Abstract
Background and aims
Although endoscopic resection (ER) is already established as a minimally invasive technique for small (< 4.0 cm) upper gastrointestinal subepithelial tumors originating from the muscularis propria layer (MP-SETs), published data of ER for large (≥ 4.0 cm) upper gastrointestinal MP-SETs are extremely rare and limited to case reports. This retrospective study aimed to evaluate the feasibility and safety of ER for large (≥ 4.0 cm) upper gastrointestinal MP-SETs in a large case series.
Methods
Between June 2012 and December 2018, 101 patients with large (≥ 4 cm) upper gastrointestinal MP-SETs were enrolled in this study. The main outcome measures included complete resection, total complications, and local residual or recurrent tumor.
Results
The rate of complete resection was 86.1%. Thirteen patients (12.9%) experienced complications including gas-related complications (6/101, 5.9%), localized peritonitis (4/101, 4.0%), esophageal/cardiac mucosal laceration (2/101, 2.0%), and delayed bleeding (1/101, 1.0%). These 13 patients recovered after endoscopic and conservative treatment. The independent risk factor for incomplete resection was tumor size (P = 0.005), and the independent risk factors for total complications were tumor size (P = 0.011) and tumor extraluminal growth (P = 0.037). During the median follow-up of 36 months, local residual tumor was detected in 1 patient. No local recurrence occurred in any patient.
Conclusions
Despite being associated with a relatively low complete resection rate, ER is an alternative therapeutic method for large (≥ 4.0 cm) upper gastrointestinal MP-SETs when performed by an experienced endoscopist. This method is especially valuable for patients who are unwilling to undergo surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The clinical pathology of upper gastrointestinal subepithelial tumors originating from the muscularis propria layer (MP-SETs) is complex. A proportion of these tumors are gastrointestinal stromal tumors (GISTs) and neuroendocrine tumors, which have the potential for malignant transformation [1, 2]. When enlarged, the tumors can cause abdominal pain, gastrointestinal obstruction, or even acute gastrointestinal hemorrhage. Therefore, based on the Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy (ASGE) guidelines, upper gastrointestinal MP-SETs that measure more than 2.0 cm in diameter or increase in size at follow-up should be resected either endoscopically or surgically [2].
Currently, endoscopic resection (ER) is widely performed to remove small (< 4.0 cm) upper gastrointestinal MP-SETs [3,4,5,6]. However, because of the difficulty of en bloc endoscopic removal of the tumor, ER has rarely been used to resect large (≥ 4.0 cm) upper gastrointestinal MP-SETs [7,8,9]. Therefore, surgical resection is still the primary therapeutic option for these patients. However, in daily clinical practice, some patients with large (≥ 4.0 cm) upper gastrointestinal MP-SETs are not willing to risk potential surgical complications, preferring endoscopic resection instead of surgical resection. Therefore, based on our previous experiences using ER for gastrointestinal MP-SETs (< 4.0 cm) and the requirements of patients, we have performed ER for large (≥ 4.0 cm) upper gastrointestinal MP-SETs since 2012 at our endoscopy center. In this study, we evaluated the feasibility and safety of ER for large upper gastrointestinal MP-SETs (≥ 4.0 cm) in a large case series.
Methods
Study design
This retrospective study was approved by the Ethics Committee of Taizhou Hospital of Zhejiang Province, Wenzhou Medical University (No. K20190401). The inclusion criteria for this study were as follows: (i) patients had upper gastrointestinal MP-SETs with clear boundaries confirmed by endoscopic ultrasonography (EUS); (ii) the lesions had a diameter of ≥ 4.0 cm; (iii) no lymph node or distant metastasis as judged by computed tomography (CT) and/or EUS; and (iv) patients could tolerate anesthesia with tracheal intubation and did not have blood coagulation disorders before the procedure. In this study, miniprobe EUS (UM-2R, 12 MHz; UM-3R, 20 MHz) was performed first to detect tumor characteristics, such as size, edge, and the internal echo of the tumor. If the tumor characteristics could not be delineated by miniprobe EUS, linear EUS was performed to further evaluate the tumor characteristics.
