Abstract
Swallowing is a sophisticated process involving the precise and timely coordination of the central and peripheral nervous systems, along with the musculatures of the oral cavity, pharynx, and airway. The role of the infratentorial neural structure, including the swallowing central pattern generator and cranial nerve nuclei, has been described in greater detail compared with both the cortical and subcortical neural structures. Nonetheless, accumulated data from analysis of swallowing performance in patients with different neurological diseases and conditions, along with results from neurophysiological studies of normal swallowing have gradually enhanced understanding of the role of cortical and subcortical neural structures in swallowing, potentially leading to the development of treatment modalities for patients suffering from dysphagia. This review article summarizes findings about the role of both cortical and subcortical neural structures in swallowing based on results from neurophysiological studies and studies of various neurological diseases. In sum, cortical regions are mainly in charge of initiation and coordination of swallowing after receiving afferent information, while subcortical structures including basal ganglia and thalamus are responsible for movement control and regulation during swallowing through the cortico-basal ganglia-thalamo-cortical loop. This article also presents how cortical and subcortical neural structures interact with each other to generate the swallowing response. In addition, we provided the updated evidence about the clinical applications and efficacy of neuromodulation techniques, including both non-invasive brain stimulation and deep brain stimulation on dysphagia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Swallowing is a sophisticated process involving the precise and timely coordination of the central and peripheral nervous systems, along with the musculatures of the oral cavity, pharynx, and airway [1]. Basic physiological research has demonstrated that the swallowing central pattern generator (sCPG) and cranial nerve nuclei at the brainstem are critical to controlling and coordinating the swallowing process [2]. However, the role played by cortical and subcortical neural components in swallowing is less clearly understood when compared with the sCPG in the brainstem.
Clinical physiological research among stroke patients has further emphasized the importance of the brainstem’s sCPG in normal swallowing. Stroke patients with brainstem lesions were found to have poorer outcomes in terms of safe oral feeding, possibly due to delayed or absent swallowing reflex leading to impaired airway protection and passage of food from the pharynx to the esophagus [3, 4]. Impaired sCPG and cranial nerve nuclei delays the swallowing reflex, with the oropharyngeal musculatures unable to contract with normal strength, timeliness, and coordination. However, the sCPG not only plays major role in involuntary swallowing by initiating the swallowing reflex but also receives various feedback and inputs from the supranuclear regions, including the somatosensory cortex, basal ganglia (BG), and thalamus [2]. Furthermore, clinical studies have revealed that patients with neurological diseases including cortical or subcortical stroke and Parkinson’s disease have altered swallowing patterns, although the sCPG should be intact [5, 6]. While Albert et al. disclosed no significant correlation between stroke location and the occurrence of aspiration, the majority of lesion-symptom mapping studies found the correlation between the lesion sites and the presentation or prognosis of swallowing disturbance among patients with cortical or subcortical stroke [4, 5, 7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Lesions including somatosensory and motor cortices, basal ganglia, insula, and internal capsules have been reported to cause dysphagia [23, 24]. These findings indicate the role of various cortical and subcortical neural structures in swallowing, in addition to sCPG.
In recent years, rapid advances in functional neuroimaging and neuromapping modalities have allowed researchers to further investigate how the human brain processes sensory and motor information. The development of modalities including functional magnetic resonance imaging (fMRI), positron emission tomography (PET), magnetoencephalography (MEG), electrocorticography (ECoG), and transcranial magnetic stimulation (TMS) has enabled a more comprehensive exploration of the neuroanatomy and neurophysiology of swallowing mediated by both cortical and subcortical structures [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. fMRI and PET detect hemodynamic and metabolic changes correlating to neural activation triggered by swallowing [23, 27]. MEG captures the magnetic fields generated by synchronized neuronal currents [32]. ECoG detects the cortical potential generated by neural oscillatory activities and has disclosed the role of the cerebral cortex in both the motor and sensory components of voluntary swallowing [41, 42]. TMS enables direct activation of cortical neurons and was used to investigate the integrity of the corticospinal and corticobulbar tracts [43]. These developments have driven enhanced understanding of how these supranuclear neural structures interact in both normal swallowing and dysphagia. Therefore, this review article aims to provide a comprehensive summary of the functions of cerebral cortices, subcortical gray matter, and subcortical white matter in swallowing control based on results from clinical studies of various central nervous system disorders and neurophysiological studies investigating the neural control of swallowing.
Role of Cerebral Cortex in Swallowing
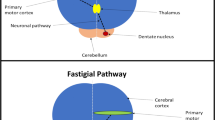
Information about the cortical control of swallowing was derived from animal studies, functional imaging studies of normal subjects, and, to a large extent, lesion studies of stroke patients. These lesion studies provide indirect indications of the role of cortical structures by demonstrating their impact on swallowing performance when damaged. However, further neurophysiological studies are necessary to precisely delineate the physiological functions of the cerebral cortex. This review article illustrates the potential contribution in swallowing of commonly reported cortical areas including sensorimotor cortices, insular cortex, parieto-temporal cortex, and cingulate cortex. These cortical regions are connected with subcortical structures including BG and the thalamus through white matter tracts, like periventricular white matter (PVWM) and corona radiata (CR). In addition, the internal capsule (IC) contains the corticobulbar tract connecting the cortical regions with the brainstem. Table 1 summarizes the main results of both neurophysiological and clinical lesion studies investigating the impact of the cerebral cortex in swallowing control, while Fig. 1 shows a schematic presentation of central nervous system (CNS) control in swallowing, with emphasis on the interplay between cortical and subcortical structures. Table 2 summarizes the potential roles of the cerebral cortex in swallowing based on the knowledge of neurophysiology.
A schematic figure showing the interplay between cortical and subcortical neural structures and the sCPG. Note the sCPG could be divided into dorsal (NTS-DSG, nucleus tractus solitarius-dorsal swallowing group) and ventral (VLM-VSG, ventrolateral medulla-ventral swallowing group) parts [44]. The NTS-DSG receives supramedullary inputs and further activates the VLM-VSG [44]. The VLM-VSG further controls motor neurons of cranial nerve nuclei at brainstem [44]. Also note that the cortico-striato-thalamo-cortical loop was presented with the red arrows and the cerebello-thalamo-cortical pathway was presented with the blue arrows
Sensorimotor Cortices
The sensorimotor cortex is composed of primary motor and primary sensory cortices. The topographic organization of the sensorimotor cortex, called the motor and sensory homunculus, contains motor or sensory representations of certain body parts, including those used in swallowing, such as the face, lips, teeth, gums, jaw, tongue, larynx, and pharynx [87,88,89,90,91]. Through the corticobulbar tract, which connects the cortical regions with the brainstem, the sensorimotor cortex initiates voluntary swallowing by activating and providing cortical inputs to the sCPG and receives afferent inputs from cranial nerve nuclei at the brainstem. [92,93,94,95]
Sensory inputs from the oral, laryngeal, pharyngeal, and esophageal areas have been shown to activate the primary sensory cortex, and this sensory information is important in providing biofeedback to ensure safe swallowing [79]. Among patients with impaired cortical sensory input due to oropharyngeal anesthesia, swallowing could still be generated by the brainstem but the swallowing response was less coordinated due to loss of cortical modulation to the sCPG [58]. Several studies have found a relationship between impaired somatosensory cortices in stroke patients and the occurrence of dysphagia [5, 10, 11]. Wilmsskoetter et al. found that impaired laryngeal vestibular closure and excessive pharyngeal residue are associated with lesions in the postcentral gyrus, while impaired laryngeal elevation is associated with lesions in the precentral gyrus [5]. In addition, lesion mapping analysis revealed that the only region that was predictive for severe dysphagia was the right postcentral gyrus, the primary sensory area for swallowing. [10]
One 2007 study investigated the influence of primary motor cortex inhibition on swallowing, using TMS to create an artificial lesion on the primary motor cortex [57]. Investigators applied an inhibitory repetitive TMS protocol to both the stronger and weaker pharyngeal motor cortex of 13 healthy volunteers. A stronger pharyngeal motor cortex was defined as the pharyngeal motor cortex producing the largest consistent motor-evoked potential at the lowest stimulus threshold, while the contralateral pharyngeal cortex was defined as the weaker one. Each participant performed voluntary swallowing tasks following electrical cues. The timing of initiation of swallowing and change of pressure wave was recorded by a pressure transducer placed at the pharynx. The time from the electrical cue to the onset of swallowing was the swallow reaction time. Results showed that the swallowing reaction time decreased significantly if inhibitory TMS stimulation was applied to the stronger pharyngeal motor cortex. The authors asserted that the reduction of swallowing reaction time might be indicative of less controlled swallowing, causing poorer manipulation of bolus and higher risk of consequent aspiration [57]. More importantly, the results indicated that the pharyngeal motor cortex produces important inhibitory inputs to the brainstem that influence swallowing initiation and modulate voluntary swallowing behavior asymmetrically [57]. A recent study used intracranial electroencephalography to record the cortical oscillatory changes induced by swallowing at the orofacial motor cortex [41]. High-gamma activity bursts coincided with voluntary swallowing and soon decreased with the completion of voluntary swallowing, indicating that the motor cortex plays a crucial role in the initiation of voluntary swallowing [41]. Similarly, several other studies also found that the motor cortex participates in swallowing initiation, as do other cortical regions including the insular cortex, cingulated cortex, and supplementary motor area, and provide significant inhibitory input to the brainstem. [26, 39, 46, 48, 49, 57, 59, 96]
Based on findings from neurophysiology studies and studies of stroke patients, the sensorimotor cortices are in charge of the initiation and coordination of the swallowing process.
