Abstract
Background and aims
Currently, published data of endoscopic resection (ER) for giant (≥ 6 cm) gastric subepithelial tumors originating from the muscularis propria layer (MP-SETs) are extremely rare and limited to only case reports. The aim of this study was thus to assess the feasibility of using ER for giant (≥ 6 cm) gastric MP-SETs in a case series.
Methods
Between July 2013 and December 2020, a total of 23 patients with giant (≥ 6 cm) gastric MP-SETs were treated with ER in the endoscopic center of Taizhou hospital. The study assessed outcomes of en bloc resection, complete resection, total complications, and local residual/recurrence of tumors.
Results
The mean procedure time was 112.2 min. En bloc resection was achieved in 22 tumors (95.7%). En bloc removal from the stomach and complete resection were achieved in 6 patients (26.1%). The rate of complete resection differed significantly depending on the minimum tumor diameter (P < 0.001). During hospitalization, 4 patients had complications, including localized peritonitis (3/23, 13.0%) and pulmonary infection (1/23, 4.3%). These 4 patients recovered successfully after conservative medical treatment. Histopathological examination revealed that 18 tumors were gastrointestinal stromal tumors (GISTs), and 5 tumors were leiomyoma. No patients were observed to have residual or recurrent tumors during the follow-up.
Conclusions
Although ER for giant (≥ 6 cm) gastric MP-SETs was associated with several technical challenges and a relatively low complete resection rate, this technique was found to be a feasible therapeutic method for selected patients with a giant (≥ 6 cm) gastric MP-SETs when performed by an experienced endoscopic team.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Although endoscopic resection (ER) is performed around the globe for the treatment of gastric subepithelial tumors originating from the muscularis propria layer (MP-SETs), published data of ER for giant (≥ 6 cm) gastric MP-SETs are extremely rare and limited to only case reports [1, 2]. This is mainly due to several technical challenges of using ER to remove giant (≥ 6 cm) tumors in the stomach. The major technical challenges include acquiring a clear endoscopic procedure view, en bloc removal of the large tumor from the narrow cardia and esophageal cavity, and closing the large gastric wall defect after tumor resection [1, 3, 4]. Therefore, laparoscopic resection is still the prevailing treatment for patients with giant (≥ 6 cm) gastric MP-SETs [4,5,6].
Our endoscopic center is one of the endoscopic submucosal dissection (ESD) training centers within the Chinese Medical Doctor Association. Our endoscopic center has treated more than 1900 cases of gastric MP-SETs using the ER method since 2005. In 2020, our recent study reported that our endoscopic center had treated 101 cases of large (≥ 4 cm) upper gastrointestinal MP-SETs with ER between June 2012 and December 2018. However, in that study, only 4 tumors were larger than 6.0 cm [7]. Based on our previous successes using ER for gastric MP-SETs, from March 2019, we began routinely applying ER to treat giant (≥ 6 cm) gastric MP-SETs in patients who preferred ER treatment. In this study, we evaluated the short-term oncologic outcomes of ER for treating patients with giant (≥ 6 cm) gastric MP-SETs and then assessed the safety and feasibility of ER in a case series of 23 patients.
Patients and methods
Study design and patients
The ethics committee of Taizhou Hospital of Zhejiang Province, Wenzhou Medical University (K.20210810) approved this retrospective study. The inclusion criteria were as follows: (1) gastric MP-SETs with clear boundaries and without lymph node or distant metastasis confirmed by endoscopic ultrasonography (EUS) and computed tomography (CT), (2) a maximum tumor diameter of more than or equal to 6.0 cm in diameter (if the tumor could not be removed from the stomach with en bloc, the tumor size was measured by CT 3D reconstruction before the procedure), (3) patients that could tolerate anesthesia with tracheal intubation with no blood coagulation disorders before the procedure, and (4) patients with a preference for endoscopic resection of the tumor.