As part of a quality assurance program, patient medical records were independently reviewed from a prospectively maintained database of our hospital by one author of this study (Dr. Peng JB). Search terms included esophageal SETs, cardia SETs, gastric SETs, duodenal SETs, and various endoscopic resection techniques. Between June 2012 and December 2018, 171 patients with large (≥ 4 cm) upper gastrointestinal MP-SETs were diagnosed in our endoscopy center. Of them, 101 patients with large (≥ 4 cm) upper gastrointestinal MP-SETs were included in this study.
All included patients provided informed consent after spoken and written detailed explanations regarding the endoscopic resection procedure and other possible treatment options. Furthermore, all patients were informed that surgery might be required if the tumor could not be removed en bloc endoscopically or that severe complications might not be managed by conservative treatment and/or endoscopic methods. Given the potential need for surgical intervention, we consulted a gastrointestinal surgeon before the procedure. If necessary, the patient could convert to a laparoscopic resection during the ER procedure. The main outcome measures for this study were; (i) complete resection, (ii) total complications, and (iii) local residual or recurrent tumor.
Endoscopic resection procedure
In this study, the selection of ESD, EFTR, and STER for upper gastrointestinal MP-SETs were mainly based on tumor location, tumor extraluminal growth or extraluminal growth, and narrow connection or extensive connection to the underlying MP or serosal layer (Fig. 1). ESD was usually performed for gastric MP-SETs with intraluminal growth, EFTR was performed for gastric MP-SETs with extraluminal growth, extensive connection with the MP layer or the serosal layer of the stomach, and submucosal tunneling endoscopic resection (STER) was applied if the lesion was located in the esophagus or in the stomach near the esophagus, as these areas are suitable for establishing a submucosal tunnel. All endoscopic resection procedures were performed by an experienced endoscopist (Dr. Ye LP) at our endoscopy center.
The main equipment and accessories included a single-channel endoscope (Q-260J, Olympus Optical) and/or a dual-channel endoscope (GTF-2TQ260M, Olympus), a high-frequency electronic cutting device (ICC 200, Erbe, Tübingen, Germany), argon plasma coagulation (APC 300, Erbe), a transparent cap (ND-201-11802, Olympus Optical), an injection needle (NM-4L-1, Olympus Optical), a hook knife (KD-620LR, Olympus Optical), an insulated-tip knife (KD-611L, IT2, Olympus Optical), a hybrid knife (Erbe), hemostatic clips (ROCC-D-26-195-C, Micro Tech, Nanjing, PRC), a snare (SD-230U-20, Olympus Optical), hot biopsy forceps (FD-410LR, Olympus Optical), an endoscopic lithotriptor (BML-4Q, Olympus Optical), and a carbon dioxide insufflator (Olympus Optical).
The details of the procedures for ESD, EFTR, and STER for resection of upper gastrointestinal MP-SETs are described in our previous study [10]. The key steps of the ESD procedure were as follows [11]. (i) Several marking dots were marked around the tumor, then a submucosal elevation was produced by the injection of several milliliters of a mixed solution into the submucosa. (ii) A circumferential incision was made along the marking dots, then the ESD technique was used to resect the tumor. If there was difficulty in exposing the dissection site between the tumor and the surrounding tissue, the clip-with-thread traction method was used. Firstly, a clip was inserted through the accessory channel after the endoscope was withdrawn. Secondly, a thread of approximately 1.5 m in length was tied to either claw of the clip. Finally, the endoscope was reinserted into the stomach and the clip was anchored with the thread on the proximal edge of the resected mucosa, which resulted in a clear visualization of the dissection site. (iii) After the tumor was completely resected, it was removed by an endoscopic lithotriptor. However, if tumors could not be removed endoscopically, laparoscopic removal was recommended. If the patient refused laparoscopic removal, the lesion was fixed in the stomach temporarily and was removed en bloc by endoscopy on the second day after ER. (iv) The mucosal defect was sealed by loop-and-clips closure. In this study, loop-and-clips closure or over-the-scope clip (OTSC) was performed on gastrointestinal defects of less than 2.0 cm in diameter. For defects of more than 2.0 cm in diameter, OTSC was performed first, and if the defect could not be completely closed safely using this method, the loop-and-clips would be continuously added until the defect was closed safely.