Parietal–Temporal Cortex
Despite the association between lesions in the posterior parietal and temporal lobes and dysphagia, the actual role of parietal–temporal cortex in swallowing has yet to be clearly identified [10,11,12,13]. These regions, including the supramarginal gyrus and angular gyrus, are traditionally known as sensory-motor integration areas, and process and relay afferent information to generate movement planning [97]. The temporal lobe was found to have a rich connection with the frontal lobe, occipital lobe, and thalamus, suggestive of its potentially integrative role for these regions [98].
Previous studies have reported the right parietal–temporal regions are related to impaired swallow response, indicated by aspiration or penetration or by poor cough reflex found during fiber-optic endoscopic evaluation of swallowing (FEES) and oropharyngeal residue [10, 11]. The results of these studies revealed the possible importance of parietal–temporal regions in relaying afferent sensory information to the swallowing motor network [10, 11]. In 2009, Steinhagen et al. found that patients with parieto-temporal infarction of either side were prone to attention deficits and those with left-side parieto-temporal infarction were more likely to have buccofacial apraxia, causing disturbance of orofacial movement during the oral stage of swallowing [12]. Furthermore, swallowing deficits including delayed or absent swallowing response, impaired laryngeal vestibular closure, and reduced hyolaryngeal excursion were more frequent among patients with parietal–temporal lesions. [13]
Insular Cortex
The insular cortex contains the primary gustatory cortex that encodes chemosensory information of food and may play a role in food preferences [80]. Moreover, insular cortex has abundant connectivity to both the cortical and subcortical brain regions [81]. Connections among the olfactory bulb, limbic system, sensory cortex, thalamus, frontal cortex, nucleus tractus solitarius, and insular cortex are both directly and indirectly related to swallowing and are implicated in functions including taste, the motivation, and initiation of swallowing and motor planning [81, 82]. For multifaceted involvement in swallowing, the insular cortex is considered to be a central integration hub of the swallowing network [13].
Several fMRI studies have shown the participation of the insular cortex in swallowing. Insular activation was found to be either bilateral or more significant in the right hemisphere [47,48,49,50, 53, 54]. The exact location of insula activated during swallowing has also been investigated but without definite conclusions. While Daniel et al. reported that the anterior insula (which directly connects to cortical and subcortical regions participating in the swallowing process) is particularly important in normal swallowing compared with posterior part, others have reported the posterior insula is activated during swallowing [48, 65]. Malandraki et al. suggested that the entire insular cortex plays a role in swallowing, with the posterior insula possibly being more activated in less voluntary and more autonomic actions, such as laryngeal closure [59]. The role of insular cortex in initiation of voluntary swallowing was suggested by Watanabe et al. [33]. In this study, long-lasting activation of insular cortex was detected by MEG before initiation of swallowing, suggesting that the activation of insular cortex might be crucial for initiation of swallowing. [33]
Clinically, stroke patients with impaired insular cortex might have prolonged dysphagia and thus be restricted from oral feeding. According to Galovic et al., stroke patients with impaired insular cortex were more often feeding tube dependent within the first 48 h after stroke onset [71]. In 2017, a lesion mapping study investigated the severity of swallowing impairment using Functional Oral Intake Scale (FOIS) among stroke patients with a follow-up period up to 4 weeks after stroke [72]. This study demonstrated that lesions of the anterior insula are associated with impaired oral intake during the second to fourth week after stroke [72]. The impact of the integrity of the insular cortex on swallowing could be explained by its integrative nature, as the insula is connected to key regions involved in the initiation and execution of swallowing, including the sensorimotor cortices, thalamus, and nucleus tractus solitarius. [72, 81, 82]
Cingulate Cortex
Different parts of the anterior cingulate cortex (ACC) have different functions related to swallowing. The rostral part of the ACC can process painful stimuli, while the affective part processes emotion and attention to volitional actions [33]. On the other hand, the more dorsal and caudal parts are considered to be involved in movement regulation, response selection, and performance of willed action [99]. However, the exact role of the ACC in swallowing is not yet fully understood and the exact site of activation in the ACC during voluntary and involuntary swallowing remains unconfirmed. In 1999, two studies from the same research team reported that both the rostral and caudal ACC could be active during water swallowing [26, 39]. Martin et al. found the rostral ACC was activated during naïve saliva swallowing, while activation of the intermediate and caudal ACC was associated with voluntary saliva and water bolus swallowing [48]. This is consistent with a subsequent study by Toogood et al. [55]. Although not conclusive, these results suggest a functional partition of the ACC corresponding to voluntary versus involuntary aspects of swallowing tasks [59].
Malandraki et al. also found the posterior cingulate cortex (PCC) is active during throat clearing [59]. In addition, PCC was activated with a volitional swallowing task [39]. The PCC integrates sensory information via reciprocal connection with the thalamus [100]. Therefore, activation of this area in conjunction with other sensory areas (including the primary somatosensory cortex, thalamus, and precuneus) during swallowing might indicate that sensory information from the oropharyngeal area and esophagus is received and processed in these areas. Furthermore, these sensory information would modulate the motor response via connections with the motor cortex and the insula. [39]
Subcortical Regions Related to Swallowing
Daniels et al. created a neural anatomic model of swallowing involving the bilateral sensorimotor cortex with descending input to the medullary swallowing center [14]. Theoretically, disruption of the connection between cortical and subcortical structures like periventricular white matter (PVWM) would lower inputs to the brainstem. Clinical observations of post-stroke dysphagia have shown that impaired neural structures including the basal ganglia, internal capsule, thalamus and PVWM led to significant swallowing disturbance [15, 94]. Also, studies investigating the swallowing disturbance of Parkinson’s disease (PD) noted the importance of the intact cortico-striatal-thalamic-cortical loop in normal swallowing [84]. The following text illustrates the potential contribution to swallowing of commonly reported subcortical structures, including BG, thalamus, PVWM, internal capsule, superior longitudinal fasciculus (SLF), and external capsule. Table 1 summarizes the main results of both neurophysiological and clinical lesion studies investigating the impact of subcortical neural structures in swallowing control. Table 2 summarizes the potential roles of subcortical neural structures in swallowing based on the knowledge of neurophysiology. Figure 1 presents the connection between the subcortical and cortical regions and the sCPG.
It should be stressed again that these lesion studies offer indirect evidence regarding the involvement of subcortical structures by showcasing the effects of their damage on swallowing performance. However, additional neurophysiological investigations are required to accurately outline the physiological functions of these structures.
Basal Ganglia
The basal ganglia (BG) is composed of interconnected nuclei including striatum, globus pallidus, subthalamic nucleus and substantia nigra located at midbrain. The BG plays a significant role in movement control, movement coordination, cognitive tasks, and limbic functions via integrating information from the cortical regions and conveying this information back to the cortical regions [101].
BG activation during swallowing has been demonstrated in several functional imaging studies [39, 51]. The brain activity of 10 healthy volunteers was recorded via fMRI, while drinking water in a study done by Hamdy et al. [39]. Although less consistent than that of the cerebral cortex, swallow-related activation was still detected at the putamen and caudate nucleus [39]. A study of pediatric brain activity found increased brain activity in both the putamen and globus pallidus when swallowing [51]. A 2004 study found activation of the right putamen and thalamus with voluntary tongue elevation [54].
