Abstract
A modified dual-task paradigm was designed to learn whether swallowing functions are selectively mediated by the left or right hemisphere. Healthy right-handed men (N = 38) were studied using videofluoroscopy to examine continuous straw drinking at baseline and with three interference conditions (silent word repetition, line orientation, finger tapping). Results indicate that activation of both right and left hemispheres can interfere with some swallowing behaviors. Findings suggest possibly different roles of the two hemispheres in the mediation of swallowing and support the notion that specific components of swallowing may be preferentially mediated by the left versus the right hemisphere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With the advent of neuroimaging techniques, it has been well documented that dysphagia, a disorder of swallowing, can result from unilateral stroke involving either cerebral hemisphere. It is unclear, however, if specific swallowing behaviors are consistently lateralized to either the right or the left hemisphere across the population, inconsistently lateralized across individuals, or nonlateralized. Thus far, lesion and functional imaging studies have yielded contradictory results; however, swallowing tasks and outcomes measured have varied in most studies, so exact comparisons between studies are difficult.
In humans, the lateralization of swallowing has primarily been based on ablation paradigms, which use computed tomography (CT) or magnetic resonance imaging (MRI) scans of focal lesions in stroke patients. All of these studies have identified that unilateral ischemic stroke of either cerebral hemisphere can produce dysphagia. Some investigators have found that different types of swallowing behaviors may be lateralized to the left and right hemispheres [1-3]. Specifically, left hemisphere damage (LHD) may be associated with oral stage dysfunction, and right hemisphere damage (RHD) may be associated with pharyngeal stage dysmotility and aspiration. Persistent dysphagia and aspiration may also be associated with RHD [4]. Other investigators have not found these hemispheric differences in swallowing behavior in the oral or pharyngeal stage of swallowing [5-8], in swallowing severity [6], increased pharyngeal transit times [9], lingual discoordination [10], or incidence of aspiration [5,7,8].
Functional imaging studies (positron emission tomography [PET], functional [f] MRI, transcranial magnetic stimulation [TMS], and magnetoencephalography [MEG]) have been used to investigate swallowing laterality in vivo in healthy adults, mostly right-handers. As with lesion studies, results have been inconsistent. That is, while most functional studies have described some degree of lateralization, lateralization is varied in regard to hemisphere and specific cytoarchitectonic site. TMS studies have found discrete somatotopic organization of swallowing musculature on the motor and premotor cortices with an asymmetric representation of swallowing within subjects [11,12]. That is, swallowing may not be consistently lateralized to one cerebral hemisphere at the population level, but within individuals one hemisphere tends to be more important than the other in mediating swallowing. Bilateral but individual asymmetric activation of the sensorimotor cortex has also been found using PET imaging [13]. In contrast, other PET [14] and fMRI studies [15,16] have identified symmetric bilateral activation of the sensorimotor cortex during swallowing. Asymmetric activation of the right insula, however, has been consistently identified across studies and may represent a critical lateralized substrate for swallowing [14–16]. Martin et al. [16] noted that the hemisphere with the larger swallowing activation varied across task within individual subjects and posited that the mechanisms underlying the functional lateralization of swallowing may be related to the behavioral context of the task. Kern et al. [17] also found asymmetric hemisphere activation dependent on the type of swallowing task. Right hemispheric activation was associated with volitional swallows, whereas left hemispheric activation was associated with reflexive swallows. One study found asymmetric left hemispheric activation of the sensorimotor cortex and insula using MEG [18] with the degree of lateralization dependent upon task complexity. Whereas volitional swallowing was strongly left lateralized, reflexive water swallowing was less lateralized, and tongue movement to the hard palate was not lateralized.
Using various imaging modalities, results thus far are inconclusive as to whether swallowing is lateralized at the cortical level. These conflicting results may be, in part, a result of the time post-onset in which stroke patients are studied, the lack of control of handedness in stroke patients, the type of neuroimaging method, and/or the type of swallowing task performed. It may be that specific components of swallowing (volitional and reflexive) may be differentially lateralized or dissociated at the cortical level.