The key steps of the EFTR procedure were as follows (Figs. 2, 3) [12]. (i) Marking dots and submucosal elevation were similar to the ESD procedure. (ii) The ESD technique was used to make a deep circumferential incision along the marking dots. (iii) A small puncture was made in the seromuscular layer after the tumor was fully revealed, then a circumferential incision was made along the puncture. (iv) A snare resection was performed to completely remove the lesion after more than three-quarters of the circumference of the tumor had been resected. (v) The gastrointestinal wall defect was closed by loop-and-clips closure or an OTSC after removal of the tumor.
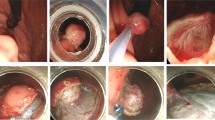
A Endoscopy showing subepithelial tumors (SETs) located in the esophagus. B, C The same tumor was evaluated by endoscopic ultrasonography (EUS) and computed tomography (CT). D, E, F The tumor was resected completely by submucosal tunneling endoscopic resection (STER) technique, and then it was removed by a endoscopic lithotriptor. G The entrance of the submucosal tunnel was closed with several clips; H the resection specimen was a 5.8-cm tumor
A Endoscopy showing SETs located in the fundus of stomach. B, C The same tumor was evaluated by EUS and CT. D A mucosal incision was made in the overlying mucosa to reveal the tumor. E Before performing complete resection, a dual-channel gastroscope was used while grasping the tumor in the gastric cavity to prevent it from falling into the peritoneal cavity. F, G After the tumor was removed from the stomach, the gastric wall defect was closed with loop-and-clips closure method. H The resection specimen was a 5.5-cm tumor
The key steps of the STER procedure were as follows (Fig. 4) [13, 14]. (i) A 2-cm longitudinal mucosal incision was made above the tumor at the entry point of the submucosal tunnel. (ii) A submucosal tunnel was created between the submucosal and muscular layers of the lesion. (iii) The lesion was resected through the submucosal tunnel using the ESD technique. (iv) The mucosal incision site was closed with several clips after removal of the tumor.
A Endoscopy showing SETs located in the descending part of the duodenum; B EUS evaluated the same tumor; C, D the tumor was resected by EFTR technique; E, F the duodenal wall defect was closed with an over-the-scope clip (OTSC); G the resection specimen was a 5.0-cm tumor; H the wound was healed 6 months after the procedure
Definitions
In this study, narrow connection was defined as when the connection width of the tumor to the MP layer was less than 50% of the maximal tumor diameter. Extensive connection was defined as a connection width of more than 50% of the maximal tumor diameter, which was evaluated by EUS. Perioperative bleeding was defined as a complication if surgical intervention was needed during the ER procedure, or a significant drop in hemoglobin (> 2 mg/dl) before and after the ER procedure was detected [3]. Delayed bleeding was defined as active bleeding from a post-procedural ulcer diagnosed by endoscopy following the procedure [3]. Esophageal/cardiac mucosal laceration was considered a complication when it occurred during tumor removal. Localized peritonitis was defined by location; in no more than two quadrants of the abdominal cavity [15]. Gas-related adverse events were considered a complication when the patient required puncture or thoracic drainage. However, if no special interventions were performed, simple pneumoperitoneum, pneumothorax, and subcutaneous emphysema were not considered complications [16].