Stroke patients with BG lesion were reported to have high incidence of swallowing disturbance and altered swallowing features. Suntrup et al. reported that 76.7% of patients with acute striatocapsular hemorrhage suffer from dysphagia [66]. The predominant feature of dysphagia was an impaired oral swallowing phase with premature leakage to the valleculae and piriform sinus [66]. Similarly, Steinhagen et al. found that basal ganglion infarctions were associated with buccofacial apraxia, leading to oral-phase dysphagia [12]. Logemann et al. found significantly prolonged pharyngeal transit time and less efficient swallowing among those with left basal ganglion infarction and noted increased oral residue and prolonged oral transit time [62]. Thus, impaired oral motor control may be a feature of swallowing disturbance among patients with BG lesions.
Dysphagia is highly prevalent among advanced PD patients, whose swallowing disturbance is possibly caused by BG dysfunction due to neuronal loss in the substantia Nigra [6]. VFSS findings of PD patients were consistent with the hallmark features of bradykinesia, hypokinesia, and difficulty in movement initiation [83], including reduced velocity of hyoid movement, prolonged swallowing time, and delayed airway closure [67, 69, 76]. Schiffer et al. reported prolonged hyoid elevation, possibly caused by delayed relaxation of suprahyoid muscle due to rigidity [77]. Moreover, increased peaks in the velocity curves of hyoid movement were found by Kim et al., which might represent incoordination of pharyngeal contractions [70]. While several studies showed decreased hyoid displacement among PD patients, the causal relationship between decreased hyoid displacement and aspiration was not significant [69, 70, 76]. On the other hand, sluggish and incoordinate hyoid movement might lead to aspiration in PD patients. [67, 83]
To sum up, BG controls and coordinates normal swallowing and its dysfunction might cause swallowing disturbance in both oral and pharyngeal phases.
Thalamus
The thalamus is responsible for relaying both sensory and motor information between cortical and subcortical neural structures and it is seen as contributing to sensory-motor integration during swallowing [47]. In the BG-thalamo-cortical loop, the sensory information of the swallowing process is conveyed by thalamus, while the BG monitors movement accuracy and progression [84, 85]. In a lesion-symptom mapping study using repetitive saliva and modified water swallowing tests, Maeshima et al. found that more than half of patients with thalamic hemorrhage had impaired swallowing efficiency or safety [68]. In 2017, Galovic et al. found an association between impaired oral intake (indicated by FOIS) lasting more than 7 days and the disrupted white matter tract, especially the projections fibers connecting thalamus and superior corona radiata [72]. Owing to the possible sensory-motor disintegration caused by disrupted thalamo-cortical fibers, prolonged oral phase of swallowing occurred [72]. Wilmskoetter et al. further investigated the relationship between acute stroke lesion locations and impairments of specific events in oropharyngeal swallowing evaluated by Modified Barium Swallow Study Impairment Profile (MBSImP) in VFSS [5]. Significant association between thalamic lesions and impaired anterior hyoid excursion was found, possibly due to sensory-motor disintegration caused by thalamic stroke [5].
In addition to the cortico-striato-thalamo-cortical loop, the thalamus might also affect the swallowing process through its connection with the cerebellum. The thalamus and the cortical areas were connected with the cerebellum via the cerebello-thalamo-cortical pathway. Deep brain stimulation (DBS) to the ventral intermediate nucleus could affect movement control of extremities and articulation, leading to gait ataxia and ataxic dysarthria [102,103,104]. In addition, a recent study found that DBS of the ventral intermediate nucleus could lead to dysphagic presentations including poor bolus control and early bolus transition from oral stage to pharyngeal stage [78]. These dysphagic presentations were attributed to a lack of coordination of the muscles of the oral cavity, possibly caused by stimulation of cerebellar-thalamic afferent fibers. [78]
Periventricular White Matter
PVWM are white matter tracts lying adjacent to the lateral ventricles of the brain. Bundles of white matter tracts that convey both motor and sensory information to cerebrum and spinal cord are contained in PVWM [105]. Currently, the role or significance of PVWM in swallowing remains unclear and there are few relevant studies. In 1992, Levine et al. investigated the impact of PVWM lesion on swallowing physiology, with VFSS showing significantly longer total swallowing duration and oral transit duration for semisolids in subjects with more severe white matter lesions [45]. In 2010, Cola et al. reported PVWM lesions among more than half of left subcortical stroke patients suffering from dysphagia [15]. Furthermore, Moon et al. suggest that PVWM lesion could be a prognostic predictor of swallowing function in elderly patients with mild stroke [73]. The severity of white matter lesions were graded using the Fazekas scale to build a prediction model using linear logistic regression analysis, with results indicating that Fazekas grade could effectively predict prolonged oral transit time and presence of penetration [73]. In a similar study including 332 patients, Fandler et al. found a higher Fazekas grade and pontine lesions to be risk factors for dysphagia [74]. Furthermore, Moon et al. found that PVWM lesions involving the corticobulbar tract (CBT) are associated with insufficient laryngeal elevation and prolonged pharyngeal transit time [16]. Based on these study results, the location and severity of PVWM lesions may determine the type of swallowing disturbance. The abnormal VFSS findings of the pharyngeal phase of swallowing are possibly caused by the disrupted connections between the cortex and cranial nerve nuclei at the brainstem.
Corona Radiata (CR)
The corona radiata is composed of both ascending and descending fibers connecting the cortical area and internal capsule. Several studies of stroke patients have shown that an injured corona radiata could cause swallowing disturbance. In 2016, Galovic et al. found that the superior corona radiata was significantly associated with impaired oral intake [71]. A later another lesion mapping analysis done by the same team found that CR lesions are associated with prolonged impaired oral intake status as assessed by the FOIS scale [72]. In addition, patients with a larger proportion of damaged corona radiata were more likely to have impaired oral intake, specifically, the majority of patients had impaired oral intake when more than 50% of corona radiate were involved in stroke [72].
Several studies have suggested a possible association between the locations of CR lesions and specific impairments in swallowing physiology as determined by VFSS. However, the results of these studies have not been consistent [5, 17, 18]. Wilmskoetter et al. reported that impaired right superior CR was associated with impaired laryngeal elevation and laryngeal vestibular closure, while right superior and posterior CR was associated with increased pharyngeal residue [5]. Lesions at the anterior corona radiata beneath the right middle frontal gyrus were found to correlate significantly with cricopharyngeal dysfunction according to Kim et al. [17]. Jang et al. analyzed the relationship between brain lesion location and chronic dysphagia in patients with supratentorial stroke, finding that delayed pharyngeal transit time correlated with lesions in right corona radiata [18]. These findings could be explained by the potential role of the CR in coordinating the sCPG of the bulbar swallowing center through integration of both central and peripheral afferent signals [17].
In terms of prognosis, Lee et al. found that stroke lesions at the bilateral corona radiata, along with bilateral BG and internal capsules, were an indicator to predict 6-month swallowing recovery [19]. The authors’ assumptions included diminished compensatory reorganization from the undamaged brain side and disruption of bilateral corticobulbar tracts, leading to possible severe pharyngeal paralysis [19].
Internal Capsule (IC)
Ascending and descending fibers, including the corticospinal tract and corticobulbar tracts extending caudally from the corona radiata, were contained in a somatotopical arrangement in the IC, with corticospinal tract localized at posterior limb and corticobulbar tract at genu of IC [106]. Stroke patients with IC lesions were reported to have a higher risk of swallowing disturbance. Gonzalez-Fernandez et al. discovered a strong correlation between IC stroke and dysphagia [8]. Galovic et al. reported that damage at the internal capsule leads to increased risk of acute aspiration after stroke [20]. In 2019, a MRI-based lesion mapping analysis done by Wilmskoetter et al. found an association between lesions at the right posterior limb and retrolenticular part of the internal capsule and increased pharyngeal residue [5]. Furthermore, as mentioned in the subsection Corona Radiata, bilateral lesions at the internal capsule, basal ganglia, and corona radiata significantly prognosticate 6-month swallowing recovery [19]. Similarly, Kim et al. reported that stroke patients with lesions at the posterior limb of the IC and caudate nucleus required longer recovery time from dysphagia [21]. Based on these data, the IC should be considered an integral part of the complex swallowing neural network. The connection between the cortex and cranial nerve nuclei at the brainstem is disrupted with a lesioned internal capsule, leading to dysphagia.