Between July 2013 and December 2020, a total of 23 patients with giant (≥ 6 cm) gastric MP-SETs were treated with ER in our endoscopic center. Before the endoscopic resection procedure, signed informed consent forms were obtained from all patients after they were informed of other possible treatment options, including laparoscopic resection or laparotomy. Patients who chose endoscopic resection were informed of the chance that the tumor might not be able to be removed en bloc by endoscopy. In this case, there were three alternative methods, including laparoscopic removal, piecemeal removal, and affixing the resected tumor to the stomach with clips temporarily to be removed en bloc with endoscopy on the second day. In addition, patients were advised that surgical intervention might be required in the event of complications that could not be managed successfully by conservative medical therapy and/or endoscopic methods.
ER procedure
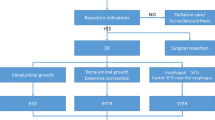
We performed ER for giant (≥ 6 cm) gastric MP-SETs using a single-channel endoscope (Q-260J, Olympus, Tokyo, Japan) with a transparent cap (ND-201-11802, Olympus) attached to the tip. First, a high-frequency electronic cutting device (VIO 200D; ERBE, Tübingen, Germany) was set to the forced coagulation mode (effect 2, output 40 W) before several dot marks were made around the lesion margin with a needle knife or a hybrid knife (ERBE, Germany). Next, several milliliters of a solution (100 mL saline + 2 mL indigo carmine + 1 mL epinephrine) were injected, and circumferential mucosal resection was performed along with the marked dots with a hybrid knife in the ENDO CUT Q mode (effect 3, duration 3, interval 2). If the tumor exhibited intraluminal growth or was not connected to the MP layer, the ESD technique was used in resection. In cases where the tumor exhibited extraluminal growth or was tightly connected to the MP layer, the endoscopic full-thickness resection (EFTR) technique was used in resection (Fig. 1). When performing the EFTR technique, we used a dual-channel endoscope (GTF-2TQ260M, Olympus) in order to take hold of the tumor and acquire a clear endoscopic resection view. If necessary, we had the option of using a position change or clip-with-thread traction method to acquire a clear endoscopic resection view. If the tumor was resected from the stomach, loop-and-clips closure or over-the-scope clip (OTSC) was applied to close the mucosal defect or to reestablish the full thickness of the stomach. Finally, in the case that the resected tumor was removed from the stomach, an endoscopic lithotripter (BML-4Q, Olympus) was used to hold the tumor steady during its removal. If it was unlikely that the resected tumor could be removed from the stomach, the tumor was removed in pieces or temporarily affixed to the stomach with clips (Fig. 2). This removal method led to the resected tumor being corroded by stomach acid and becoming smaller, which then allowed removal of the tumor from the stomach using an endoscopic lithotripter the following day (Fig. 3).
A Endoscopy showed a giant gastric subepithelial tumor located in the fundus of stomach. B The tumor was evaluated by EUS. C Ulceration could be found at the overlying mucosa of the tumor. D An insulated-tip knife being used to make a circumferential resection along the edge of the tumor. E–G OTSC combined with clips being performed to close the gastric wall defect. H The largest size of the tumor was 6.5 cm, and the shortest size of the tumor was 5.2 cm
A A giant gastric subepithelial tumor was found in the anterior wall of the gastric body. B The tumor was evaluated by EUS. C After measurement by CT, the largest size of the tumor was revealed to be 8.6 cm and the shortest size of the tumor was 5.8 cm. D, E An insulated-tip knife being used to make a circumferential resection along the edge of the tumor. F The tumor was completely resected from the stomach. G, H The loop-and-clips method being used to close the large gastric mucosal defect
A A giant gastric subepithelial tumor was detected in the gastric fundus. B After measurement by CT, the largest size of the tumor was revealed to be 6.7 cm and the shortest size of the tumor was 5.4 cm. C The tumor was resected by ESD technique. D The large gastric mucosal defect was closed by loop-and-clips method. E the resected tumor was temporarily affixed to the stomach with clips. F, G The resected tumor was removed en bloc using an endoscopic lithotripter on the following day. and then H The largest size of the tumor was about 3.3 cm and the shortest size of the tumor was about 2.5 cm
Definitions
This study assessed the outcomes of en bloc resection and complete resection while also observing total complications and local residual or recurrence of tumors. En bloc resection was characterized by tumors being resected en bloc with no apparent residual tumor at the resection site, as evaluated by the endoscopic operator. Complete resection was characterized by the en bloc removal of a resected tumor with negative margins, as evaluated by 2 pathologists[8]. A complication was defined as a procedure-related adverse event, which occurred intraoperatively or postoperatively and required additional treatments, such as a prolonged duration of antibiotics and endoscopic or surgical intervention[7].
