Abstract
Background
The key step of the endoscopic full-thickness resection (EFTR) procedure is the successful closure of any gastric wall defect which ultimately avoids surgical intervention. This report presents a new method of closing large gastric defects left after EFTR, using metallic clips and novel endoloops by means of single-channel endoscope.
Methods
We retrospectively analyzed 68 patients who were treated for gastric fundus gastrointestinal stromal tumors originating from the muscularis propria layer at four institutes between April 2014 and February 2015 and consequently underwent EFTR. The large gastric post-EFTR defects were completely closed with metallic clips and novel endoloops using single-channel endoscope, and all the patients were discharged with subsequent endoscopic and clinical follow-up. Patient characteristics, tumor size, en bloc resection rate, closure operation time, and postoperative adverse events were evaluated.
Results
EFTR was successfully performed on 68 patients [41 male (60 %), 27 female (40 %); median age 61 years, range 38–73], and the en bloc resection rate was 100 %. Complete closure of all the gastric post-EFTR defects was achieved (success rate 100 %). The mean closure operation time was 13 min (range 9–21 min). The mean maximum size of the lesions was 2.6 cm (range 2.0–3.5 cm). One Mallory–Weiss syndrome and one delayed bleeding were resolved with nonsurgical treatment. The wounds were healed in all cases 1 month after the procedure.
Conclusions
The use of metallic clips and novel endoloops with single-channel endoscope is a relatively safe, easy, and feasible method for repairing large gastric post-EFTR defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Most gastric subepithelial tumors (SETs) originating from the muscularis propria (MP) layer are gastrointestinal stromal tumors (GISTs), which is now regarded as potentially malignant [1, 2]. The latest National Comprehensive Cancer Network (NCCN) task force report has suggested that all GISTs 2 cm or larger should be resected [3]. With the progressive increase in experience in endoscopic submucosal dissection (ESD), endoscopic full-thickness resection (EFTR) without laparoscopic assistance has been applied to remove GISTs with extra-luminal growth and/or close proximity to the serosa [4–9]. The key step of the EFTR procedure is the complete closure of wall defects left by full-thickness resection and thereby avoiding surgical intervention. The endoscopic purse-string suture (EPSS) method using endoloops and metallic clips is an effective and safe closure technique when perforations are larger than 2 cm [10–12]. However, this technique requires the use of dual-channel endoscope, which is not usually available in most endoscopy units. This report presents a new simple method of closing large gastric defects left after EFTR for the treatment of GISTs arising from the MP, using LeClamp™ endoloops and metallic clips by means of single-channel endoscope.
Materials and methods
Patient information
We retrospectively analyzed 68 patients who were treated at four institutes from April 2014 to February 2015 with gastric fundus GISTs arising from the MP and who consequently underwent EFTR, with the resulting large gastric defects being closed using novel endoloops (LeClamp™ Loop-20 and Loop-30; Leo, Changzhou, China) and metallic clips (HX-600-135; Olympus). Before the procedure, computed tomography (CT) scans and endoscopic ultrasound (EUS) were used to evaluate the patients. Contraindications for procedure were as follows: (1) possible high-risk EUS features including irregular border, cystic spaces, ulceration, echogenic foci, and heterogeneity; (2) lymph nodes or distal metastases; (3) blood coagulation disorders (international normalized ratio >2.0, platelet count <70,000/mm3); (4) inability to give informed consent for procedure; and (5) no tolerance to anesthesia with tracheal intubation. Patient characteristics, tumor size, en bloc resection, and post-operative complications were evaluated in all the patients. Detailed clinicopathological data are listed in Table 1.
The research was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University, the People’s Hospital of Donghai County, the First Affiliated Hospital of Xinjiang Medical University, and the First People’s Hospital of Yancheng City in accordance with the Helsinki Declaration, and written informed consent was obtained from each patient.