Superior Longitudinal Fasciculus (SLF)
The SLF is an association tract connecting the frontal, occipital, parietal, and temporal lobes and transmitting cortical neural signals across long and short distances [107]. Through the connection between different cortical sensory-motor regions (e.g., the supramarginal gyrus and premotor and prefrontal regions) it might play a significant role in motor control, including swallowing [13]. Currently, few studies have examined the role of SLF in the swallowing process. In 2015, Suntrup et al. evaluated the swallowing function of 200 stroke patients with FEES within 96 h from admission. 165 were diagnosed with dysphagia, with a significant correlation with lesion at the SLF [10]. Galovic et al. used voxel-based lesion-symptom mapping to investigate the association between lesion pattern and dysphagia [71, 72]. Lesion at the SLF was found to be significantly associated with both acute tube dependency (within 48 h of stroke onset) and persistence of impaired oral intake based on FOIS scale after 7 days of stroke onset [71, 72]. More recently, Wilmskoetter et al. investigated the relationship between stroke lesions and swallowing physiology assessed by VFSS using Modified Barium Swallow Study Impairment Profile (MBSImP). Voxels at the right SLF were associated with impaired laryngeal elevation, increased pharyngeal residue, and higher penetration–aspiration scale (PAS) score. [5]
Although the exact underlying mechanism is unclear, dysphagia caused by impaired SLF might be the result of disrupted signal transmissions from the temporal–parietal swallowing regions to the frontal motor areas. [13]
External Capsule (EC)
The EC is composed of white matter fibers and is located between the putamen and claustrum, with potentially diverse physiological functions. Both cortico-cortical association fibers and striatal fibers connecting the primary sensorimotor cortex with the putamen were found in EC [86]. Therefore, EC is considered to be a key connection between the cortical motor regions and the basal ganglia, and it might contribute to the engagement of the basal ganglia in motor control. Several lesion-symptom mapping studies showed the relationship between damaged EC and swallowing impairment [5, 71, 72]. Galovic et al. found that lesion at the EC was significantly associated with impaired oral intake within 2 days or lasting more than one week [71, 72]. In addition, Wilmskoetter et al. used VFSS to identify an association between injured EC and impaired laryngeal elevation and impaired laryngeal vestibule closure [5].
Hemispheric Dominance of Swallowing Control
Hemispheric dominance, or functional lateralization, plays a crucial role in the efficient and rapid access of neural resources for variable tasks, including swallowing, by preventing interhemispheric neural conduction delay [108]. Studies using EEG, functional neuroimaging, and TMS have reported hemispheric dominance of swallowing control, with interhemispheric asymmetry of cortical representation of mylohyoid, pharyngeal, and esophageal musculature on the motor and premotor cortex [90, 109]. Both right and left hemispheric dominance have been reported by functional neuroimaging studies and the side of hemispheric dominance was different among individuals [26, 30, 38, 47, 48, 52, 54, 60]. Furthermore, the presentation of hemispheric dominance seemed related to the cortical regions activated during swallowing [39]. Hamdy et al. found a clear asymmetric activation of the right premotor cortex and insula during volitional swallowing but a more bilateral activation of sensorimotor areas [39]. Nonetheless, one functional near-infrared spectroscopy study displayed no lateralization effect during motor execution or in swallowing imagery [110].
Additionally, task-dependent hemispheric dominance of swallowing control was revealed by several neuroimaging studies [30, 47, 48, 56, 61, 111]. Kristine et al. showed that the hemispheric dominance noted during dry or wet swallows shifted alternatively between 6 out of 8 subjects [47]. In another study, right hemispheric dominance of insula activation was found only during voluntary saliva swallowing but not during naïve saliva and water bolus swallowing [48]. Mistry et al. showed that the primary motor cortex, predominantly right lateralized, was strongly activated during water swallowing [61]. By contrast, the activation of the premotor cortex and supplementary motor cortex was predominantly detected at the left hemisphere during tongue elevation and saliva swallowing [61]. Lastly, Daniels et al. used a modified dual-task paradigm to investigate whether the swallowing performance would be affected by left hemisphere- or right hemisphere-specific tasks [56]. The results showed that different components of swallowing showing differential lateralization [56]. Left hemisphere tasks reduced the volume of swallowing while right hemisphere tasks reduced the rate of swallowing [56]. These results indicate a task-dependent hemispheric dominance of swallowing control.
Furthermore, lesion-symptom mapping studies of stroke patients have shown that the side of the lesioned hemisphere is associated with the features or severity of dysphagia [5, 10, 11, 22, 63, 64, 75]. Right hemispheric stroke was possibly associated with impaired pharyngeal phase of swallowing and more severe dysphagia while left hemispheric stroke was associated with impaired oral phase [5, 63, 64, 75]. Nonetheless, impaired pharyngeal phase of swallowing could still be detected in a right hemispheric stroke, including increased pharyngeal stasis, delayed pharyngeal swallow, and reduced hyoid elevation [63, 64, 75], suggesting that both right and left cerebral networks are crucial for neural control of swallowing [5].
Plastic Change Induced by Non-invasive Brain Stimulation (NIBS) and Deep Brain Stimulation (DBS) and the Corresponding Clinical Implication
Neuroplasticity refers to the brain’s ability to adaptively change its structure or function in response to intrinsic or extrinsic stimuli [112]. Plastic change of the motor cortex at the damaged hemisphere in stroke patients has been found to enable the recovery of swallowing function [113]. Available evidence indicates that non-invasive brain stimulation including repetitive TMS (rTMS) and transcranial direct current stimulation (tDCS) could induce neural plastic change in the pharyngeal motor cortex of normal subjects [57, 114, 115]. Furthermore, recently published meta-analysis has disclosed the positive effect induced by NIBS on recovery of dysphagia in stroke patients [116, 117]. Nonetheless, at present there is no available standard protocol or guideline to provide the best treatment practices for patients suffering from post-stroke dysphagia. High-frequency rTMS stimulating the affected or unaffected motor cortex could improve swallowing function of patients with post-stroke dysphagia [118,119,120,121]. In addition, some studies have revealed that bilateral hemisphere high-frequency rTMS stimulation outperforms unilateral stimulation in terms of improving swallowing function [122, 123]. More specific investigation about the effect of stimulation frequency and stimulation hemisphere of unilateral rTMS for post-stroke dysphagia was done by Cheng et al. in a recently published meta-analysis [124]. The effect size of high-frequency rTMS over ipsilesional hemisphere was larger than low-frequency rTMS over contralesional hemisphere and high-frequency rTMS over contralesional hemisphere [124]. tDCS can improve swallowing in post-stroke dysphagia but may be inferior to rTMS in reducing aspiration risk [117]. The meta-analysis done by Cheng et al. disclosed that the anodal stimulation on contralesional hemisphere was superior to stimulation on ipsilesional hemisphere [124]. The discrepancy of the stimulation protocol between rTMS and tDCS could be explained by the bimodal balance recovery model of post-stroke neural plasticity [125]. In this model, if the damaged hemisphere has low structural reserve due to more severe damage, the input from the unaffected hemisphere is crucial to compensate for the lost function. Therefore, stimulatory NIBS applied on the unaffected hemisphere could lead to better functional outcome in this scenario [125]. On the other hand, it is possible that stimulatory NIBS applied on the damaged hemisphere or inhibitory NIBS applied on the contralesional hemisphere will lead to better outcome if the structural reserve is high [125, 126].
