Abstract
Background
Gastric subepithelial tumors originating from muscularis propria (MP) are usually benign, but some have malignant potential.
Aims
The aim of this study was to evaluate the utility of endoscopic enucleation for the diagnosis and treatment of MP tumors.
Patients and Methods
From January 2010 to February 2018, eighty patients with gastric MP tumors underwent endoscopic enucleation at our hospital. Band ligation and resection (BLR) or endoscopic muscularis resection (EMD) was performed based on considerations of tumor size (≤ 12 mm or > 12 mm). Tumor characteristics, procedure times, complete resection rates, adverse events and recurrence were analyzed.
Results
Eighty patients with 82 lesions were eligible for inclusion in this study. BLR was used to treat 41 lesions. For these lesions, mean tumor size was 9.5 mm, median procedural time was 17.6 min (range 4–52), and the endoscopic complete resection rate was 100% (41/41). Perforation was developed in four patients, and was closed by endoscopic clipping. EMD was used to treat 41 lesions. Median procedure time was 66.1 min (range 12–260) and the endoscopic complete resection rate was 85.4% (35/41). Perforation occurred in eight patients, four patients received endoscopic treatment and four underwent surgery. Tumor recurrence was not observed in any patient over follow-up (mean 26.3 months).
Conclusion
Endoscopic enucleation appears to offer an effective, relatively safe means for diagnosing and treating gastric subepithelial tumors originating from the MP, and BLR provides a straightforward, effective, and relatively safe treatment for small MP tumors (≤ 12 mm).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Subepithelial tumors (SET) of the stomach are mostly asymptomatic and are found incidentally during routine endoscopy; for routine upper endoscopy, the incidence of SET is about 0.36–0.76% [1,2,3]. Although most SETs are considered benign, some tumors, especially those originating from the muscularis propria (MP) layer, have malignant potential. Furthermore, because the preoperative diagnosis and assessment of malignant potential are difficult, selecting an optimal management strategy is difficult. For hypoechoic MP tumors, the most important point is that gastrointestinal stromal tumor (GIST) be differentiated from benign tumors because of its uncertain malignant potential [4]. Although endoscopic ultrasound (EUS) provides good visualization of MP tumors, it does not enable the differentiation of GIST and other mesenchymal tumors, such as, leiomyoma and schwannoma.
Small hypoechoic MP tumors (< 20 mm) are generally followed up until their size is greater than 20 mm even if optimal management remains controversial [5,6,7], because small GISTs (< 20 mm) have a low risk of malignancy and metastasis, and other benign diseases, such as, leiomyoma and schwannoma, can be followed-up. However, endoscopic surveillance has its limitations. First, in the absence of a definitive diagnosis, regular lifelong follow-ups are needed, and this imposes tremendous emotional and economic burdens on patients, who can become preoccupied with the risk of malignancy. Second, if an MP tumor increases in size during follow-up, the opportunity for less invasive treatment, such as endoscopic treatment, might be missed. Third, small GISTs (< 20 mm) do not always adopt a benign course.
A definitive diagnosis of MP tumor can only be made based on the immunochemical staining findings of tissue specimens. Several tissue sampling techniques have been developed, including bite-on-bite biopsy, EUS guided fine needle aspiration (EUS-FNA) and EUS-guided core needle biopsy [3]. However, these techniques are not always sufficient for diagnosis. Endoscopic biopsies are frequently non-diagnostic [8] and EUS-FNA is preferred, but it is difficult to perform and diagnostic yields are low when tumors are small [9]. Even when GIST is diagnosed using such methods, they exhibited a wide variety of clinical behaviors that range from benign to malignant. In fact, 10–30% of all GISTs are malignant and the malignant features can be predicted by tumor size, mitotic index, cellularity and proliferation index [10, 11]. Therefore, en bloc specimens are needed to accurately assess the biologic behavior of GISTs.