Endoscopic equipment and accessories
Standard single-accessory-channel endoscopy (GIF-Q260J; Olympus, Tokyo, Japan) and high frequency generator electronic cutting device (ICC 200; ERBE, Tubingen, Germany) were used during the procedure. A transparent cap (ND-201-11802; Olympus) was attached to the tip of the endoscope. An insulated-tip knife and a dual knife (KD-611L and KD-650L, respectively; Olympus) were used to dissect the submucosal and MP layer to reveal the tumor. Other equipment included injection needles (NM-4L-1), forceps (FG-8U-1), snares (SD-230U-20), hot biopsy forceps (FD-410LR), metallic clips (HX-600-135), and carbon dioxide insufflator (MAJ-339) (all Olympus).
Procedures
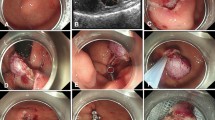
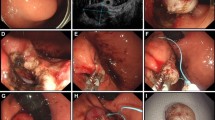
EFTR without laparoscopic assistance was performed as previously described [4, 7]. The post-resection large defect was closed immediately by means of LeClamp™ endoloops and metallic clips and using a single-channel endoscope. The key steps of closure were: (1) An endoloop was inserted into the gastric cavity by forceps through the single-channel therapeutic endoscope; (2) the endoloop was anchored onto the full thickness of the defect’s distal margin with the clip, followed by insertion of several additional clips to anchor the endoloop at different sides of the margin; (3) the delivery system was inserted and the removable hook was connected with the endoloop; (4) the endoloop was tightened by slight pulling of all the edges together; (5) the delivery system was removed from the endoloop and withdrawn; (6) other clips were used if any clip was not accurately positioned or the purse-string suture was not tight; and (7) finally, the lesion was irrigated with physiologic saline followed by air inflation of the gastric cavity to ensure the integrity of the suture and exclude bleeding. An example of closure procedure is shown in Fig. 1. A 20-gauge needle was used to relieve the pneumoperitoneum during and after the procedure. In addition, endoscopic carbon dioxide insufflation was applied during the procedure.
Key steps of closure: A The defect left after EFTR; B An endoloop was inserted into the gastric cavity by forceps through the single-channel therapeutic endoscope; C The endoloop was anchored onto the full thickness of the defect’s distal margin with the clip, followed by insertion of several additional clips to anchor the endoloop at different sides of the margin; D The delivery system was inserted; E The removable hook was connected with the endoloop and the endoloop was tightened by slight pulling of all the edges together; and F The delivery system was removed from the endoloop and the defect was completely closed
Patients were asked to remain in a semi-reclined position after the procedure. A gastrointestinal decompression drainage tube was routinely placed for detection of any postoperative hemorrhaging. Post-EFTR medication included proton pump inhibitors (PPIs), antibiotics, and hemocoagulase injections. Patients were allowed a liquid diet and received PPIs in an oral form from the third day onwards unless serious complications occurred. Contrast roentgenography was routinely performed on all patients on post-procedure day 3 to identify any gastrointestinal tract leakage.
Pathologic evaluation
Paraffin-embedded tumor specimens were sectioned and stained with hematoxylin and eosin. Additionally, immunohistochemical staining was performed on paraffin-embedded tissue sections with DAKO antibodies (DAKO, Carpinteria, Calif). Positive reactions for c-KIT (CD117) or DOG-1, and CD34 were considered diagnostic of a GIST. GIST malignancy potential was categorized on the basis of tumor size and mitotic counts per 50 high-power field according to the National Institutes of Health consensus and classification [13, 14]. Tumor resection was deemed microscopically complete when both lateral and deep resection margins were negative for tumor tissue.
Follow-up
Surveillance endoscopy was performed to observe healing of the wound and to check for any tumor residual or recurrence at 1, 3, 6, and 12 months after the endoscopic resection and then annually thereafter. Endoscopic ultrasound was performed whenever a residual or recurrent lesion at the resection site was observed. Abdominal ultrasound and/or CT and chest radiography were carried out to evaluate distant metastasis every 12 months.
