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W (2010) Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc 71:722–727
Schlag C, Wilhelm D, von Delius S, Feussner H, Meining A (2013) EndoResect study: endoscopic full-thickness resection of gastric subepithelial tumors. Endoscopy 45:4–11
Jeong ID, Jung SW, Bang SJ, Shin JW, Park NH, Kim DH (2011) Endoscopic enucleation for gastric subepithelial tumors originating in the muscularis propria layer. Surg Endosc 25:468–474
Zhang Y, Ye LP, Zhou XB, Mao XL, Zhu LH, He BL, Huang Q (2013) Safety and efficacy of endoscopic excavation for gastric subepithelial tumors originating from the muscularis propria layer: results from a large study in China. J Clin Gastroenterol 47:689–694
Al-Bawardy B, Rajan E, Wong Kee Song LM (2017) Over-the-scope clip-assisted endoscopic full-thickness resection of epithelial and subepithelial GI lesions. Gastrointest Endosc 85:1087–1092
Shi D, Li R, Chen W, Zhang D, Zhang L, Guo R, Yao P, Wu X (2017) Application of novel endoloops to close the defects resulted from endoscopic full-thickness resection with single-channel gastroscope: a multicenter study. Surg Endosc 31:837–842
Liu BR, Song JT, Qu B, Wen JF, Yin JB, Liu W (2012) Endoscopic muscularis dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria. Surg Endosc 26:3141–3148
Franquemont DW (1995) Differentiation and risk assessment of gastrointestinal stromal tumors. Am J Clin Pathol 103:41–47
Karaca C, Daglilar ES, Soyer OM, Gulluoglu M, Brugge WR (2017) Endoscopic submucosal resection of gastric subepithelial lesions smaller than 20 mm: a comparison of saline solution-assisted snare and cap band mucosectomy techniques. Gastrointest Endosc 85:956–962
Polkowski M, Gerke W, Jarosz D, Nasierowska-Guttmejer A, Rutkowski P, Nowecki ZI, Ruka W, Regula J, Butruk E (2009) Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: a prospective study. Endoscopy 41:329–334
Hunt GC, Smith PP, Faigel DO (2003) Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc 57:68–72
Cantor MJ, Davila RE, Faigel DO (2006) Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc 64:29–34
Hoda KM, Rodriguez SA, Faigel DO (2009) EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc 69:1218–1223
Philipper M, Hollerbach S, Gabbert HE, Heikaus S, Bocking A, Pomjanski N, Neuhaus H, Frieling T, Schumacher B (2010) Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy 42:300–305
Schmidt A, Bauder M, Riecken B, von Renteln D, Muehleisen H, Caca K (2015) Endoscopic full-thickness resection of gastric subepithelial tumors: a single-center series. Endoscopy 47:154–158
Acknowledgements
We sincerely thank the staff of the endoscopy unit at General Hospital of Southern Theater Command of PLA, the First Affiliated Hospital of Soochow University, and the Clifford Hospital for their excellent technical assistance in conducting this study.
Funding
This study was supported by combined grants from the National Natural Science Foundation of China (No. 81602170), the Natural Science Foundation of Guangdong province (No. 2014A030313595), the Science and Technology Plan Project of Guangzhou City (Nos. 201804010039 and 201607010077), and the Youth Medical Key Talent Project of Jiangsu province (No. QNRC2016705).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Hongwu Zhu, Dongtao Shi, Hong Song, Meihua Zhou, Dayong Sun, Rui Li, and Yagang Zhao have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, H., Shi, D., Song, H. et al. Snare-assisted endoscopic resection of gastric subepithelial tumors originating from the muscularis propria layer: a multicenter study. Surg Endosc 34, 3827–3832 (2020). https://doi.org/10.1007/s00464-019-07147-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-07147-5