In the past, surgical intervention was the standard treatment for MP tumors, and endoscopy was used only for diagnosis, because endoscopic treatment was associated with risks of positive margins, tumor spillage and perforation [7, 12]. However, recent studies have demonstrated endoscopic resection of MP tumors has very low recurrence rates and reasonable complication rates [13,14,15,16], and thus, based on these results and the less invasive nature of endoscopic treatment, it has been suggested it may provide a better means of treating small MP tumors [16]. Therefore, we undertook to respectively evaluate the utility of endoscopic enucleation for the diagnosis and treatment of SETs originating from muscularis propria.
Patients and Method
Patients
The medical records of 80 patients (82 lesions) with a gastric SET originating from the MP layer treated at Inha University hospital between January 2010 and February 2018 were retrospectively reviewed. SETs arising from submucosal or muscularis mucosa layers were excluded. Charts were reviewed for clinical characteristics, endoscopic and EUS findings, clinical outcomes, and follow-up events. Informed consent for endoscopic enucleation was obtained from all patients before the procedure, and this study was approved by the institutional review board of Inha University Hospital (2014-01-102).
Endoscopic Procedure
Before endoscopic resection, all patients underwent endoscopic ultrasound (GF-UC240P-AL5, Olympus Optical Co, Tokyo, Japan) to determine the layers of origin and accurate tumor sizes. All lesions included in this study were well circumscribed, hypoechoic, homogeneous masses originating in gastric MP. All procedures were performed by one of three experienced endoscopists (H.K, Kim, Y.W. Shin, and B.W. Bang) on an inpatient basis under conscious sedation.
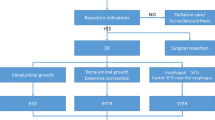
Endoscopic methods were chosen based on considerations of tumor size (> 12 mm or ≤ 12 mm) because only MP tumors with a diameter of < 12 mm could be managed using the band device. Band ligation and resection (BLR) was performed using a video endoscope (GIF-Q260J, Olympus, Tokyo, Japan) and a transparent ligator cap (Olympus Optical Co., Tokyo, Japan) to treat small MP tumors (≤ 12 mm).
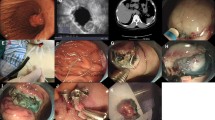
BLR was performed as follows (Fig. 1a). After the target lesion had been identified, the lesion was fully aspirated into the transparent cap, and the band was then released to ligate the lesion fully. Endoscopic resection was performed using a polypectomy snare with electrocoagulation below the elastic band. When there was concern of delayed perforation or bleeding, several hemoclips (Olympus, Tokyo) were applied to the stump. Resected specimens were then withdrawn for pathological diagnosis.
a Band ligation and resection. When a subepithelial tumor was encountered, the lesion was sucked into the transparent cap and ligated with a rubber band. The mass below the band was removed using a polypectomy snare. b Endoscopic muscular dissection (EMD). The target lesion was marked by argon plasma coagulator. After submucosal saline injection as in conventional endoscopic submucosal dissection, incision and dissection were performed around the lesion. Subsequently, the tumor and muscularis propria layer were separated using various knives
When an MP tumor was more than 12 mm or could not aspirate to cap, Endoscopic muscularis dissection (EMD) was performed (Fig. 1b) [17]. Briefly, marker dots were placed around the target lesion using an argon plasma coagulator (ERBE Elektromedizin GmbH, Tübingen, Germany). After injecting saline submucosally, mucosa was incised and dissected using a flex knife (KD-630L, Olympus, Tokyo, Japan) and an IT-2 knife (KD-611L, Olympus, Tokyo, Japan) to expose the MP tumor. Submucosal tissue beneath the lesion was then gradually dissected and the MP layer was peeled from the edge of the tumor using various knives. When the lesion had been resected completely from the MP layer, the resection site was observed carefully to check for any residual tumor or perforation. If perforation was suspected, it was closed endoscopically using clips. If necessary, several hemoclips were placed in the enucleation sites to reduce the risk of delayed perforation. If perforation occurred, it was occluded using clips, and second-look endoscopy was performed the day after the procedure to check for complications such as bleeding and perforation.
Follow-Up Evaluation
All patients underwent endoscopy 3–6 months after the procedure. Patients diagnosed with a benign tumor were not recommended for regular follow-up, but were recommended for upper endoscopy every 2 years as provided by the Korean National Cancer Screening Program. Patients with GIST were recommended to undergo abdominal computed tomography (CT) and upper endoscopy annually. GIST patients enrolled in the present study not followed at our institute were contacted by telephone and asked when they last underwent an endoscopic examination and whether they had experienced recurrence.