Results
A total of 68 patients [27 female (40 %), 41 male (60 %); median age 61 years, range 38–73] successfully underwent EFTR, and the en bloc resection rate was 100 %. Complete closure of all the gastric full-thickness defects was achieved (success rate 100 %). The time required for the closure procedure was measured from the time point of the resection of the lesion to complete closure of the defect. The mean suture operation time was 13 min (range 9–21 min). The mean total procedure time was 41 min (range 23–118 min). The mean maximum size of the lesions was 2.6 cm (range 2.0–3.5 cm). Pathological examination confirmed that all of the lesions were GISTs. All the resected lesions showed both lateral and vertical tumor-free margins. The tumors were all of low risk or very low risk, with a low mitotic index. All the patients had vague abdominal pain or discomfort within the first day after the operation and experienced fever with the body temperatures 38 °C below, recovering within 1–3 days. One patient (1.47 %, 1/68) with Mallory–Weiss syndrome caused by too much air inflation during the procedure was successfully treated by endoscopic clipping and spraying with norepinephrine. Another patient (1.47 %, 1/68) experienced delayed bleeding and was treated successfully by conservative treatment. No other patient experienced severe complications, such as delayed bleeding, peritonitis, or abdominal abscesses. Contrast roentgenography 3 days after the procedure showed that no patients had gastrointestinal tract leakage or disturbed gastric emptying. No further treatment was given. The mean hospital stay after the procedure was 5.4 days (range 3–9).All the patients paid follow-up visits, and the wounds were healed in all cases 1 month after the procedure. Many of the endoclips and endoloops fell off spontaneously, but parts of the metallic clips and endoloops remained in situ for several months without any side effects. The remaining clips and endoloops were removed using foreign body forceps. No residual tumor or tumor recurrence was observed during the follow-up period (range 3–13 months).
Discussion
GISTs are the most common mesenchymal tumors of the gastrointestinal tract. In adult GISTs, the stomach (60 %) is the most common primary site. The consensus is that all GISTs are potentially malignant which is strongly correlated with their size and mitotic activity [3, 15]. Based on long-term follow-up of more than 1800 patients, the largest retrospective series of gastric GISTs, Miettinen et al. [2] suggested guidelines for the risk stratification of primary GISTs based on mitotic index, size, and site. According to this study, overall tumor-specific mortality was 17 %, and the mortality for those tumors not larger than 2 cm was zero. In tumors with mitotic activity not greater than 5/50 HPFs, the mortality was only 3 %. However, the tumor-specific mortality was significantly higher for tumors with mitotic activity >5/50 HPFs (P < 0.0001) and was overall 46 % for such tumors. Among these tumors, the tumor-specific mortality rose by tumor size, being 16 % for tumors 2–5 cm, 49 % for tumors 5–10 cm, and 86 % for tumors >10 cm. But, it was difficult to make the precise differentiation between benign and malignant GISTs before surgery. Preoperative biopsy was not routinely recommended because of associated risk of tumoral rupture and/or intraperitoneal dissemination. Thus, postoperative pathology assessment is essential to confirm the diagnosis after removal of any suspected GISTs. Based on the above reasons, most guidelines strongly recommend that all GISTs larger than 2 cm be resected [3, 16–20].
GISTs can be effectively treated by a complete gross resection of the tumor. Radical gastric resection, formal lymph node dissection, and wider resection of uninvolved tissue show no apparent benefit. Lymphadenectomy is usually not indicated because lymph node metastases are rare with GISTs in general. For the past years, open or laparoscopic wedge resection has usually been the mainstay of treatment for patients with primary GISTs with no evidence of metastasis [21]. Tumors near the gastroesophageal junction, especially those located at the posterior wall of the stomach, are sometimes difficult to remove by simple wedge resection and may need proximal gastrectomy, which can cause post-gastrectomy functional sequelae [21]. Thus, a minor invasive treatment without loss of curability, such as EFTR which is developed from ESD, is desirable for GISTs 2–5 cm of the gastric fundus with low tumor-related mortality (2 %). The most common type of gastric wall involvement of GISTs was from muscularis propria bulging outward from the stomach, which could not be resected by standard ESD procedure [2]. The significant advantages of EFTR over ESD include complete resection of the muscularis propria layer, prevention of recurrence, and pathologic confirmation of the lesion’s margins [22]. In this study, all lesions were resected en bloc with both lateral and vertical tumor-free margins. One Mallory–Weiss syndrome and one delayed bleeding were resolved with nonsurgical treatment. No residual tumor or tumor recurrence was observed during the follow-up period. These results indicate that EFTR is a safe and effective procedure for providing accurate histopathologic evaluation and curative treatments of patients with gastric GISTs originating from the MP layer.