A residual tumor was defined as a tumor found within the endoscopic resection site within 6 months after the procedure. Local recurrence was defined as a tumor that reappeared within the endoscopic resection site more than 6 months after the procedure[7].
Postoperative management
After the endoscopic procedure, patients were directed to fast and underwent gastrointestinal decompression and intravenous infusion of esomeprazole and antibiotics. Every patient within the study underwent a routine abdominal CT examination the day following the procedure. If no abnormal radiographic results were found and if the patient had no symptoms of a stomach ache or abdominal distension, the nasogastric tube was removed, and a fluid-only diet was prescribed for the patient. Generally, if the patients underwent ESD without gas-related adverse events, a fluid-only diet was prescribed the day following the procedure. If the patients underwent EFTR and had gas-related adverse events during the procedure, gastrointestinal tract leakage or disturbance of gastric emptying needed to first be ruled out before a fluid-only diet could be prescribed.
Follow-up
Endoscopy was performed to check whether there was a residual local tumor or tumor recurrence at 3, 6, and 12 months, and abdominal CT scans were performed to monitor for residual tumor, tumor recurrence, or distant metastasis at 3 and 12 months over the year following discharge. Subsequently, for patients with GISTs, endoscopy and/or EUS was performed to detect for recurrent lesions, while abdominal CT was used to evaluate distant metastasis every year for at least 5 years, indefinitely. Meanwhile, for patients with leiomyoma, endoscopy and/or abdominal CT was performed to find recurrent lesions every 1 or 2 years for 5 years.
Statistical analysis
For descriptive statistics, normally distributed variables are expressed as mean ± SD, while those with a skewed distribution are expressed as median (interquartile range). Associations involving parametric data were assessed with Student’s t test. Dichotomous nonparametric data were assessed with the χ2 test or Fisher’s exact test. Statistical analysis was performed using SPSS 24.0 software (IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics of patients
In this study, 23 patients with giant (≥ 6 cm) gastric MP-SETs were treated with ER. The clinical characteristics and therapeutic outcomes of these 23 patients are shown in Table 1. The median age of the patients was 61 years (interquartile range 46–70 years). Of these 23 patients, 10 patients had symptoms of gastrointestinal bleeding, including 2 cases of hematemesis and melena and 8 cases of melena. One patient had taken aspirin 1 week prior to the endoscopic procedure. The mean maximum tumor diameter was 6.4 ± 0.5 cm, and the mean minimum tumor diameter was 4.8 ± 0.8 cm. Of the 23 tumors, 12 tumors were located in the fundus of the stomach, 8 in the body, and 3 in the antrum. Endoscopic findings showed ulceration on the surface mucosa of 13 tumors. The mucosa of the remaining 10 tumors were intact. In all, 15 tumors showed intraluminal growth while the remaining 8 tumors showed extraluminal growth.
Therapeutic outcomes and complications
In this study, 12 tumors were treated with ESD, and the other 11 tumors were treated with EFTR. The mean procedure time was 112.2 min. En bloc resection was achieved in 22 tumors (95.7%), and piecemeal resection was performed in 1 tumor. In the 22 tumors that underwent en bloc resection, en bloc removal from the stomach and complete resection were achieved in 6 patients (26.1%). The other 16 tumors were temporarily affixed to the stomach with clips and then removed en bloc using an endoscopic lithotripter on the following day.