Statistical Analyses
Categorical data was compared using the Chi squared test or Fisher’s exact test and continuous data using the t test. Non-normally distributed data were analyzed using the Mann–Whitney U-test. Statistical significance was accepted for p values < 0.05 and the analysis was conducted SPSS ver. 18.0 (SPSS Inc., Chicago, IL).
Results
Characteristics of Patients and Tumors
Eighty patients with 82 gastric SETs originating in gastric MP were enrolled in this study. Patient baseline characteristics are summarized in Table 1. There were 30 men (36.6%) and 50 women (63.4%) of overall mean age 51.9 ± 10.4 years. Mean tumor size was 14.9 ± 9.2 mm (range 5–40 mm). Histologic diagnoses were leiomyoma (n = 42, 51.2%), GIST (n = 35, 42.7%), schwannoma (n = 2, 2.4%) and other tumors (n = 3, 3.7%)). The most commonly affected site was the gastric body followed by fundus and the antrum was least affected (1.2%, 1/82).
Endoscopic Treatment Outcomes and Adverse Events
BLR was used to treat 41 lesions. For these lesions, mean tumor size was 9.2 ± 2.4 mm and median procedural time was 12.0 min (range 4–52). Endoscopic complete resection and en bloc resection rates were 100% (41/41) and 80.8% (35/41), respectively (Table 2). MP tumors were not completely removed by BLR in six patients, and remnant tumors were completely removed by additional BLR (n = 1) or EMD (n = 5). The histologic complete resection rate was 78.0% (30/41). Perforation occurred in 4 patients (9.8% 4/41); all were treated by endoscopic clipping without serious complication.
EMD was used to treat 41 lesions. For these lesions, mean tumor size was 20.3 ± 9.1 mm and median procedural time was 46 min (range 12–260). Endoscopic complete resection and en bloc resection rates were 85.4% (35/41) and 75.6% (31/41), respectively. However, the histologic complete resection rate was only 48.8% (20/41). Perforation occurred in 8 patients (18.5%, 8/41). Four of the 8 were treated successfully using endoscopic hemoclips. However, the remaining four patients received laparoscopic wedge resection (n = 3) or primary closure (n = 1) because of incomplete tumor resection (n = 3) or incomplete endoscopic closure (n = 1).
During follow-up (mean 26.3 months, range 1–89 months), no patients with an MP tumor experienced local recurrence or metastasis.
Tumor Characteristics and Endoscopic Outcomes According to Tumor Location
Table 3 provides details of tumor characteristics and clinical outcomes according to tumor location. Almost all hypoechoic MP layer tumors arising in cardia were leiomyomas (22/26, 84.6%) and GIST was uncommon (4/26, 15.3%). Tumors in cardia were larger and had longer procedural time than non-cardia tumors (p = 0.059). However, endoscopic and histologic complete resection and en bloc resection rates were no different between cardia and non-cardia tumor.
Clinical Outcomes and Follow-Up Finding of Patients with GIST
GIST was diagnosed in 35 patients (Table 4). According to Fletcher’s classification [18], 24 patients were classified to be at very low risk (68.6%), 6 were at low risk (17.1%) for a malignancy, and 5 patients were at intermediate to high risk. Surgical treatment was recommended to these 5 patients but all refused. One of the 5 died of stroke during follow-up, and no recurrence was observed by close follow-up of the other four. No recurrence was evident in any of the 35 patients with GIST over a mean follow-up of 29.9 months (range 1–88).
Discussion
In the present study, endoscopic method was determined according to tumor size (< 12 mm or ≥ 12 mm), because tumors of less than 12 mm in diameter could be aspirated into the transparent cap. Sun et al. [19] treated 29 patients with small GISTs by endoscopic band ligation. MP tumors were ligated with an elastic band and allowed to slough off spontaneously, and in all cases, tumors were completely removed without serious complication. Endoscopic band ligation is worth considering as a treatment tool for small MP tumors because of its simplicity and effectiveness. However, it has its drawbacks, as deep biopsy is needed to confirm histologic diagnosis before band ligation and, in reality, it is not easy to obtain a proper specimen of small MP tumors by biopsy and the diagnosis rate is low [8]. In addition, it is difficult to obtain enough material by forcep biopsy to determine mitotic count and cellularity.