DBS has been applied in the treatment of various kinds of CNS diseases, including PD, essential tremor, and Alzheimer disease [126]. In addition to immediate response, DBS also produces persistent effects by inducing neuroplasticity [127, 128]. However, few studies have investigated the effects of DBS on swallowing function. A narrative review summarized the effects of DBS on swallowing function in PD patients [129], with a majority of the recruited studies showing positive effects of subthalamic nucleus (STN) DBS [129]. Improvements in both oral and pharyngeal phases were found. The possible underlying mechanism might be direct activation of the nigrostriatal dopaminergic pathway through activating the glutamatergic neurons in the STN and subsequent stimulation of substantia Nigra [130]. Other mechanisms, such as reversing the phenomenon of excessive beta oscillations and extinction of theta and gamma rhythms in the striatum through STN DBS, have also been reported [131]. Furthermore, Agarwal et al. showed that low-frequency STN DBS could disrupt the pathological oscillations observed in the STN of PD patients, leading to a more reliable relay of cortical input to thalamic neurons [132]. The impact of different DBS locations and frequencies on swallowing function of patients with different movement disorders was investigated by Yu et al. [133]. While contradictory effects were found with high-frequency STN DBS [133], a more consistent improvement was found with low-frequency STN DBS in PD [133]. Xie et al. firstly found that low-frequency bilateral STN DBS could reduce aspiration frequency in PD and the beneficial effect could last for an average of 6 weeks [134]. Nonetheless, when the follow-up period was extended to approximately one year, the beneficial effect of low-frequency STN over routine 130-Hz stimulation in reducing aspiration frequency or swallowing difficulty perception was not observed [135]. The effect of globus pallidus internus (GPi) DBS on swallowing function was compared with STN DBS in 2 studies of PD patients [136, 137]. The retrospective chart review from Troche et al. showed that the PAS worsened significantly in STN DBS group but not in GPi DBS group [136]. Robertson et al. found that STN DBS decreased voluntary jaw velocity in PD patients, whereas GPi DBS had the opposite effect [137]. It is important to note that DBS did not generate significant beneficial effect on PD patients with dysphagia according to a recently published meta-analysis [138]. However, only 3 DBS randomized controlled trials were recruited in the subgroup analysis [138]. Therefore, further larger numbers of randomized controlled trials are needed to elucidate the effect of DBS on swallowing function of PD patients.
Both NIBS and DBS could induce neural plasticity. Providing more precise and individualized brain stimulation requires the establishment of brain stimulation protocols based on a comprehensive understanding of the neurophysiology of swallowing and the pathophysiology of dysphagia, given the variability of lesion patterns and the corresponding clinical deficits in stroke patients and in patients with movement disorders.
Conclusion
Swallowing is a sophisticated process relying on coordination and participation of various neural structures, including the cerebral cortex, subcortical white and gray matters, sCPG, brainstem nuclei, and peripheral cranial nerves. Although the roles of both the cerebral cortex and subcortical neural structures are less well studied than brainstem structures, understanding of the participation and specific contribution of these neural structures in swallowing is gradually improving with accumulating data from analysis of altered swallowing performance in multiple neurological diseases, especially the lesion mapping studies of stroke patients, and the advancement of both structural and functional neural image modalities [139]. However, the inconsistent results of lesion mapping studies have led to a poor definition of the relationship between lesion location and specific impaired components of the swallowing process. A more thorough understanding of the neurophysiology of swallowing will help clinicians develop more individualized and precise treatment protocols via non-invasive brain stimulation, for example, rTMS or transcranial direct current stimulation, or deep brain stimulation to induce neuroplasticity and further improvement of swallowing performance.
References
Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19(4):691–707. https://doi.org/10.1016/j.pmr.2008.06.001.
Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929–69. https://doi.org/10.1152/physrev.2001.81.2.929.
Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756–63. https://doi.org/10.1161/01.STR.0000190056.76543.eb.
Lee HT, Lim OK, Park KD, Lee JK. Dysphagia pattern according to stroke location. J Korean Dysphagia Soc. 2014;4(1):28–36.
Wilmskoetter J, Bonilha L, Martin-Harris B, Elm JJ, Horn J, Bonilha HS. Mapping acute lesion locations to physiological swallow impairments after stroke. Neuroimage Clin. 2019;22:101685. https://doi.org/10.1016/j.nicl.2019.101685.
Suttrup I, Warnecke T. Dysphagia in Parkinson’s disease. Dysphagia. 2016;31(1):24–32. https://doi.org/10.1007/s00455-015-9671-9.
Flowers HL, AlHarbi MA, Mikulis D, et al. MRI-based neuroanatomical predictors of dysphagia, dysarthria, and aphasia in patients with first acute ischemic stroke. Cerebrovasc Dis Extra. 2017;7(1):21–34. https://doi.org/10.1159/000457810.
Gonzalez-Fernandez M, Kleinman JT, Ky PK, Palmer JB, Hillis AE. Supratentorial regions of acute ischemia associated with clinically important swallowing disorders: a pilot study. Stroke. 2008;39(11):3022–8. https://doi.org/10.1161/STROKEAHA.108.518969.
Alberts MJ, Horner J, Gray L, Brazer SR. Aspiration after stroke: lesion analysis by brain MRI. Dysphagia. 1992;7(3):170–3. https://doi.org/10.1007/BF02493452.
Suntrup S, Kemmling A, Warnecke T, et al. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: dysphagia incidence, severity and aspiration. Eur J Neurol. 2015;22(5):832–8. https://doi.org/10.1111/ene.12670.
Suntrup-Krueger S, Kemmling A, Warnecke T, et al. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 2: oropharyngeal residue, swallow and cough response, and pneumonia. Eur J Neurol. 2017;24(6):867–74. https://doi.org/10.1111/ene.13307.
Steinhagen V, Grossmann A, Benecke R, Walter U. Swallowing disturbance pattern relates to brain lesion location in acute stroke patients. Stroke. 2009;40(5):1903–6. https://doi.org/10.1161/STROKEAHA.108.535468.
Wilmskoetter J, Daniels SK, Miller AJ. Cortical and subcortical control of swallowing-can we use information from lesion locations to improve diagnosis and treatment for patients with stroke? Am J Speech Lang Pathol. 2020;29(2S):1030–43. https://doi.org/10.1044/2019_AJSLP-19-00068.
Daniels SK, Foundas AL. Lesion localization in acute stroke patients with risk of aspiration. J Neuroimaging. 1999;9:91–8.
Cola MG, Daniels SK, Corey DM, Lemen LC, Romero M, Foundas AL. Relevance of subcortical stroke in dysphagia. Stroke. 2010;41(3):482–6. https://doi.org/10.1161/STROKEAHA.109.566133.
Moon HI, Kim GS, Lee E. Is the location of white matter lesions important in the swallowing function of older patients with mild stroke? Dysphagia. 2019;34(3):407–14. https://doi.org/10.1007/s00455-018-9955-y.
Kim JY, Yoon SY, Kim J, Wook KY. Neural correlates of cricopharyngeal dysfunction after supratentorial stroke: a voxel-based lesion-symptom mapping with propensity score matched case-control. Int J Stroke. 2022;17(2):207–17. https://doi.org/10.1177/17474930211006300.
Jang S, Yang HE, Yang HS, Kim DH. Lesion characteristics of chronic dysphagia in patients with supratentorial stroke. Ann Rehabil Med. 2017;41(2):225–30. https://doi.org/10.5535/arm.2017.41.2.225.
Lee WH, Lim MH, Seo HG, Seong MY, Oh BM, Kim S. Development of a novel prognostic model to predict 6-month swallowing recovery after ischemic stroke. Stroke. 2020;51(2):440–8. https://doi.org/10.1161/STROKEAHA.119.027439.
Galovic M, Leisi N, Muller M, et al. Lesion location predicts transient and extended risk of aspiration after supratentorial ischemic stroke. Stroke. 2013;44(10):2760–7. https://doi.org/10.1161/STROKEAHA.113.001690.
Kim JH, Oh SH, Jeong HJ, Sim YJ, Kim DG, Kim GC. Association between duration of dysphagia recovery and lesion location on magnetic resonance imaging in patients with middle cerebral artery infarction. Ann Rehabil Med. 2019;43(2):142–8. https://doi.org/10.5535/arm.2019.43.2.142.
Wilmskoetter J, Martin-Harris B, Pearson WG Jr, et al. Differences in swallow physiology in patients with left and right hemispheric strokes. Physiol Behav. 2018;194:144–52. https://doi.org/10.1016/j.physbeh.2018.05.010.
Malandraki GA, Johnson S, Robbins J. Functional MRI of swallowing: from neurophysiology to neuroplasticity. Head Neck. 2011;33(Suppl 1):S14-20. https://doi.org/10.1002/hed.21903.
Lima MS, Mangilli LD, Sassi FC, Andrade CR. Functional magnetic resonance and swallowing: critical literature review. Braz J Otorhinolaryngol. 2015;81(6):671–80. https://doi.org/10.1016/j.bjorl.2015.08.006.
Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: a systematic review. Dysphagia. 2007;22(3):266–75. https://doi.org/10.1007/s00455-007-9080-9.
Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG. Identification of the cerebral loci processing human swallowing with H2(15)O PET activation. J Neurophysiol. 1999;81(4):1917–26. https://doi.org/10.1152/jn.1999.81.4.1917.
Harris ML, Julyan P, Kulkarni B, et al. Mapping metabolic brain activation during human volitional swallowing: a positron emission tomography study using [18F]fluorodeoxyglucose. J Cereb Blood Flow Metab. 2005;25(4):520–6. https://doi.org/10.1038/sj.jcbfm.9600042.
David H, Zald JVP. The functional neuroanatomy of voluntary swallowing. Ann Neurol. 2001;46:281–6. https://doi.org/10.1002/1531-8249(199909)46:3%3c281::AID-ANA2%3e3.0.CO;2-L.
Abe S, Wantanabe Y, Shintani M, Tazaki M, Takahashi M, Yamane GY, Ide Y, Yamada Y, Shimono M, Ishikawa T. Magnetoencephalographic study of the starting point of voluntary swallowing. Cranio. 2003;21:46–9.
Dziewas R, Sörös P, Ishii R, et al. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage. 2003;20(1):135–44. https://doi.org/10.1016/s1053-8119(03)00285-4.
Furlong PL, Hobson AR, Aziz Q, et al. Dissociating the spatio-temporal characteristics of cortical neuronal activity associated with human volitional swallowing in the healthy adult brain. Neuroimage. 2004;22(4):1447–55. https://doi.org/10.1016/j.neuroimage.2004.02.041.
Loose R, Hamdy S, Enck P. Magnetoencephalographic response characteristics associated with tongue movement. Dysphagia. 2001;16(3):183–5. https://doi.org/10.1007/s00455-001-0062-z.
Watanabe Y, Abe S, Ishikawa T, Yamada Y, Yamane GY. Cortical regulation during the early stage of initiation of voluntary swallowing in humans. Dysphagia. 2004;19(2):100–8. https://doi.org/10.1007/s00455-003-0509-5.
Ertekin C, Turman B, Tarlaci S, Celik M, Aydogdu I, Secil Y, Kiylioglu N. Cricopharyngeal sphincter muscle responses to transcranial magnetic stimulation in normal subjects and in patients with dysphagia. Clin Neurophysiol. 2001;112:86–94.
Rödel RMW, Laskawi R, Markus H. Tongue representation in the lateral cortical motor region of the human brain as assessed by transcranial magnetic stimulation. Ann Otol Rhinol Laryngol. 2003;112:71–6.
Hiraoka K. Movement-related cortical potentials associated with saliva and water bolus swallowing. Dysphagia. 2004;19(3):155–9. https://doi.org/10.1007/s00455-004-0002-9.
Huckabee M, Deecke L, Cannito MP, Gould HJ, Mayr W. Cortical control mechanisms in volitional swallowing: the bereitschaftspotential. Brain Topogr. 2003;16:3–17.
Satow AI T, Yamamoto J-I, Begum T, Thuy DHD, Matsuhashi M, Mima T, Nagamine T, Baba K, Mihara T, Inoue Y, Miyamoto S, Hashimoto N, Shibasaki H. Role of primary sensorimotor cortex and supplementary motor area involitional swallowing: a movement-related cortical potential study. Am J Physiol Gastrointest Liver Physiol. 2003. https://doi.org/10.1152/ajpgi.00323.2003.
Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitionalswallowing: an event-related fMRI study. Am J Physiol-Gastrointest Liver Physiol. 1999;277:219–25.
Tae WS, Lee S, Choi S, Pyun SB. Effects of aging on brain networks during swallowing: general linear model and independent component analyses. Sci Rep. 2021;11(1):1069. https://doi.org/10.1038/s41598-020-79782-1.
Hashimoto H, Takahashi K, Kameda S, et al. Swallowing-related neural oscillation: an intracranial EEG study. Ann Clin Transl Neurol. 2021;8(6):1224–38. https://doi.org/10.1002/acn3.51344.
Hashimoto H, Takahashi K, Kameda S, et al. Motor and sensory cortical processing of neural oscillatory activities revealed by human swallowing using intracranial electrodes. iScience. 2021;24(7):102786. https://doi.org/10.1016/j.isci.2021.102786.
Groppa S, Oliviero A, Eisen A, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012;123(5):858–82. https://doi.org/10.1016/j.clinph.2012.01.010.
Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol. 2003;114(12):2226–44. https://doi.org/10.1016/s1388-2457(03)00237-2.
Levine R, Robbins JA, Maser A. Periventricular white matter changes and oropharyngeal swallowing in normal individuals. Dysphagia. 1992;7:142–7.
Mosier K, Patel R, Liu WC, Kalnin A, Maldjian J, Baredes S. Cortical representation of swallowing in normal adults: functional implications. Laryngoscope. 1999;109(9):1417–23. https://doi.org/10.1097/00005537-199909000-00011.
Mosier KM, Liu W-C, Maldjian JA, Shah R, Modia B. Lateralization of cortical function in swallowing: a functional MR imaging study. Am J Neuroradiol. 1999;20:1520–6.
Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85:938–50.
Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res. 2001;140(3):280–9. https://doi.org/10.1007/s002210100813.
Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol. 2001;280:531–8.
Hartnick C, Rudolph C, Willging J, Holland S. Functional magnetic resonance imaging of the pediatric swallow: imaging the cortex and the brainstem. Laryngoscope. 2001. https://doi.org/10.1097/00005537-200107000-00010.
Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol. 2001;280(3):G354–60. https://doi.org/10.1152/ajpgi.2001.280.3.G354.
Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003;18(2):71–7. https://doi.org/10.1007/s00455-002-0088-x.
Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol. 2004;92:2428–43. https://doi.org/10.1152/jn.01144.2003.
Toogood JA, Barr AM, Stevens TK, Gati JS, Menon RS, Martin RE. Discrete functional contributions of cerebral cortical foci in voluntary swallowing: a functional magnetic resonance imaging (fMRI) “Go, No-Go” study. Exp Brain Res. 2005;161(1):81–90. https://doi.org/10.1007/s00221-004-2048-1.
Daniels SK, Corey DM, Fraychinaud A, DePolo A, Foundas AL. Swallowing lateralization: the effects of modified dual-task interference. Dysphagia. 2006;21(1):21–7. https://doi.org/10.1007/s00455-005-9007-2.
Mistry S, Verin E, Singh S, et al. Unilateral suppression of pharyngeal motor cortex to repetitive transcranial magnetic stimulation reveals functional asymmetry in the hemispheric projections to human swallowing. J Physiol. 2007;585(Pt 2):525–38. https://doi.org/10.1113/jphysiol.2007.144592.
Teismann IK, Steinstraeter O, Stoeckigt K, et al. Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci. 2007;8:62. https://doi.org/10.1186/1471-2202-8-62.
Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp. 2009;30(10):3209–26. https://doi.org/10.1002/hbm.20743.
Peck KK, Branski RC, Lazarus C, et al. Cortical activation during swallowing rehabilitation maneuvers: a functional MRI study of healthy controls. Laryngoscope. 2010;120(11):2153–9. https://doi.org/10.1002/lary.21125.
Mistry S, Michou E, Singh S, et al. Using diffusion weighted MR imaging to dissect the neuroanatomy of human swallowing related behaviours. Gut. 2011;60(Suppl 1):A39–40. https://doi.org/10.1136/gut.2011.239301.78.
Logemann JA, Shanahan T, Rademaker AW, Kahrilas PJ, Lazar R, Halper A. Oropharyngeal swallowing after stroke in the left basal ganglion/internal capsule. Dysphagia. 1993;8:230–4.
Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB. Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil. 1993;74(12):1295–300. https://doi.org/10.1016/0003-9993(93)90082-l.
Daniels SK, Foundas AL, Iglesia GC, Sullivan MA. Lesion site in unilateral stroke patients with dysphagia. J Stroke Cerebrovasc Dis. 1996;6(1):30–4. https://doi.org/10.1016/s1052-3057(96)80023-1.
Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia. 1997;12:146–56. https://doi.org/10.1007/PL000095298.
Suntrup S, Warnecke T, Kemmling A, et al. Dysphagia in patients with acute striatocapsular hemorrhage. J Neurol. 2012;259(1):93–9. https://doi.org/10.1007/s00415-011-6129-3.