Of the 23 patients, the mucosal defect or the gastric wall defect was closed with clips (in 4 cases), loop-and-clips closure (in 14 cases), OTSC combined with clips (in 4 cases), and OTSC combined with loop-and-clips closure (in 1 case). During the stay in the hospital, 4 patients presented with complications (17.4%), which included localized peritonitis (3/23, 13.0%) and pulmonary infection (1/23, 4.3%). These 4 patients recovered successfully after conservative medical treatment. The median hospital stay following the ER procedure was 8 days (interquartile range 7–11 days).
Factors associated with complete resection and the total complications
The rate of complete resection differed significantly depending on the minimum tumor diameter (P < 0.001). Other analyzed factors in this study, including (P = 0.079), gender (P > 0.999), GI bleeding symptom (P = 0.124), maximum tumor diameter (P = 0.684), tumor location (P = 0.680), tumor growth pattern (P = 0.369), and tumor mucosal ulceration (P = 0.341; Table 2), showed no statistical relation to complete resection. Among the analyzed factors associated with the total complications (Table 3), there were no statistical differences in the total complications depending these factors, which included age (P = 0.274), gender (P = 0.565), GI bleeding symptom (P = 0.557), maximum diameter of tumor (P = 0.060), minimum tumor diameter (P = 0.330), tumor location (P = 0.300), tumor growth pattern (P = 0.589), tumor mucosal ulceration (P = 0.604), histologic diagnosis (P > 0.999), and resection method (P = 0.317).
Follow-up
Following histopathology examination, 18 tumors were found to be GISTs with a mitotic count of < 5 per high power field, and the remaining 5 tumors were leiomyoma. Among the 18 patients with GISTs, 8 patients with uncomplete resection were treated with imatinib mesylate to prevent recurrence or metastasis after the ER procedure, and the 10 other patients with GISTs were unable to take imatinib mesylate due to the expense of medications. In this study, all 23 patients were followed up regularly by our follow-up team after discharge, and the median follow-up period after the ER procedure was 18 months (interquartile range 13–24 months). During the follow-up period, no patients presented with residual tumor or recurrence.
Discussion
ER was originally used for the treatment of patients with superficial GI lesions, such as GI polyps or early GI cancer. With the application of new endoscopic accessories for ER and the development of endoscopic techniques, ER has recently been used for removing large (≥ 4.0 cm) upper GI MP-SETs [1, 2, 7, 9]. It should be noted that in these studies, only a few tumors were larger than 6.0 cm in size [1, 2]. Several previous studies have reported on techniques involved in laparoscopic and endoscopic combined surgery for treating giant gastric MP-SETs [10]. Compared with laparoscopic resection in treating gastric MP-SETs, laparoscopic and endoscopic combined surgery allows for more accurate resection, reducing the risk of potential complications. However, laparoscopic and endoscopic combined surgery requires greater coordination between the endoscopic and laparoscopic teams. Moreover, several complications related to laparoscopic resection are unavoidable. In this study, we applied endoscopic resection without laparoscopic assistance for giant (≥ 6 cm) gastric MP-SETs, which provides a new therapeutic option for patients with giant (≥ 6 cm) gastric MP-SETs.
In this study, a total of 23 patients with giant (≥ 6 cm) gastric MP-SETs were treated with ER. En bloc resection was achieved in 22 tumors (95.7%), and en bloc removal from the stomach with complete resection was achieved in 6 patients (26.1%). During the stay in the hospital, 4 patients had complications (17.4%) but recovered successfully after conservative medical treatment. In this study, 16 tumors with en bloc resection could not be removed from the stomach, resulting in a reduced rate of complete resection. Importantly, no patient from this subgroup presented with residual tumors or recurrence throughout the 18-month median follow-up period. Therefore, in regard to long-term outcomes, endoscopic resection without laparoscopic assistance may be a useful therapeutic option for patients with giant (≥ 6 cm) gastric MP-SETs.