Complete resection was defined as when the tumor was resected en bloc with tumor-free lateral and basal margins (confirmed by two gastrointestinal pathologists) [12]. Gastroscopy and/or EUS was used to check for the presence of a submucosal protrusion lesion within 3 cm of the endoscopic resection site at 3, 6, and 12 months during the first year after the ER procedure. Subsequently, gastroscopy and/or EUS was performed every year. If a submucosal protrusion lesion was detected, using a biopsy forceps to create a defect in the overlying mucosa of the lesion and then obtain 3 to 5 deeper biopsy specimens to identify if it was a residual or recurrent tumor. Residual tumor was defined as the tumor was found within 3 cm of the endoscopic resection site at less than 6 months after procedure, while local recurrence was defined as the tumor was reappeared within 3 cm of the endoscopic resection site at more than 6 months after procedure.
Statistical analysis
For descriptive statistics, mean ± standard deviation (SD) was used for normally distributed variables, whereas the median (interquartile range) was used for variables with a skewed distribution. Univariate analyses were performed with Chi-square tests or Mann–Whitney U tests to determine the effect associations of tumor features with incomplete resection and total complications. Variables for which P < 0.1 were then entered in the bivariate logistic regression analysis. Statistical analysis was performed using SPSS 18.0 software (SPSS, Chicago, IL).
Results
Clinical characteristics of patients
In Table 1, the characteristics of the enrolled 101 patients (male 62, female 39) with large (≥ 4 cm) upper gastrointestinal MP-SETs are summarized. The mean age of the patients was 55.5 ± 13.0 (range 19 to 84) years. Six patients had taken aspirin or other anticoagulant drugs in the week preceding the ER procedure. Gastrointestinal symptoms were experienced by 61 patients, including 22 with gastrointestinal bleeding, 21 with upper abdominal pain, 11 with abdominal distention and nausea, 4 with a choking feeling, and 2 with upper abdominal pain and emesis. The other 40 patients had no obvious clinical symptoms and their tumors were found during routine endoscopic examination. In total, 71 lesions showed intraluminal growth and 30 lesions showed extraluminal growth. EUS examination showed that 41 tumors had a narrow connection, whereas the other 60 tumors had an extensive connection to the MP layer. In this study, 9 patients received EUS-guided fine needle aspiration (EUS-FNA) for preoperative pathological diagnosis. Of the 9 upper gastrointestinal MP-SETs, 5 tumors were leiomyoma and 4 were GISTs. The pathological results of EUS-FNA were consistent with the postoperative pathological diagnosis.
Therapeutic outcomes and complications
In this study, 21 tumors were resected by ESD, 51 were resected by EFTR, and 29 were resected by STER (Fig. 5). All gastrointestinal wall or mucous defects were closed using the endoscopic method, including clips (41 cases), loop-and-clips closure (30 cases), OTSC (24 cases), and OTSC combined with loop-and-clips closure (6 cases). The rate of en bloc resection was 92.1% (93/101). Of the 93 tumors with en bloc resection, 88 tumors were removed en bloc, 1 was laparoscopically removed, and the other 4 tumors were fixed in the stomach with several clips then removed en bloc on the second day. After histological assessment, the rate of complete resection was 86.1% (87/101). Fourteen tumors did not achieve complete resection, including 8 resected in piece by endoscopy, 1 removed by laparoscopy and 4 removed using the endoscopic method on the second day (Table 2).
In this study, 13 patients (12.9%) had complications including gas-related complications (6/101, 5.9%), localized peritonitis (4/101, 4.0%), esophageal/cardiac mucosal laceration (2/101, 2.0%), and delayed bleeding (1/101, 1.0%). All 13 patients with complications recovered after conservative treatment. No patient had massive bleeding or any other serious complications. The median hospitalization cost was 3641.6 USD (interquartile range, 2894.8 – 5041.1.0 USD).