Lin CW, Chang YC, Chen WS, Chang K, Chang HY, Wang TG. Prolonged swallowing time in dysphagic Parkinsonism patients with aspiration pneumonia. Arch Phys Med Rehabil. 2012;93(11):2080–4. https://doi.org/10.1016/j.apmr.2012.07.010.
Maeshima S, Osawa A, Yamane F, Ishihara S, Tanahashi N. Dysphagia following acute thalamic haemorrhage: clinical correlates and outcomes. Eur Neurol. 2014;71(3–4):165–72. https://doi.org/10.1159/000355477.
Ellerston JK, Heller AC, Houtz DR, Kendall KA. Quantitative measures of swallowing deficits in patients with Parkinson’s disease. Ann Otol Rhinol Laryngol. 2016;125(5):385–92. https://doi.org/10.1177/0003489415617774.
Kim YH, Oh BM, Jung IY, Lee JC, Lee GJ, Han TR. Spatiotemporal characteristics of swallowing in Parkinson’s disease. Laryngoscope. 2015;125(2):389–95. https://doi.org/10.1002/lary.24869.
Galovic M, Leisi N, Muller M, et al. Neuroanatomical correlates of tube dependency and impaired oral intake after hemispheric stroke. Eur J Neurol. 2016;23(5):926–34. https://doi.org/10.1111/ene.12964.
Galovic M, Leisi N, Pastore-Wapp M, et al. Diverging lesion and connectivity patterns influence early and late swallowing recovery after hemispheric stroke. Hum Brain Mapp. 2017;38(4):2165–76. https://doi.org/10.1002/hbm.23511.
Moon HI, Nam JS, Leem MJ, Kim KH. Periventricular white matter lesions as a prognostic factor of swallowing function in older patients with mild stroke. Dysphagia. 2017;32(4):480–6. https://doi.org/10.1007/s00455-017-9788-0.
Fandler S, Gattringer T, Eppinger S, et al. Frequency and predictors of dysphagia in patients with recent small subcortical infarcts. Stroke. 2017;48(1):213–5. https://doi.org/10.1161/STROKEAHA.116.015625.
May NH, Pisegna JM, Marchina S, Langmore SE, Kumar S, Pearson WG Jr. Pharyngeal swallowing mechanics secondary to hemispheric stroke. J Stroke Cerebrovasc Dis. 2017;26(5):952–61. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.11.001.
Lee WH, Lim MH, Nam HS, et al. Differential kinematic features of the hyoid bone during swallowing in patients with Parkinson’s disease. J Electromyogr Kinesiol. 2019;47:57–64. https://doi.org/10.1016/j.jelekin.2019.05.011.
Schiffer BL, Kendall K. Changes in timing of swallow events in Parkinson’s disease. Ann Otol Rhinol Laryngol. 2019;128(1):22–7. https://doi.org/10.1177/0003489418806918.
Lapa S, Claus I, Reitz SC, et al. Effect of thalamic deep brain stimulation on swallowing in patients with essential tremor. Ann Clin Transl Neurol. 2020;7(7):1174–80. https://doi.org/10.1002/acn3.51099.
Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev. 2008;14(2):77–86. https://doi.org/10.1002/ddrr.12.
Oliveira-Maia AJ, de Araujo IE, Monteiro C, Workman V, Galhardo V, Nicolelis MA. The insular cortex controls food preferences independently of taste receptor signaling. Front Syst Neurosci. 2012;6:5. https://doi.org/10.3389/fnsys.2012.00005.
Gogolla N. The insular cortex. Curr Biol. 2017;27(12):R580–6. https://doi.org/10.1016/j.cub.2017.05.010.
Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol. 2017;34(4):300–6. https://doi.org/10.1097/WNP.0000000000000377.
Wei KC, Hsiao MY, Wang TG. The kinematic features of hyoid bone movement during swallowing in different disease populations: a narrative review. J Formos Med Assoc. 2022. https://doi.org/10.1016/j.jfma.2022.04.007.
Ghaemi H, Sobhani-Rad D, Arabi A, Saifpanahi S, Ghayoumi AZ. Role of basal ganglia in swallowing process: a systematic review. Iran Rehabil J. 2017;14(4):239–45. https://doi.org/10.18869/nrip.irj.14.4.239.
Lenz FA, Gracely RH, Zirh TA, Leopold DA, Rowland LH, Dougherty PM. Human thalamic nucleus mediating taste and multiple other sensations related to ingestive behavior. J Neurophysiol. 1997;77(6):3406–9. https://doi.org/10.1152/jn.1997.77.6.3406.
Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. https://doi.org/10.1196/annals.1444.017.
Brown S, Ngan E, Liotti M. A larynx area in the human motor cortex. Cereb Cortex. 2008;18(4):837–45. https://doi.org/10.1093/cercor/bhm131.
Huang CS, Hiraba H, Murray GM, Sessle BJ. Topographical distribution and functional properties of cortically induced rhythmical jaw movements in the monkey (Macaca fascicularis). J Neurophysiol. 1989;61(3):635–50. https://doi.org/10.1152/jn.1989.61.3.635.
Li WQ, Lin T, Li X, et al. TMS brain mapping of the pharyngeal cortical representation in healthy subjects. Brain Stimul. 2020;13(3):891–9. https://doi.org/10.1016/j.brs.2020.02.031.
Hamdy S, Aziz Q, Rothwell JC, et al. The cortical topography of human swallowing musculature in health and disease. Nat Med. 1996;2(11):1217–24. https://doi.org/10.1038/nm1196-1217.
Morecraft RJ, Stilwell-Morecraft KS, Rossing WR. The motor cortex and facial expression: new insights from neuroscience. Neurologist. 2004;10(5):235–49. https://doi.org/10.1097/01.nrl.0000138734.45742.8d.
Gonzalez-Fernandez M, Ottenstein L, Atanelov L, Christian AB. Dysphagia after stroke: an overview. Curr Phys Med Rehabil Rep. 2013;1(3):187–96. https://doi.org/10.1007/s40141-013-0017-y.
Ertekin C. Voluntary versus spontaneous swallowing in man. Dysphagia. 2011;26(2):183–92. https://doi.org/10.1007/s00455-010-9319-8.
Leopold NA, Daniels SK. Supranuclear control of swallowing. Dysphagia. 2010;25(3):250–7. https://doi.org/10.1007/s00455-009-9249-5.
Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17(3):166–71. https://doi.org/10.1097/MOO.0b013e32832b255e.
Cheng I, Takahashi K, Miller A, Hamdy S. Cerebral control of swallowing: An update on neurobehavioral evidence. J Neurol Sci. 2022;442:120434. https://doi.org/10.1016/j.jns.2022.120434.
Ariani GWM, Lingnau A. Decoding internally and externally driven movement plans. J Neurosci. 2015;35(42):14160–71. https://doi.org/10.1523/JNEUROSCI.0596-15.2015.
Kiernan JA. Anatomy of the temporal lobe. Epilepsy Res Treat. 2012;2012:176157. https://doi.org/10.1155/2012/176157.
Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995. https://doi.org/10.1093/brain/118.1.279.
Yukie M. Neural connections of auditory association cortex with the posterior cingulate cortex in the monkey. Neurosci Res. 1995;22:179–87.
Florio TM, Scarnati E, Rosa I, et al. The Basal Ganglia: More than just a switching device. CNS Neurosci Ther. 2018;24(8):677–84. https://doi.org/10.1111/cns.12987.
Mücke D, Hermes A, Roettger TB, et al. The effects of thalamic deep brain stimulation on speech dynamics in patients with essential tremor: an articulographic study. PLOS ONE. 2018;13(1):e0191359. https://doi.org/10.1371/journal.pone.0191359.
Chiu SY, Nozile-Firth K, Klassen BT, et al. Ataxia and tolerance after thalamic deep brain stimulation for essential tremor. Parkinsonism Relat Disord. 2020;80:47–53. https://doi.org/10.1016/j.parkreldis.2020.09.009.
Aumann TD. Cerebello-thalamic synapses and motor adaptation. Cerebellum. 2002;1(1):69–77. https://doi.org/10.1080/147342202753203104.
Marmarou CR. Periventricular white matter. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of clinical neuropsychology. New York: Springer; 2011. p. 1914–5.
Hazzaa NM, Mancini L, Thornton J, Yousry TA. Somatotopic organization of corticospinal/corticobulbar motor tracts in controls and patients with tumours: a combined fMRI-DTI study. Neuroimage Clin. 2019;23:101910. https://doi.org/10.1016/j.nicl.2019.101910.