Another way to study lateralization is with a dual-task paradigm. The dual-task paradigm has been used extensively in neuropsychology to investigate lateralized functions. This paradigm indirectly evaluates lateralized cortical systems by comparing performance on tasks at baseline and with a concurrent or competitive condition. The most common paradigm is the verbal-manual interference paradigm. In this paradigm the subject performs a motor task, such as finger tapping, with the dominant and nondominant hands at baseline (without interference) and with interference. The interference task consists of a verbal output such as repeating a series of words. Because distal hand movements are mediated by the contralateral primary motor cortex, right-handers will be activating the left primary motor cortex when tapping with the right hand and vice versa when tapping with the left hand [19]. In the baseline condition, right-handers typically tap faster with the right than with the left hand. Concurrent verbalization (verbal-manual interference condition) yields a decrement in right-hand finger-tapping rate compared with baseline finger-tapping rate [20,21]. There are several theories that have been proposed to explain verbal-manual interference. The most widely accepted theory is the “functional cerebral space model” which indicates that coactivation of functionally overlapping neural substrates will yield a decline in response in one of the two concurrent activities [22]. Others have posited that “hemispheric overload” [23] or “competition of resources” [24] produces a decrement in response. That is, allocation of attentional or processing resources is compromised because of two tasks sharing resources within the same hemisphere. The neural system has limited functional capacity; therefore, there is a decrement in one concurrent behavioral response from the baseline condition.
In a previous study, we used a modified dual-task paradigm to investigate swallowing lateralization [25]. In the study, we measured swallowing at baseline and during two concurrent tasks: finger tapping (left and right hands) and silent word repetition (left hemispheric activating task) in 14 right-handed men. Tapping rate decreased in both right and left hands during concurrent swallowing, and volume per swallow decreased with concurrent silent repetition. These results offered partial support for the bilateral representation of swallowing and also suggested left hemisphere involvement. However, this study did not adequately address the potential right hemisphere contribution because a right hemisphere-lateralized interference task comparable to silent word repetition was not included.
The primary aim of the present investigation was to continue to define lateralization of swallowing using a modified dual-task paradigm. We expanded our earlier study design by including a visuospatial line-orientation task known to be right hemisphere-lateralized. Based on the results of our initial study [25], we hypothesized that there would be a significant decline in volume per swallow with the silent repetition task (higher-order left hemisphere task). Neural regions activated for swallowing and motor speech are anatomically close and functionally overlapping [26,27]. The cerebral space model suggests that coactivation of functionally overlapping and anatomically close neural substrates will yield a decline in response in one of the two concurrent activities [22]. It was predicted that there would not be significant differences between baseline swallowing averages and averages with a concurrent visuospatial task (higher-order right hemisphere task) because the motor neural regions for swallowing and visuospatial processing, which is predominantly an occipitoparietal right hemisphere function, are not anatomically close. Based on our previous findings, we also posited that finger-tapping rates would be slower with concurrent swallowing compared with baseline tapping rates, with tapping rates similarly reduced for the two hands with swallowing interference.
Materials and Methods
Subjects
Healthy right-handed men (N = 38, average age = 49.27 years, SD = 25.82) participated in the study. Handedness was confirmed using a survey comprising 12 questions about hand preference for unimanual tasks [28]. Exclusion criteria were history of dysphagia, head and neck structural damage, neurologic disorders, and left-handed parents or siblings. The study was approved by the Institutional Review Board at Tulane University Health Sciences Center and by the Veterans Affairs Medical Center in New Orleans, and all subjects provided written consent.