To overcome this disadvantage, a modification of band ligation was devised for MP tumors [20]. According to this method, MP tumors were first ligated and then lesions were unroofed with an electrocautery snare to obtain sufficient specimen; main lesions sloughed off spontaneously. This technique produced good results without complication, although only a small number (n = 7) of patients were enrolled. However, when band ligation is used without a resection technique, the operator is not aware of complications such as bleeding, uncomplete resection, or perforation, and delayed perforation requiring surgical treatment has been reported in several case reports [21, 22].
Therefore, we first ligated small tumors (≤ 12 mm) with an elastic band and then resected them using a polypectomy snare to obtain specimens and immediately checked for complications. When delayed perforation was a concern or perforation was identified, hemoclipping was performed. In the present study, an excellent endoscopic complete resection rate (100%, 41/41) and a relatively short mean procedural time (12 min) were achieved by BLR. In particular, the histologically complete resection rate (78% 30/41) obtained was high. These observations convinced us that BLR should be considered as a promising diagnostic and therapeutic tool for small MP tumors (≤ 12 mm). Perforation developed in four patients treated by BLR, and these tumors were located in the fundus (n = 1) and high body (n = 3), that is, in the thinnest areas of the stomach wall. Hence, more caution is needed when treating such lesions and it should be borne in mind that excessive suction increases the risk of perforation. In one case, the tumor was partially removed by the first BLR, then a second BLR procedure performed on the remnant tumor resulted in perforation. After the first BLR, mucosa and the submucosal layer were removed by peeling, and thus the stomach wall was too thin to perform second BLR. After experience of this case, we performed additional EMD instead of second BLR when remnant tumor was detected after the first BLR.
In the present study, EMD was performed to treat MP tumors of diameter > 12 mm. EMD is not widely used because of the high risk of incomplete resection and an elevated risk of perforation [7, 23]. However, EMD has being increasingly used to treat MP tumors [13,14,15]. EMD for MP tumors is less invasive than surgical resection and has been reported to have confident diagnostic yield [16]. Although the perforation risk is significant, perforations that occur are usually small and detected early, and thus, can be managed endoscopically. In the present study, perforation occurred in 8 of 41 lesions (18.5%), and four of these were managed endoscopically. To reduce the risk of perforation, proper selection of endoscopic treatment is important. In particular, MP tumors of the subserosal type are difficult to dissect and present a high risk of perforation [15], which suggests EUS should be performed to determine the feasibility of EMD and for risk stratification. A previous study showed endoscopic resection of schwannoma has a high risk of perforation because of poor tumor capsule and tight adhesion [15]. In the present study, perforation occurred in both patients with schwannoma, indicating EMD of gastric schwannoma is unadvisable. Delayed bleeding was not encountered in the present study, presumably due to narrow resection areas and the use of prophylactic hemoclipping.
Although GISTs can arise everywhere in the gastrointestinal tract, they most commonly occur in the stomach (50–60%). In a previous study, gastric GIST usually affected the body and rarely the gastroesophageal junction or cardia, which both had rates of < 5% [24], whereas in the present study, GIST was less common in cardia (15.4%, 4/26) and usually affected the body or fundus, which is consistent with previous observations. Most of MP tumors located in cardia were leiomyoma (84.6%, 22/26).
GISTs are the most common type of MP tumor and are the primary target of treatment. If a small sized GIST is deemed to have a high potential for malignancy and postoperative recurrence, surgical resection is essential to achieve sufficient deep and lateral margins. However, endoscopic complete resection can be considered equivalent to complete resection [25], and thus, if a small GIST is completely removed by endoscopic resection and histopathological examination indicates very low risk of a malignancy, no further treatment is necessary. In the present study, the histologic complete resection rate was relatively low (45.7%, 16/35). However, most of these were classified to be pathologically very low or at low risk of a malignancy and achieved endoscopic complete resection. Therefore, we decided to follow up without additional treatment. It should be added that if a resected tumor has a high risk of malignancy, further treatment should be considered.