Pathology results
The median maximal tumor size was 4.3 cm (range 4.0–7.0 cm; interquartile range 4.10–4.95 cm). In total, 82 tumors had an ovoid morphology and the other 19 tumors were non-ovoid. Pathological examination showed that 47 tumors were GISTs (31 low risk, 11 intermediate risk, 5 high risk), 41 were leiomyomas, 6 were Brunner’s gland adenomas, 3 were schwannomas, 2 were heterotopic pancreas tissue, 1 was a glomus tumor, and 1 was an angiolipoma. Of the 101 tumors, 70 were located in the stomach (45 GISTs, 19 leiomyomas, 3 schwannoma, 2 heterotopic pancreas tissue, and 1 glomus tumor), 21 were located in the esophagus (20 leiomyomas and 1 GIST), and 10 were located in the duodenum (6 Brunner’s gland adenomas, 2 leiomyomas, 1 GIST, and 1 angiolipoma).
Risk factors associated with incomplete resection and the total complications
Among the analyzed factors related to incomplete resection, only tumor size (P = 0.005) was met the criteria for inclusion in the bivariate logistic regression (Table 3). Therefore, univariate logistic regression analysis (enter method) was performed with one independent variable: tumor size. Among the analyzed factors related to the total complications, tumor size (P = 0.016) and tumor extraluminal growth (0.054) met the criteria for inclusion in the bivariate logistic regression. Therefore, multivariate logistic regression analysis (Forward LR method) was performed with two independent variable: tumor size and tumor extraluminal growth.
Univariate logistic regression analysis demonstrated that tumor size was a risk factor for incomplete resection (OR 5.490; 95% CI 1.681–17.934; P = 0.005) (Table 4), and multivariate logistic regression analysis indicated that tumor size (OR 5.186; 95% CI 1.459–18.438; P = 0.011) and tumor extraluminal growth (OR 3.851; 95% CI 1.085–13.661; P = 0.037) were the independent risk factors for total complications.
Follow-up outcomes
The median hospital stay after the ER procedure was 7 days (interquartile range, 5–8 days) and the median follow-up period after the ER procedure was 36 months (interquartile range 27.5–58.0 months). During the follow-up period, a local residual tumor was detected in 1 patient, who subsequently underwent laparoscopic resection. No recurrent tumors and no deaths related to ER were observed in any patients.
Discussion
In this study, the rate of complete resection by ER was 86.1%. Compared with our previous study of ER for small upper gastrointestinal MP-SETs, this complete resection rate was relatively low [3]. However, it should be noted that due to the difficulty in removing the resected tumor from the gastrointestinal tract, piecemeal removal or laparoscopic removal might be applied for some tumors with en bloc resection, which reduces the complete resection rate. Therefore, a complete resection rate of 86.1% is acceptable. Thirteen patients (12.9%) had complications. These patients recovered successfully after endoscopy and conservative treatment. During the follow-up (median, 36 months), 1 patient (1.0%) was found to have a residual tumor and was treated with surgical resection. Overall, no local recurrence and no deaths were associated with ER for any patient. Therefore, ER is an alternative therapeutic approach for large (≥ 4.0 cm) upper gastrointestinal MP-SETs, especially for patients who are unwilling to take on the potential risks of surgery.
Although ER is a feasible, minimally invasive treatment for large (≥ 4.0 cm) upper gastrointestinal MP-SETs, several technical difficulty should be noted. The first is maintaining a clear endoscopic view, especially when the digestive tract collapses after puncture during the EFTR procedure [17]. A clear endoscopic view is essential for success and to avoid accidental damage to adjacent organs. Position change is considered the simplest approach to acquiring a clear endoscopic view, but it is challenging to obtain a satisfactory operation field in most cases [18]. Traction is another useful method for maintaining a satisfactory endoscopic view [19,20,21]. Several traction techniques have been described in recent literature, among which the clip-with-thread method is simple, economic, and useful for ER of large upper gastrointestinal MP-SETs [20].