Swallowing
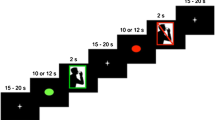
Videofluoroscopic swallowing samples were recorded on a Super-VHS videocassette recorder. Lateral radiographic views of swallowing were obtained with the fluoroscopic tube focus encompassing the oral cavity (rostral to the lips) and the pharynx (caudal to the UES). Two 10-s trials were completed for each condition and task order was randomized and counterbalanced. For each swallowing trial, 300 ml of diluted liquid barium (2:1, juice to barium) was ingested through a straw with the examiner holding the cup and straw. Subjects were instructed to continually swallow at a normal rate without pausing until signaled to stop. Number of swallows during sequential swallowing was totaled using the video recordings and was averaged across the two trials. Volume per swallow was calculated by dividing the total amount ingested during each trial by the number of swallows.
Interference Conditions
Interference conditions consisted of a higher-order left hemisphere-activating task (silent repetition), higher-order right hemisphere-activating task (silent line orientation), and motor tasks (right- and left-hand finger-tapping rate). The left primary motor cortex is activated during silent repetition [29], whereas research has identified impairment in judging line orientation to be associated with right hemispheric lesions [30,31]. fMRI studies have demonstrated that finger tapping activated motor regions contralateral to the performing hand [19].
For silent repetition, subjects silently repeated a three-word set (wolf, butterfly, duck) as they continuously swallowed. That is, they did not vocalize the words during this condition; rather, they were instructed to rapidly and repeatedly think the three-word set. Confirmation of silent repetition was obtained after each trial. With line orientation, subjects silently matched line stimuli to the identical slope in a “sun ray” of lines [32] as they repeatedly swallowed. Subjects were instructed to think of the answer but not vocalize it. Stimuli were presented every 3 s for a total of four stimuli presented in each of the two 10-s trials. Confirmation of the visuospatial task performance was ascertained after each trial. Only 23 of the 38 subjects completed the spatial task. For the motor task, subjects used their index finger to tap on a board that had an attached counter switch to register the number of taps while concurrently swallowing. Number of taps (right and left hand) was recorded at baseline and while swallowing.
Statistical Analyses
Testing Manual, Verbal, and Visuospatial Interference Effects on Swallowing
Multivariate repeated-measures analysis of variance (MANOVA) was used to test all interference effects. The single independent variable (IV) was interference and dependent variables (DVs) were volume per swallow and number of swallows. Because only a subset of participants completed the line-orientation task, these conditions were analyzed in a separate MANOVA. The MANOVA was therefore run twice: first with the IV manual-verbal interference (baseline, right-hand finger tapping, left-hand finger tapping, word repetition) and then with the IV visuospatial interference (baseline, visuospatial interference). Where indicated by significant MANOVA effects, repeated-measures analyses of variance (ANOVA) were used to test univariate interference effects.
Testing Swallowing Interference Effects on Finger Tapping
The effect of swallowing on finger tapping was tested via a two-way repeated-measures ANOVA with IVs of swallowing (baseline, swallowing) and tapping hand (left, right). The DV was number of taps.
For each ANOVA described above, Huynh-Feldt-corrected degrees of freedom were used when the sphericity assumption was violated. Pairwise comparisons were made using the estimated marginal means method [33]. An alpha level of 0.05 was required for significance on all analyses.
Results
Manual and Verbal Interference Effects on Swallowing
Means for swallowing variables are shown in Figure 1 for each level of manual-verbal interference. There was a significant multivariate effect of interference, Wilkes’ Lambda = 0.571, F(6,32) = 4.00, p = 0.004. Univariate effects were significant for both volume per swallow (F[2.572] = 4.98, p = 0.005) and number of swallows (F[2.572] = 3.19, p = 0.039). Volume per swallow was significantly lower during word repetition than at baseline (p = 0.004), during right-hand finger tapping (p = 0.003), and during left-hand finger tapping (p = 0.021). Number of swallows was significantly lower for right- (p = 0.030) and left- (p = 0.024) hand finger tapping conditions than at baseline.