Incomplete closure of GI wall perforations may lead to serious morbidity and is probably the major safety consideration of the clinical implementation of EFTR. The secure closure of the GI wall defect is considered to be the major obstacle in performing an effective EFTR. The use of endoclips is still a widely accepted closure technique, which is limited to small perforations. A large variety of methods and devices for GI wall closures have been studied [23–29]. Previous studies introduced the endoloop–metallic clips method to close the large gastric wall defect after EFTR treated gastric SETs arising from the MP [30]. This closure method is based on clip closure plus endoloop ligature, which would tightly close both sides of the defect and prevent postoperative gastric leaks and peritonitis. However, this method has many disadvantages. First, this technique requires the use of dual-channel endoscope, which is not usually available in most Chinese endoscopy units. Second, the most commonly used endoloops (MAJ339-340, Olympus) require installation in advance of insertion, and the operation is complicated. Moreover, if purse-string suture is not tight when the delivery system is removed from the endoloops, it is difficult to connect the endoloops with the delivery system again and then the closure operation fails.
In this study, complete closure of all the gastric post-EFTR defects was achieved (success rate 100 %). The mean closure operation time was 13 min (range 9–21 min). All wounds were healed in all cases 1 month after the procedure. Compared with Olympus endoloops, the novel LeClamp™ endoloops which we used in this study demonstrated certain advantages (Table 2): (1) It can be performed using single-channel endoscope, and it is good news for endoscopy units that are not equipped with dual-channel endoscope. Through the endoscope channel, endoloops can be put anywhere in the stomach. (2) It is not needed to be preloaded prior to insertion, and during closure procedures, the loop can be conveniently introduced with the hemostasis clip and then closed by a removable hook from the delivery system. (3) The ring-pull at the tail end of loops is larger than that of Olympus, so it can be conveniently connected with the hook of the delivery system and can greatly reduce the procedure time. Even if the purse-string suture is not tight, it could be immediately remedied.
Based on our experiences, we have a number of recommendations on the use of endoloops for repairing large gastric post-EFTR defects. First, before the procedure, the operative field should be separated from the gastric fluid by changing position to get a satisfactory view of the lesion. Second, fluid in the stomach should be sucked out as the incision reaches the serosal layer to avoid the gastric fluid escaping into peritoneal cavity and to reduce postoperative peritoneal infection. Third, the clips should anchor the full thickness of the defect margins to ensure a tight purse-string suture with the endoloops. Moreover, gas in the gastric cavity should be sucked out before the endoloops are tightened to reduce the tension of the defect. And the endoloop should be tightened by slight pulling of all the edges together to prevent the collapse of the clips.
Our study was limited by the lack of randomization, the retrospective design, a small sample size, and a relatively short follow-up period. For these reasons, a randomized, controlled, prospective, and long-term follow-up study is required to fully evaluate the safety and efficiency of this technique.
In summary, according to our results of this multicenter study, the use of metallic clips and novel endoloops with single-channel endoscope is a relatively safe, easy, and reliable technique for repairing large gastric post-EFTR defects. Being a simple manipulation without requiring complex or specialized equipment, this technique may have potential value in the treatment of gastrointestinal perforation, fistula, and leakage.