The en bloc removal of large tumors from the narrow cardia and esophageal cavity is another technical difficulty. Based on our experience, we have several recommendations. Firstly, we used an endoscopic lithotriptor for the removal of large tumors, which requires a steady hold during the removal process. Secondly, the tumor was pulled slowly and steadily close to the cardia before removing it from the stomach to the esophagus. When the cardia is relaxed and the lesion can be accommodated, the tumor is immediately pulled from the stomach to the esophagus using an endoscopic lithotriptor. Thirdly, the patient’s chin was lifted, which keeps the month, neck, and thorax aligned before removing the lesion from the esophagus. This reduces the resistance of the esophageal export. During the removal process, undue force should be avoided, otherwise the tumor may rupture or injury to the esophagus/cardia may occur. For tumors that cannot be removed en bloc by endoscopy, laparoscopic removal should first be considered. Piecemeal removal using the endoscopic method is an alternative approach for patients who refuse laparoscopic removal and fit the inclusion criteria. However, piecemeal resection of a large lesion using the endoscopic method requires an extended period of time. In this study, we temporarily affixed the resected tumor to the stomach with clips. The tumor became small after corrosion by stomach acid, then it was removed using the endoscopic method on the second day. This method was suited to patients who could not tolerate a long operation duration. Four tumors were removed using this method in this study. During the follow-up, no residual or recurrent tumors occurred in these four patients.
Although a growing body of evidence has demonstrated that gastrointestinal defects after ER can be effectively managed by endoscopy, the closure of large gastrointestinal defects is still technically demanding for most endoscopists [17, 22]. Moreover, current management strategies for gastrointestinal defects are still not well established. As previously reported, clips combined with an omental patch can be used for the closure of large defects, although the safety of this approach has not been fully verified with large scale clinical evidence [23]. Recently, several novel suturing devices have been reported for the management of large defects. However, these devices are expensive, complex, and not readily available [24]. Therefore, a simple and reliable suturing method should first be considered. Loop-and-clips closure and OTSC are effective suturing techniques that are safe and simple to perform [16, 17, 25].
Due to its technical difficulties, ER of large (≥ 4.0 cm) upper gastrointestinal MP-SETs is associated with certain complications. In this study, the types of complications included gas-related complications (5.9%), localized peritonitis (4.0%), esophageal/cardiac mucosal laceration (2.0%), and delayed bleeding (1.0%). Additionally, we analyzed the risk factors associated with ER-related adverse events, including incomplete resection and total complications. The analyses showed that tumor size was an independent risk factor for incomplete resection. Furthermore, tumor size was an independent risk factor for total complications. Our study also showed extraluminal growth to be another independent risk factor for total complications. These results indicate that tumor extraluminal growth and tumor size can be used for risk assessment when carrying out ER for large (≥ 4.0 cm) upper gastrointestinal MP-SETs.
In this study, the constituent ratios of GISTs in the esophagus, stomach, and duodenum were 4.8%, 64.3%, and 10.0%, respectively. These results are consistent with several previous studies in which the stomach was the most common location of GISTs in the upper gastrointestinal tract [3,4,5]. EUS-FNA is widely performed to obtain tissue and to make a pathological diagnosis preoperatively for gastrointestinal MP-SETs before the procedure, and has a high diagnostic yield in the evaluation of gastrointestinal SETs (≥ 2.0 cm) [26]. In this study, EUS-FNA showed high accuracy in the diagnosis of large gastrointestinal MP-SETs (≥ 4.0 cm). Therefore, EUS-FNA may be an effective pathological diagnosis method, which could assist with treatment decision-making for large gastrointestinal MP-SETs prior to surgery.
For intermediate-risk or high-risk GISTs, further surgical therapy and imatinib mesylate were recommended after pathological diagnosis. In this study, 3 patients with high-risk GISTs received imatinib mesylate for the prevention of recurrence or metastasis, as none of these patients were willing to have further surgery. After discharge, all patients with GISTs received strict follow-up examinations, which were described in our previous study [27]. In this study, residual tumor was detected in 1 patient, and this patient underwent laparoscopic resection. No recurrent lesions were detected in any patient.