The main challenges of using ER for giant (≥ 6 cm) gastric MP-SETs involves the risk of intraoperative pulsating bleeding, difficulties in closing a large gastric wall defect after tumor resection, and managing the resected tumor specimen if it is impossible to remove it entirely from the stomach. Pulsating bleeding often occurred during ER for giant (≥ 6 cm) gastric MP-SETs, which usually could be managed effectively by coagrasper (FD-410LR; Olympus) and/or clips. This occurrence required urgent attention because of the risk of massive bleeding blurring the endoscopic resection view. In the event of this situation, it is advised to halt the ER procedure and switch to the laparoscopic resection technique [8, 11]. In this study, all pulsating bleeding that occurred during the procedure was managed successfully with coagrasper and/or clips.
The closure of large gastric wall defects is a major challenge of using ER for giant (≥ 6 cm) gastric MP-SETs [12]. The defects can be closed with clips, loop-and-clips closure, OTSC, or OTSC combined with loop-and-clips closure [7, 13,14,15,16]. Because there is no standard for the selection of closure methods for gastric wall defects following EFTR, the experience of individual endoscopists and the size of the gastric wall defect are the main factors in choosing which method to employ. In this study, the most useful method for large gastric wall defects was the loop-and-clips closure method. Compared to the OTSC method, the loop-and-clips closure was not dependent on the size of the gastric wall defect. When the defect was too large and could not be entirely closed the first time with loop and clips, additional application of loop and clips was conducted to secure the first loop and clips and the remaining unclosed site of the defect. Where necessary, we used loop-and-clips a third time to reinforce the closure site of the defect. Recently, several previous studies have reported the use of an endoscopic suturing device, such as that from Apollo Endosurgery, for closure larger gastric wall defects [17, 18]. However, these endoscopic suturing devices have only been approved for use in a small number of countries. In addition, the omental-patch method could be an alternative suture method for closure of the defect [14, 19]. However, there have been few cases reported in the literature relating to the use of the omental-patch method for large gastric wall defects due to the difficulties of performing this technique.
Another major controversy in the use of ER for giant (≥ 6 cm) gastric MP-SETs involves the best way to manage large tumors after complete resection [1, 12]. In this study, a small portion of giant (≥ 6 cm) gastric MP-SETs could be removed from the stomach to facilitate complete resection. Our analyses showed that the minimum diameter of tumor was an independent risk factor for incomplete resection. This result indicates that the minimum diameter of tumor is important for the assessing the risk related to whether a tumor can undergo en bloc removal from the stomach to achieve complete resection. When giant (≥ 6 cm) gastric MP-SETs could not be removed from the stomach, several clips were used to affix the tumor to the gastric wall so that it could be removed from the stomach 1 day after the initial procedure. It is important to note that the outer membrane of tumor was corroded by stomach acid, which might lead to tumor implantation metastasis in the GI tract due to GISTs having malignant potential. In addition, this tumor removal method had also impacted on the pathological assessment of the tumor. In this study, all 23 patients were followed up regularly by our follow-up team. Although no patients who underwent this tumor resection method presented with residual tumors or recurrence during follow-up, the safety of the method requires rigorous investigation.