Endoscopic treatment of MP tumors has been performed mostly in China [12, 19, 26, 27]. In a large-scale study of modified ESD treatment in 144 patients with an MP tumor, the complete resection rate was 92.4% and no recurrence was detected during follow-up [26]. Others have reported complete resection rates of 74.3–96.8% without recurrence during follow-up, but with relatively high rates of perforation (ranging from 5.7 to 27.2%), which were usually treated by endoscopic clipping [12]. In the present study, BLR and EMD were used to diagnose and treat MP tumors, but recently, various modified endoscopic methods have been introduced. Endoscopic full-thickness resection allows en bloc resection by resecting the tumor and the surrounding serosal layer, but general anesthesia is necessary, primary closure may be difficult, and perforation, serious bleeding and peritonitis may occur, which contraindicates its use for small MP tumors [12, 28, 29]. Several studies have reported submucosal tunnel endoscopic resection (a modification of peroral endoscopic myotomy) can be safely and effectively used to resect MP tumors of cardia or of the esophagogastric junction [12, 30]. Telescoping caps with over-the-scope clip (OTSC) was introduced for full-thickness resection of subepithelial tumors. By using overlapping caps, large lesions can be sucked in with sufficient margin. The use of OTSC at the end of the cap prevents perforation and bleeding. Once the OTSC is released, subepithelial tumors can be resected with a needle knife or snare polypectomy [31, 32].
The present study has some limitations. First, it is inherently limited by its retrospective, single-center design, although a relatively large number of SETs originating in the MP layer were enrolled. Second, no recurrence was detected during follow-up after the endoscopic resection of gastric GIST even when margins were histologically positive, but since not all patients underwent follow-up computed tomography, it is difficult to conclude that there was no recurrence. Third, the follow-up period (mean 26.3 months) was inadequate, and thus, we suggest a large-scale, long-term follow-up study be conducted to confirm our results.
Summarizing, the present study shows endoscopic enucleation is feasible and relatively safe for the treatment of SETs originating in the gastric MP layer, and that for small MP tumors (≤ 12 mm), BLR is straightforward, relatively safe, and requires short procedure times. These findings indicate endoscopic enucleation should be considered a diagnostic and therapeutic option that can replace the ‘wait-and-see’ approach for small MP tumors. Although the risk of perforation after endoscopic enucleation was found to be less than satisfactory (14.6%, 12/82), most were managed endoscopically without serious complication.
References
Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. Surg Endosc. 1991;5:20–23.
Lim YJ, Son HJ, Lee JS, et al. Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J Gastroenterol. 2010;16:439–444.
Hwang JH, Kimmey MB. The incidental upper gastrointestinal subepithelial mass. Gastroenterology. 2004;126:301–307.
Huang ZG, Zhang XS, Huang SL, Yuan XG. Endoscopy dissection of small stromal tumors emerged from the muscularis propria in the upper gastrointestinal tract: Preliminary study. World J Gastrointest Endosc. 2012;4:565–570.
Casali P, Jost L, Reichardt P, Schlemmer M, Blay J. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20:64–67.
Blackstein ME, Blay J, Corless C, et al. Gastrointestinal stromal tumours: consensus statement on diagnosis and treatment. Can J Gastroenterol Hepatol. 2006;20:157–163.
Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Cancer Netw. 2010;8:S1-41. quiz S42-4.
Ji JS, Lee BI, Choi KY, et al. Diagnostic yield of tissue sampling using a bite-on-bite technique for incidental subepithelial lesions. Korean J Intern Med. 2009;24:101–105.
Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–2082.
Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Archiv. 2010;456:111–127.
Antonescu CR. Targeted therapy of cancer: new roles for pathologists in identifying GISTs and other sarcomas. Mod Pathol. 2008;21:S31.
Kim SY, Kim KO. Management of gastric subepithelial tumors: the role of endoscopy. World J Gastrointest Endosc. 2016;8:418–424.
Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, et al. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012;75:276–286.
Chun SY, Kim KO, Park DS, et al. Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: a preliminary analysis of appropriate indications. Surg Endosc. 2013;27:3271–3279.
Du Jeong I, Jung SW, Bang S, Shin JW, Park NH, Kim DH. Endoscopic enucleation for gastric subepithelial tumors originating in the muscularis propria layer. Surg Endosc. 2011;25:468–474.
Feng F, Liu Z, Zhang X, et al. Comparison of endoscopic and open resection for small gastric gastrointestinal stromal tumor. Transl Oncol. 2015;8:504–508.
Liu BR, Song JT, Qu B, Wen JF, Yin JB, Liu W. Endoscopic muscularis dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria. Surg Endosc. 2012;26:3141–3148.
Hornick JL, Fletcher CD. The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol. 2007;38:679–687.
Sun S, Ge N, Wang C, Wang M, Lü Q. Endoscopic band ligation of small gastric stromal tumors and follow-up by endoscopic ultrasonography. Surg Endosc. 2007;21:574–578.
Huang W, Feng C, Lai H, et al. Endoscopic ligation and resection for the treatment of small EUS-suspected gastric GI stromal tumors. Gastrointest Endosc. 2010;71:1076–1081.
Xing X, Wang J, Chen M, Cui Y. Perforation posterior to endoscopic band ligation of a gastric submucosal tumor. Endoscopy. 2012;44:E296–E297.
Siyu S, Sheng W, Guoxin W, Nan G, Jingang L. Gastric perforations after ligation of GI stromal tumors in the gastric fundus. Gastrointest Endosc. 2010;72:615–616.
Sepe PS, Brugge WR. A guide for the diagnosis and management of gastrointestinal stromal cell tumors. Nat Rev Gastroenterol Hepatol. 2009;6:363.
Emory TS, Sobin LH, Lukes L, Lee DH, O’leary TJ. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999;23:82–87.
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58.
He Z, Sun C, Wang J, et al. Efficacy and safety of endoscopic submucosal dissection in treating gastric subepithelial tumors originating in the muscularis propria layer: a single-center study of 144 cases. Scand J Gastroenterol. 2013;48:1466–1473.
Zhang Y, Ye LP, Zhou XB, et al. Safety and efficacy of endoscopic excavation for gastric subepithelial tumors originating from the muscularis propria layer: results from a large study in China. J Clin Gastroenterol. 2013;47:689–694.
Zhou P, Yao L, Qin X, et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926–2931.
Sun M, Song J, Song X, Liu B. Endoscopic full-thickness resection for gastric subepithelial tumors originating from the muscularis propria: A 69-case series. Surg Laparosc Endosc Percutan Tech. 2018;28:e12–e17.
Zhai YQ, Li HK, Linghu EQ. Endoscopic submucosal tunnel dissection for large superficial esophageal squamous cell neoplasms. World J Gastroenterol. 2016;22:435–445.
Abu Dayyeh BK, Wong Kee Song L, Rajan E, Buttar N. Telescoping caps with over-the-scope clip for full-thickness resection of GI lesions (Xtender technique). Gastrointest Endosc. 2015;81:1005.
Rajan E, Wong Kee Song LM. Endoscopic full thickness resection. Gastroenterology. 2018;154:1925.e2–1937.e2.
Authors’ contribution
Eun Jung Ko and Byoung Wook Bang made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. Kye Sook Kwon and Yong Woon Shin made substantial contribution to acquisition of data. Hyung Kil Kim has been involved in drafting the manuscript or revising it critically for important intellectual content.
Funding
This work was supported by Inha University Research Grant (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This was a retrospective study. Informed consent was obtained from all patients before the procedure, and this study was approved by the institutional review board of Inha University Hospital. (2014-01-102).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Ko, E.J., Bang, B.W., Kwon, K.S. et al. Endoscopic Enucleation Is Effective and Relatively Safe in Small Gastric Subepithelial Tumors Originating from Muscularis Propria. Dig Dis Sci 64, 524–531 (2019). https://doi.org/10.1007/s10620-018-5348-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5348-1