Visuospatial Interference Effects on Swallowing
Means for swallowing variables are shown in Figure 2 for each level of visuospatial interference. There was a significant multivariate effect of visuospatial interference, Wilkes’ Lambda = 0.575, F(2,21) = 7.75, p = 0.003. At the univariate level, there was a significant effect of visuospatial interference on number of swallows (F[1,22] = 16.17, p = 0.001) but not on volume per swallow (F[1,22] = 1.21, p = 0.283).
Swallowing Interference Effects on Finger Tapping
Right- and left-hand finger-tapping means are shown in Figure 3 for each level of swallowing interference. Participants tapped significantly more with the right hand than with the left hand, F(1,37) = 39.48, p < 0.0005 for tapping hand main effect. Neither the swallowing interference main effect nor the swallowing interference × tapping hand interaction was statistically significant.
Discussion
The notion of swallowing lateralization is controversial, and two competing hypotheses have been put forth. One theory asserts that there is bilateral control of swallowing with perhaps some hemispheric specialization of deglutition. The other hypothesis suggests that there is a dominant hemisphere for swallowing, although the pattern of lateralization may not be consistent across individuals. Another view of the cortical organization of swallowing could be that different components of swallowing are differentially lateralized. For example, the left hemisphere may more selectively mediate the oral phase and volitional components, whereas the right hemisphere may contribute more to the pharyngeal phase and automatic-reflexive aspects of swallowing. Empirical studies have supported each hypothesis. The present study was undertaken to further study swallowing lateralization using a modified dual-task paradigm in healthy right-handed adults. It is the first study using a dual-task paradigm to measure the effects of higher-order left and right hemisphere-activating tasks and left and right hemisphere motor tasks on swallowing behavior.
Using this paradigm, we found right and left cerebral hemisphere contributions to swallowing. The two hemispheres, however, may mediate different components of swallowing. Silent repetition, which primarily activates the left hemisphere, and visuospatial processing, which primarily activates the right hemisphere, interfered with swallowing behavior in different ways. The left hemisphere task induced a decrease in swallowing volume, while the right hemisphere task reduced swallowing rate. Both right- and left-hand finger tapping, which selectively activates the left and right sensorimotor cortex, respectively, interfered with swallowing. These results are consistent with some prior functional imaging and lesion studies as well as with our earlier dual-task study, demonstrating that swallowing may not be consistently lateralized and some components may be bilaterally represented at the cortical level. The implications of these results will be discussed in more detail below.
Results on swallowing behavior differed in each of the cognitive tasks. Silent repetition, a higher-order left hemisphere-activating task, yielded a significant change in the volume per swallows, while line orientation, a higher-order right hemisphere-activating task, impacted the number of swallows. These findings suggest perhaps a more complex nature of swallowing lateralization and might indicate that the two hemispheres have different roles in swallowing behavior. The finding of decreased volume per swallow with silent repetition suggests that the left hemisphere plays a functional role in swallowing. Based on our results and the findings reported by others [2,3,11], it may be that the left hemisphere plays a major role in volitional aspects of swallowing and the oral phase. Awareness and regulation of bolus size may be a volitional component of swallowing mediated during the oral phase of swallowing. Both of these selective functions may be left lateralized. In contrast, it may be that the timing of swallows represents a more automatic-reflexive swallowing behavior that is associated with right hemispheric neural systems. Other factors may be that the two cognitive tasks varied in complexity and may activate different anatomic loci within the hemispheres, with the language task more frontal and the visuospatial task more posterior cortical.
Particular lesion localization studies offer support for these findings. Robbins et al. [2, 3] identified oral stage dysfunction “swallowing apraxia” to be associated with LHD and pharyngeal stage dysfunction to be associated with RHD. Daniels et al. [1] also found that RHD damage was associated with pharyngeal stage dysmotility, but they did not identify oral stage dysmotility with LHD. In contrast, numerous other studies have not found laterality differences in oral versus pharyngeal stage dysmotility patterns [5,7]. This lack of consistent findings in the stroke literature may indicate that in some people different components of swallowing may lateralize differently and may explain individual variation. These results may also relate to findings from functional imaging studies in which swallowing lateralization varied as a function of task within individuals [16] and across groups [17]. Our results support the notion that specific components of swallowing may be preferentially mediated by the left versus right hemisphere, i.e., oral-volitional components may be left lateralized and pharyngeal-reflexive components may be more right lateralized.