Our study has several limitations, including the possibility of selection bias. This was a retrospective, single-center study, although data were derived from a prospectively maintained database. Moreover, some patients with large (≥ 4 cm) upper gastrointestinal MP-SETs did not undergo ER between June 2012 and December 2018. Our institution is a tertiary endoscopic center in Zhejiang Province and is the ESD training center of the Chinese Medical Doctor Association. All ER procedures were performed by an experienced surgeon Ye LP, who is a tutor for the ESD training center of the Chinese Medical Doctor Association. Hence, the results of this study may not apply to other endoscopic centers, which may reduce their generalizability. Additionally, this study was not randomized and did not include control samples. Thus, it was impossible to make a cost-effectiveness analysis.
In conclusion, our study highlights ER as an alternative therapeutic method for large (≥ 4.0 cm) upper gastrointestinal MP-SETs when performed by an experienced endoscopist. This method was especially valuable for patients who were unwilling to assume the potential risks of surgery.
References
Miettinen M, Sobin LH, Lasota J (2005) Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 29:52–68
Faulx AL, Kothari S, Acosta RD, Agrawal D, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Gurudu SR, Khashab MA, Lightdale JR, Muthusamy VR, Shaukat A, Qumseya BJ, Wang A, Wani SB, Yang J, DeWitt JM (2017) The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc 85:1117–1132
Ye LP, Zhang Y, Luo DH, Mao XL, Zheng HH, Zhou XB, Zhu LH (2016) Safety of endoscopic resection for upper gastrointestinal subepithelial tumors originating from the muscularis propria layer: an analysis of 733 tumors. Am J Gastroenterol 111:788–796
Bialek A, Wiechowska-Kozlowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpinska K, Lawniczak M, Starzynska T (2012) Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc 75:276–286
Li QL, Yao LQ, Zhou PH, Xu MD, Chen SY, Zhong YS, Zhang YQ, Chen WF, Ma LL, Qin WZ (2012) Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc 75:1153–1158
Andalib I, Yeoun D, Reddy R, Xie S, Iqbal S (2018) Endoscopic resection of gastric gastrointestinal stromal tumors originating from the muscularis propria layer in North America: methods and feasibility data. Surg Endosc 32:1787–1792
Wang HY, Shih SC, Chen YB, Chang CW, Chen MJ (2013) En bloc resection of large gastric submucosal tumor with endoscopic submucosal dissection. Gastrointest Endosc 78:940–941
Saxena P, El Zein M, Makary M, Kumbhari V, Khashab MA (2016) Submucosal tunneling and en bloc endoscopic resection facilitates laparoscopic transgastric removal of a large GI stromal tumor at the esophagogastric junction. Gastrointest Endosc 84:179–180
Peng W, Tang X, Fu X (2018) Submucosal tunneling endoscopic resection of a large esophageal leiomyoma using endoscopy with near-focus mode. Dig Endosc 30:680
Zhang Y, Ye LP, Mao XL (2015) Endoscopic treatments for small gastric subepithelial tumors originating from muscularis propria layer. World J Gastroenterol 21:9503–9511
Zhang Y, Ye LP, Zhou XB, Mao XL, Zhu LH, He BL, Huang Q (2013) Safety and efficacy of endoscopic excavation for gastric subepithelial tumors originating from the muscularis propria layer: results from a large study in China. J Clin Gastroenterol 47:689–694
Ye LP, Yu Z, Mao XL, Zhu LH, Zhou XB (2014) Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surg Endosc 28:1978–1983
Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY (2014) Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc 28:524–530
Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ, Yao LQ (2012) Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 75:195–199