Janelle F, Iorio-Morin C, D’Amour S, Fortin D. Superior longitudinal fasciculus: a review of the anatomical descriptions with functional correlates. Front Neurol. 2022;13:794618. https://doi.org/10.3389/fneur.2022.794618.
Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4(4):331–43. https://doi.org/10.1093/cercor/4.4.331.
Aziz Q, Furlong PL, Barlow J, et al. Topographic mapping of cortical potentials evoked by distension of the human proximal and distal oesophagus. Electroencephalogr Clin Neurophysiol. 1995;96(3):219–28. https://doi.org/10.1016/0168-5597(94)00297-r.
Kober SE, Wood G. Changes in hemodynamic signals accompanying motor imagery and motor execution of swallowing: a near-infrared spectroscopy study. Neuroimage. 2014;93(Pt 1):1–10. https://doi.org/10.1016/j.neuroimage.2014.02.019.
Soros P, Inamoto Y, Martin RE. Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Hum Brain Mapp. 2009;30(8):2426–39. https://doi.org/10.1002/hbm.20680.
Mateos-Aparicio P, Rodriguez-Moreno A. The impact of studying brain plasticity. Front Cell Neurosci. 2019;13:66. https://doi.org/10.3389/fncel.2019.00066.
Hamdy S, Aziz Q, Rothwell JC, Power M, Singh KD, Nicholson DA, Tallis RC, Thompson DG. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115(5):1104–12. https://doi.org/10.1016/s0016-5085(98)70081-2.
Gow D, Rothwell J, Hobson A, Thompson D, Hamdy S. Induction of long-term plasticity in human swallowing motor cortex following repetitive cortical stimulation. Clin Neurophysiol. 2004;115(5):1044–51. https://doi.org/10.1016/j.clinph.2003.12.001.
Jefferson S, Mistry S, Singh S, Rothwell J, Hamdy S. Characterizing the application of transcranial direct current stimulation in human pharyngeal motor cortex. Am J Physiol Gastrointest Liver Physiol. 2009;297(6):G1035–40. https://doi.org/10.1152/ajpgi.00294.2009.
Yang W, Cao X, Zhang X, Wang X, Li X, Huai Y. The effect of repetitive transcranial magnetic stimulation on dysphagia after stroke: a systematic review and meta-analysis. Front Neurosci. 2021;15:769848. https://doi.org/10.3389/fnins.2021.769848.
Li L, Huang H, Jia Y, et al. Systematic review and network meta-analysis of noninvasive brain stimulation on dysphagia after stroke. Neural Plast. 2021;2021:3831472. https://doi.org/10.1155/2021/3831472.
Du J, Yang F, Liu L, et al. Repetitive transcranial magnetic stimulation for rehabilitation of poststroke dysphagia: a randomized, double-blind clinical trial. Clin Neurophysiol. 2016;127(3):1907–13. https://doi.org/10.1016/j.clinph.2015.11.045.
Khedr EM, Abo-Elfetoh N, Rothwell JC. Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol Scand. 2009;119(3):155–61. https://doi.org/10.1111/j.1600-0404.2008.01093.x.
Michou E, Mistry S, Jefferson S, Tyrrell P, Hamdy S. Characterizing the mechanisms of central and peripheral forms of neurostimulation in chronic dysphagic stroke patients. Brain Stimul. 2014;7(1):66–73. https://doi.org/10.1016/j.brs.2013.09.005.
Park JW, Oh JC, Lee JW, Yeo JS, Ryu KH. The effect of 5Hz high-frequency rTMS over contralesional pharyngeal motor cortex in post-stroke oropharyngeal dysphagia: a randomized controlled study. Neurogastroenterol Motil. 2013;25(4):324-e250. https://doi.org/10.1111/nmo.12063.
Zhang C, Zheng X, Lu R, Yun W, Yun H, Zhou X. Repetitive transcranial magnetic stimulation in combination with neuromuscular electrical stimulation for treatment of post-stroke dysphagia. J Int Med Res. 2019;47(2):662–72. https://doi.org/10.1177/0300060518807340.
Park E, Kim MS, Chang WH, et al. Effects of bilateral repetitive transcranial magnetic stimulation on post-stroke dysphagia. Brain Stimul. 2017;10(1):75–82. https://doi.org/10.1016/j.brs.2016.08.005.
Cheng I, Sasegbon A, Hamdy S. Effects of neurostimulation on poststroke dysphagia: a synthesis of current evidence from randomized controlled trials. Neuromodulation. 2021;24(8):1388–401. https://doi.org/10.1111/ner.13327.
Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597–608. https://doi.org/10.1038/nrneurol.2014.162.
Lyons MK. Deep brain stimulation: current and future clinical applications. Mayo Clin Proc. 2011;86(7):662–72. https://doi.org/10.4065/mcp.2011.0045.
Vedam-Mai V, van Battum EY, Kamphuis W, Feenstra MGP, Denys D, Reynolds BA, Okun MS, Hol EM. Deep brain stimulation and the role of astrocytes. Mol Psychiatr. 2012;17(2):124–31. https://doi.org/10.1038/mp.2011.61.
McIntyre CC, Anderson RW. Deep brain stimulation mechanisms: the control of network activity via neurochemistry modulation. J Neurochem. 2016;139(Suppl 1):338–45. https://doi.org/10.1111/jnc.13649.
Chang MC, Park JS, Lee BJ, Park D. The effect of deep brain stimulation on swallowing function in Parkinson’s disease: a narrative review. Dysphagia. 2021;36(5):786–99. https://doi.org/10.1007/s00455-020-10214-y.
Lester DB, Rogers TD, Blaha CD. Neuronal pathways involved in deep brain stimulation of the subthalamic nucleus for treatment of Parkinson’s disease. Annu Int Conf IEEE Eng Med Biol Soc. 2009;2009:3302–5. https://doi.org/10.1109/IEMBS.2009.5333771.
Adam EM, Brown EN, Kopell N, McCarthy MM. Deep brain stimulation in the subthalamic nucleus for Parkinson’s disease can restore dynamics of striatal networks. Proc Natl Acad Sci U S A. 2022;119(19):e2120808119. https://doi.org/10.1073/pnas.2120808119.
Agarwal R, Sarma SV. The effects of DBS patterns on basal ganglia activity and thalamic relay : a computational study. J Comput Neurosci. 2012;33(1):151–67. https://doi.org/10.1007/s10827-011-0379-z.
Yu H, Takahashi K, Bloom L, Quaynor SD, Xie T. Effect of deep brain stimulation on swallowing function: a systematic review. Front Neurol. 2020;11:547. https://doi.org/10.3389/fneur.2020.00547.
Xie T, Vigil J, MacCracken E, et al. Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology. 2015;84(4):415–20. https://doi.org/10.1212/WNL.0000000000001184.
Xie T, Bloom L, Padmanaban M, et al. Long-term effect of low frequency stimulation of STN on dysphagia, freezing of gait and other motor symptoms in PD. J Neurol Neurosurg Psychiatry. 2018;89(9):989–94. https://doi.org/10.1136/jnnp-2018-318060.
Troche MS, Brandimore AE, Foote KD, et al. Swallowing outcomes following unilateral STN vs GPi surgery: a retrospective analysis. Dysphagia. 2014;29(4):425–31. https://doi.org/10.1007/s00455-014-9522-0.
Robertson LT, St George RJ, Carlson-Kuhta P, Hogarth P, Burchiel KJ, Horak FB. Site of deep brain stimulation and jaw velocity in Parkinson disease. J Neurosurg. 2011;115(5):985–94. https://doi.org/10.3171/2011.7.JNS102173.
Cheng I, Sasegbon A, Hamdy S. Dysphagia treatments in Parkinson’s disease: a systematic review and meta-analysis. Neurogastroenterol Motil. 2022. https://doi.org/10.1111/nmo.14517.
Simons A, Hamdy S. The use of brain stimulation in dysphagia management. Dysphagia. 2017;32(2):209–15. https://doi.org/10.1007/s00455-017-9789-z.
Funding
Nil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, KC., Wang, TG. & Hsiao, MY. The Cortical and Subcortical Neural Control of Swallowing: A Narrative Review. Dysphagia 39, 177–197 (2024). https://doi.org/10.1007/s00455-023-10613-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-023-10613-x