Abdominal infection relating to the ER procedure is another major concern. In previous studies, the rate of abdominal infections, including peritonitis and abdominal abscesses ranged from 0% to 10.0%, indicating a high degree of variability [13,14,15, 20]. In a previous study where EFTR was used to treat 69 patients with gastric MP-SETs, 2 patients (2/69, 2.9%) developed peritonitis [15]. In another similar study, 4 patients (4/61, 6.6%) developed abdominal infection [21]. In our study, 3 patients developed peritonitis (3/23, 13.0%). The differences in the rate of abdominal infection may be due to the variability in inclusion criteria and definitions of abdominal infection in these studies. In the present study, most of the abdominal infections were mild and were able to be cured successfully after intravenous infusion of antibiotics without the need for abdominal drainage or surgical intervention. Only a few abdominal infections associated with delayed perforation are relatively severe, and these cases usually need to treated with peritoneal catheterization and endoscopic repair of the gastric defect [15]. In this study, all 3 patients with localized peritonitis recovered successfully after conservative medical treatment without surgical intervention.
Several limitations of this study should be noted. First, as patients with giant (≥ 6 cm) gastric MP-SETs were referred for surgical resection in our institution, selection bias might an issue. Second, 17 tumors (GISTs, 15 cases; leiomyoma, 2 cases) could not be removed from the stomach via en bloc due to their large size. Although no patient presented with residual tumors or recurrence, a larger, randomized, controlled and multicenter study is still needed to ascertain the long-term safety of the method of removing tumors that may be malignant, such as GISTs, on the day after the initial procedure. Finally, our endoscopic center and endoscopists are highly experienced in the treatment of gastric MP-SETs with ER. In this study, all procedures were performed by an experienced endoscopist (LP Ye). Therefore, the results of this study may not be applicable to other endoscopic centers. Other limitations include the lack of randomization and control samples, the relatively small sample size, and the short follow-up period.
In conclusion, ER without laparoscopic assistance for giant (≥ 6 cm) gastric MP-SETs has several drawbacks related to its technical challenges and its relatively low complete resection rate. However, this technique seems to be a feasible therapeutic option for a select number of patients with giant (≥ 6 cm) gastric MP-SETs when performed by an experienced endoscopic team.
References
Perales J, Visrodia K, Sethi A (2021) Managing endoscopic challenges on the road to resect a large ulcerated gastric GI stromal tumor. VideoGIE 6:219–221
Nabi Z, Ramchandani M, Darisetty S, Reddy DN (2021) Endoscopic resection of a large submucosal tumor causing intermittent gastric outlet obstruction using a novel radiofrequency enabled device. Dig Dis (Basel, Switzerland) 40:119
Grande G, Pigò F, Caruso A, Russo S, Lupo M, Piccoli M, Conigliaro R (2021) Too big to go out: gastric endoscopic submucosal dissection of giant lipoma complicated by intestinal obstruction. Endoscopy. https://doi.org/10.1055/a-1346-8122
Faulx AL, Kothari S, Acosta RD, Agrawal D, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Gurudu SR, Khashab MA, Lightdale JR, Muthusamy VR, Shaukat A, Qumseya BJ, Wang A, Wani SB, Yang J, DeWitt JM (2017) The role of endoscopy in subepithelial lesions of the GI tract. Gastroint Endosc 85:1117–1132
Hsiao CY, Yang CY, Lai IR, Chen CN, Lin MT (2015) Laparoscopic resection for large gastric gastrointestinal stromal tumor (GIST): intermediate follow-up results. Surg Endosc 29:868–873
Yu M, Wang DC, Wei J, Lei YH, Fu ZJ, Yang YH (2021) Meta-analysis on the efficacy and safety of laparoscopic surgery for large gastric gastrointestinal stromal tumors. Am Surg 87:450–457