References
Akahoshi K, Sumida Y, Matsui N et al (2007) Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol 13(14):2077–2082
Miettinen M, Sobin LH, Lasota J (2005) Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 29(1):52–68
Demetri GD, von Mehren M, Antonescu CR et al (2010) NCCN Task Force Report: update on the management of patients with gastrointestinal stromal tumors. J Natl Comp Cancer Netw 8(Suppl 2):S1–S41
Zhou PH, Yao LQ, Qin XY et al (2011) Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc 25(9):2926–2931
von Renteln D, Rösch T, Kratt T et al (2012) Endoscopic full-thickness resection of submucosal gastric tumors. Dig Dis Sci 57(5):1298–1303
Schlag C, Wilhelm D, von Delius S et al (2012) EndoResect study: endoscopic full-thickness resection of gastric subepithelial tumors. Endoscopy 45(1):4–11
Schmidt A, Bauder M, Riecken B et al (2015) Endoscopic full-thickness resection of gastric subepithelial tumors: a single-center series. Endoscopy 47(2):154–158
Kopelman Y, Siersema PD, Bapaye A et al (2012) Endoscopic full-thickness GI wall resection: current status. Gastrointest Endosc 75(1):165–173
Li QL, Yao LQ, Zhou PH et al (2012) Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc 75(6):1153–1158
Zhang Y, Wang X, Xiong G et al (2014) Complete defect closure of gastric submucosal tumors with purse-string sutures. Surg Endosc 28(6):1844–1851
Shi Q, Chen T, Zhong YS et al (2013) Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy 45(5):329–334
Ye LP, Yu Z, Mao XL et al (2014) Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surg Endosc 28(6):1978–1983
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 23(2):70–83
Joensuu H (2008) Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 39:1411–1419
Cioffi A, Maki RG (2015) GI stromal tumors: 15 years of lessons from a rare cancer. J Clin Oncol 33(16):1849–1854
Sepe PS, Brugge WR (2009) A guide for the diagnosis and management of gastrointestinal stromal cell tumors. Nat Rev Gastroenterol Hepatol 6(6):363–371
Nishida T, Hirota S, Yanagisawa A et al (2008) Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol 13(5):416–430
Hwang JH, Rulyak SD, Kimmey MB, Institute Association (2006) American Gastroenterological American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 130(7):2217–2228
Blay JY, Bonvalot S, Casali P GIST Consensus Meeting Panelists et al (2005) Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol 16(6):566–578
ESMO/European Sarcoma Network Working Group (2012) Gastrointestinal stromal tumors: ESMO clinical practice guide-lines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):vii49–vii55
Kong SH, Yang HK (2013) Surgical treatment of gastric gastrointestinal stromal tumor. J Gastric Cancer 13(1):3–18
Shen C, Chen H, Yin Y et al (2015) Endoscopic versus open resection for small gastric gastrointestinal stromal tumors: safety and outcomes. Med (Baltimore) 94(1):1–7
Raju GS (2011) Gastrointestinal perforations: role of endoscopic closure. Curr Opin Gastroenterol 27(5):418–422
Raju GS (2014) Endoscopic clip closure of gastrointestinal perforations, fistulae, and leaks. Dig Endosc 26(Suppl 1):95–104
Al Ghossaini N, Lucidarme D, Bulois P (2014) Endoscopic treatment of iatrogenic gastrointestinal perforations: an overview. Dig Liver Dis 46(3):195–203
Mangiavillano B, Viaggi P, Masci E (2010) Endoscopic closure of acute iatrogenic perforations during diagnostic and therapeutic endoscopy in the gastrointestinal tract using metallic clips: a literature review. J Dig Dis 11(1):12–18
Kantsevoy SV, Bitner M, Mitrakov AA et al (2014) Endoscopic suturing closure of large mucosal defects after endoscopic submucosal dissection is technically feasible, fast, and eliminates the need for hospitalization (with videos). Gastrointest Endosc 79(3):503–507
Paspatis GA, Dumonceau JM, Barthet M et al (2014) Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy 46(8):693–711
Schmidt A, Damm M, Caca K (2014) Endoscopic full-thickness resection using a novel over-the-scope device. Gastroenterology 147(4):740–742
Samarasena JB, Nakai Y, Park DH et al (2012) Endoscopic closure of an iatrogenic duodenal perforation: a novel technique using endoclips, endoloop, and fibrin glue. Endoscopy 44(Suppl 2):E424–E425
Acknowledgments
The authors would like to thank the patients with gastric stromal tumors for their participation and cooperation. This study was supported in part by the grants from Natural Science Foundation of Jiangsu Province Education Department (14KJB320014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dongtao Shi, Rui Li, Weichang Chen, Deqing Zhang, Lei Zhang, Rui Guo, Ping Yao and Xudong Wu have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, D., Li, R., Chen, W. et al. Application of novel endoloops to close the defects resulted from endoscopic full-thickness resection with single-channel gastroscope: a multicenter study. Surg Endosc 31, 837–842 (2017). https://doi.org/10.1007/s00464-016-5041-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5041-4