Finger-tapping results revealed a decrease in the number of swallows with concurrent right and left finger tapping. The right and left finger-tapping tasks used in this paradigm are similar in task demands and represent an automatic motor control task that becomes self-paced and rhythmic. This task may interfere with the timing of the swallow, thus reducing swallowing rate (numbers of swallows) in both the left and right conditions. These motor interference tasks also yielded a decremental response in the volume per swallow. Because both swallowing rate and volume were affected, it may be that the anatomic substrates for finger tapping and swallowing are anatomically overlapping to a greater degree than the cognitive tasks used in our paradigm.
Swallowing and finger tapping are mediated by the primary motor cortex, with modulation of response provided by somatosensory and peripheral sensory input [19,33]. The motor hand representation is anatomically close to the representation of the face, larynx, and pharynx along the length of the primary motor cortex (precentral gyrus, Brodmann’s area 4). Thus, coactivation of these anatomically close neural substrates during the concurrent finger-tapping-swallowing task may have contributed to the decline in the number of swallows with right and left finger tapping.
When considering swallowing mediation, factors in addition to hemisphere must be considered. Daniels and Foundas [7] have suggested that location may be more important than hemisphere in determining dysphagia in stroke patients. Lesions located anterior to the central sulcus and subcortical periventricular white matter sites were common in patients with risk of aspiration. Thus, it may be that an interaction between hemisphere, lesion location, and even lesion size determines the incidence of dysphagia, dysmotility pattern, aspiration, and recovery. No study as of yet has had a large enough sample size to fully determine whether one of these variables or a specific combination of variables is more important than others.
References
Daniels SK, Foundas AL, Iglesia GC, Sullivan MA: Lesion site in unilateral stroke patients with dysphagia. J Stroke Cerebrovas Dis 6:30-34, 1996
Robbins J, Levine R: Swallowing after unilateral stroke of the cerebral cortex: preliminary experiences. Dysphagia 3:11-17, 1988
Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB: Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil 74:1295-1300, 1993
Smithard DG, O’Neill PA, Martin DF, England R: Aspiration following stroke: is it related to the side of stroke? Clin Rehabil 11:73-76, 1997
Alberts MJ, Horner J, Gray L, Brazer SR: Aspiration after stroke: lesion analysis by brain MRI. Dysphagia 7:170-173, 1992
Chen MYM, Ott DJ, Peele VN, Gelfand DW: Oropharynx in patients with cerebrovascular disease: evaluation with videofluoroscopy. Radiology 176:641-643, 1990
Daniels SK, Foundas AL: Lesion localization in acute stroke patients with risk of aspiration. J Neuroimaging 9:91-97, 1999
Perlman AL, Booth BM, Grayhack JP: Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia 9:90-95, 1994
Johnson ER, McKenzie SW, Rosenquist J, Liebermann JS, Sievers AE: Dysphagia following stroke: quantitative evaluation of pharyngeal transit times. Arch Phys Med Rehabil 73:419-423, 1992
Daniels SK, Brailey K, Foundas AL: Lingual discoordination and dysphagia following acute stroke: analyses of lesion localization. Dysphagia 14:85-92, 1999
Hamdy S, Aziz Q, Rothwell JC, Singh KD, Barlow J, Hughes DG, et al.: The cortical topography of human swallowing musculature in health and disease. Nat Med 2:1217-1224, 1996
Hamdy S, Aziz Q, Rothwell JC, Crone R, Hughes D, Tallis RC, et al.: Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet 350:686-692, 1997