Zhang Y, Peng JB, Mao XL, Zheng HH, Zhou SK, Zhu LH, Ye LP (2021) Endoscopic resection of large (≥ 4 cm) upper gastrointestinal subepithelial tumors originating from the muscularis propria layer: a single-center study of 101 cases (with video). Surg Endosc 35:1442–1452
Zhang Y, Ye LP, Zhou XB, Mao XL, Zhu LH, He BL, Huang Q (2013) Safety and efficacy of endoscopic excavation for gastric subepithelial tumors originating from the muscularis propria layer: results from a large study in China. J Clin Gastroenterol 47:689–694
Chen Q, Yu M, Lei Y, Zhong C, Liu Z, Zhou X, Li G, Zhou X, Chen Y (2020) Efficacy and safety of endoscopic submucosal dissection for large gastric stromal tumors. Clin Res Hepatol Gastroenterol 44:90–100
Saxena P, El Zein M, Makary M, Kumbhari V, Khashab MA (2016) Submucosal tunneling and en bloc endoscopic resection facilitates laparoscopic transgastric removal of a large GI stromal tumor at the esophagogastric junction. Gastrointest Endosc 84:179–180
Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ, Yao LQ (2012) Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 75:195–199
Yamamoto Y, Uedo N, Abe N, Mori H, Ikeda H, Kanzaki H, Hirasawa K, Yoshida N, Goto O, Morita S, Zhou P (2018) Current status and feasibility of endoscopic full-thickness resection in Japan: results of a questionnaire survey. Dig Endosc 30(Suppl 1):2–6
Dong HY, Wang YL, Jia XY, Li J, Li GD, Li YQ (2014) Modified laparoscopic intragastric surgery and endoscopic full-thickness resection for gastric stromal tumor originating from the muscularis propria. Surg Endosc 28:1447–1453
Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW, Liu JZ (2011) Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc 25:2926–2931
Sun M, Song J, Song X, Liu B (2018) Endoscopic full-thickness resection for gastric subepithelial tumors originating from the muscularis propria: a 69-case series. Surg Laparosc Endosc Percutaneous Techn 28:e12–e17
Zhang Y, Ye LP, Mao XL (2015) Endoscopic treatments for small gastric subepithelial tumors originating from muscularis propria layer. World J Gastroenterol 21:9503–9511
Han S, Wani S, Kaltenbach T, Soetikno R, Hammad H (2019) Endoscopic suturing for closure of endoscopic submucosal dissection defects. VideoGIE 4:310–313
Kolb JM, Hammad H (2020) The use of the overstitch to close endoscopic resection defects. Gastrointest Endosc Clin N Am 30:163–171
Hashiba K, Carvalho AM, Diniz G Jr, Barbosa de Aridrade N, Guedes CA, Siqueira Filho L, Lima CA, Coehlo HE, de Oliveira RA (2001) Experimental endoscopic repair of gastric perforations with an omental patch and clips. Gastrointest Endosc 54:500–504
Ye LP, Zhang Y, Luo DH, Mao XL, Zheng HH, Zhou XB, Zhu LH (2016) Safety of endoscopic resection for upper gastrointestinal subepithelial tumors originating from the muscularis propria layer: an analysis of 733 tumors. Am J Gastroenterol 111:788–796
Zhang H, Huang X, Qu C, Bian C, Xue H (2019) Comparison between laparoscopic and endoscopic resections for gastric submucosal tumors. Saudi J Gastroenterol 25:245–250
Acknowledgements
This study was supported by the Major R&D Project of Zhejiang Province (no. 2019C03040) and the Zhejiang Province Bureau of Health (no. 2020KY1030).
Author information
Authors and Affiliations
Contributions
YZ drafted the manuscript. QM enrolled patients and acquired the follow-up data. X-BZ performed postoperative management. GC performed preoperative consultation. L-HZ enrolled patients and acquired the follow-up data. X-LM designed the study and performed postoperative management. L-PY performed and analyzed the procedure.
Corresponding authors
Ethics declarations
Disclosures
Yu Zhang, Qian Meng, Xian-Bin Zhou, Guang Chen, Lin-Hong Zhu, Xin-Li Mao, and Li-Ping Ye have no conflicts of interest to declare relating to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 40863 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Meng, Q., Zhou, XB. et al. Feasibility of endoscopic resection without laparoscopic assistance for giant gastric subepithelial tumors originating from the muscularis propria layer (with video). Surg Endosc 36, 3619–3628 (2022). https://doi.org/10.1007/s00464-022-09019-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09019-x