Rebibo L, Ebosse I, Iederan C, Mahjoub Y, Dupont H, Cosse C, Regimbeau JM (2017) Does drainage of the peritoneal cavity have an impact on the postoperative course of community-acquired, secondary, lower gastrointestinal tract peritonitis? Am J Surg 214:29–36
Rajan E, Wong Kee Song LM (2018) Endoscopic full thickness resection. Gastroenterology 154:1925–1937.e1922
Yamamoto Y, Uedo N, Abe N, Mori H, Ikeda H, Kanzaki H, Hirasawa K, Yoshida N, Goto O, Morita S, Zhou P (2018) Current status and feasibility of endoscopic full-thickness resection in Japan: results of a questionnaire survey. Dig Endosc 30(Suppl 1):2–6
Lee BI (2013) Debates on colorectal endoscopic submucosal dissection—traction for effective dissection: gravity is enough. Clin Endosc 46:467–471
Zhang Q, Cai JQ, Wang Z, Xiao B, Bai Y (2019) Snare combined with endoscopic clips in endoscopic resection of gastric submucosal tumor: a method of tumor traction. Endosc Int Open 7:E1150–e1162
Li J, Meng Y, Ye S, Wang P, Liu F (2019) Usefulness of the thread-traction method in endoscopic full-thickness resection for gastric submucosal tumor: a comparative study. Surg Endosc 33:2880–2885
Lü MH, Fu KI, Wang ZQ, Liu Y, Xia HF, Deng MM (2016) Traction with snare during endoscopic submucosal dissection of a gastrointestinal stromal tumor in the gastric fundus. Endoscopy 48(Suppl 1):E183–185
Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW, Liu JZ (2011) Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc 25:2926–2931
Monkemuller K, Sarker S, Baig KR (2014) Endoscopic creation of an omental patch with an over-the-scope clip system after endoscopic excavation and resection of a large gastrointestinal stromal tumor of the stomach. Endoscopy 46:E451–452
Aslanian HR, Sethi A, Bhutani MS, Goodman AJ, Krishnan K, Lichtenstein DR, Melson J, Navaneethan U, Pannala R, Parsi MA, Schulman AR, Sullivan SA, Thosani N, Trikudanathan G, Trindade AJ, Watson RR, Maple JT (2019) ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE 4:343–350
Shi Q, Chen T, Zhong YS, Zhou PH, Ren Z, Xu MD, Yao LQ (2013) Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy 45:329–334
Lee JH, Cho CJ, Park YS, Ahn JY, Kim DH, Na HK, Choi KD, Song HJ, Lee GH, Jung HY (2016) EUS-guided 22-gauge fine needle biopsy for the diagnosis of gastric subepithelial tumors larger than 2 cm. Scand J Gastroenterol 51:486–493
Zhang Y, Mao XL, Zhou XB, Yang H, Zhu LH, Chen G, Ye LP (2018) Long-term outcomes of endoscopic resection for small (</= 4.0 cm) gastric gastrointestinal stromal tumors originating from the muscularis propria layer. World J Gastroenterol 24:3030–3037
Acknowledgements
This study was supported by the Major R&D Project of Zhejiang province (2019C03040) and the project of Taizhou Science and Technology Bureau (1701KY10).
Author information
Authors and Affiliations
Contributions
YZ drafted the manuscript. JBP performed EUS examination and acquired the follow-up data. XLM designed the study and performed postoperative management. SKZ performed laparoscopic procedure. HHZ acquired the histopathologic data. LHZ enrolled patients and acquired the follow-up data. LPY performed and analyzed the procedure.
Corresponding author
Ethics declarations
Disclosures
Yu Zhang, Jin-Bang Peng, Xin-Li Mao, Hai-Hong Zheng, Shen-Kang Zhou, Lin-Hong Zhu, Li-Ping Ye have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 34698 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Peng, JB., Mao, XL. et al. Endoscopic resection of large (≥ 4 cm) upper gastrointestinal subepithelial tumors originating from the muscularis propria layer: a single-center study of 101 cases (with video). Surg Endosc 35, 1442–1452 (2021). https://doi.org/10.1007/s00464-020-08033-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08033-1