Hamdy S, Rothwell JC, Brooks DH, Bailey D, Aziz Q, Thompson DG: Identification of the cerebral loci processing human swallowing with H 152 O PET activation. J Neurophysiol 81:1917-1926, 1999
Zald DH, Pardo JV: The functional neuroanatomy of voluntary swallowing. Ann Neurol 46:281-286, 1999
Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, et al.: Cortical activation during human volitional swallowing: an event-related MRI study. Am J Physiol 277:G219-G225, 1999
Martin RE, Goodyear BG, Gati JS, Menon RS: Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 85:938-950, 2001
Kern MK, Jaradeh S, Arndorfer RC, Shaker R: Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol 280:G354-G360, 2001
Dziewas R, Soros P, Ishii R, Chau W, Henningsen H, Ringelstein EB, et al.: Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. NeuroImage 20:135-144, 2003
Rao SM, Binder JR, Hammneke TA, Bandettini PA, Bobholz HA, et al.: Somatotopic mapping of the human primary motor cortex with functional magnetic resonance imaging. Neurology 45:919-924, 1995
Kee DW, Bathurst K, Hellige JB: Lateralized interference of repetitive finger tapping: influence of familial handedness, cognitive load, and verbal production. Neuropsychologia 21:617-624, 1983
Dalen K, Hugdahl K: Inhibitory versus facilitory interference for finger-tapping to verbal and nonverbal, motor, and sensory tasks. J Clin Exp Neuropsychol 8:627-636, 1986
Kinsbourne M, Hicks RE: Functional cerebral space: a model for overflow, transfer, and interference effects in human performance. In: Requin J (ed.) Attention and Performance VII. Hillsdale, NJ: Erlbaum, 1978, pp 345-362
Dalby IJ Hemispheric timesharing: verbal and spatial loading with concurrent unimanual activity. Cortex 16:567-573, 1980
Kee DW, Bathurst K, Hellige JB: Lateralized interference in finger tapping: assessment of block design activities. Neuropsychologia 22:197-203, 1984
Daniels SK, Corey DM, Barnes CL, Faucheaux NM, Priestly DH, Foundas AL: Cortical representation of swallowing: a modified dual task paradigm. Percept Mot Skills 94:1029-1040, 2002
Miller AJ: The Neuroscientific Principles of Swallowing and Dysphagia. San Diego, CA: Singular, 1999
Carpenter MB: Core Text of Neuroanatomy, 3rd edn. Baltimore, MD: Williams & Wilkins, 1985
Briggs GG, Nebes RD: Patterns of hand preference in a student population. Cortex 11:230-238, 1974
Warburton E, Wise RJS, Price CH, Weiller C, Hadar U, Ramsay S, et al.: Noun and verb retrieval by normal subjects: studies with PET. Brain 119:159-179, 1996
Benton AL, Hannay J, Varney NR: Visual perception of line direction in patients with unilateral brain disease. Neurology 25:907-910, 1975
Warrington EK, Rabin P: Perceptual matching in patients with cerebral lesions. Neuropsychologia 8:475-487, 1970
Benton AL, Hamsher K, Varney NR, Spreen O: Contributions to Neuropsychological Assessment. New York: Oxford University Press, 1983
Searle SR, Speed FM, Milliken GA: Population marginal means in the linear model: an alternative to least squares means. Am Stat 34:216-221, 1980
Acknowledgments
This research was supported in part by the National Institutes of Health (NIH) National Institute of Deafness and Communication Disorders Grant DC00135 (ALF), the Tulane-Charity-LSU General Clinical Research Center Grant RR5096, and the Department of Veterans Affairs South Central MIRECC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daniels, S.K., Corey, D.M., Fraychinaud, A. et al. Swallowing Lateralization: The Effects of Modified Dual-Task Interference. Dysphagia 21, 21–27 (2006). https://doi.org/10.1007/s00455-005-9007-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-005-9007-2