Abstract
Dysphagia is found in up to 80% of acute stroke patients. To date most studies have focused on ischemic stroke only. Little is known about the incidence and pattern of dysphagia in hemorrhagic stroke. Here we describe the characteristics of dysphagia in patients with striatocapsular hemorrhage. Fiberoptic Endoscopic Evaluation of Swallowing (FEES) was carried out in 30 patients with acute striatocapsular hemorrhage. Dysphagia was classified according to the six-point Fiberoptic Endoscopic Dysphagia Severity Scale (FEDSS) within 72 h after admission. Lesion volume, hemisphere and occurrence of ventricular rupture were determined from computer tomography scans. Data on initial NIH-SS, clinical symptoms, need for endotracheal intubation, diagnosis of pneumonia and feeding status on discharge were recorded. Swallowing impairment was observed in 76.7% of patients (n = 23). Mean FEDSS score was 3.1 ± 1.5. Main findings were penetration or aspiration of liquids as well as leakage to valleculae and piriform sinus. Incidence of pneumonia was 30.0% (n = 9). Age, NIH-SS and hematoma volume did not correlate with dysphagia severity. None of the clinical characteristics was predictive for dysphagia. On discharge after 12.9 ± 5.3 days, a two-point improvement on the FEDSS was seen in seven patients, (30.4%) and five patients (21.7%) had gained at least one point. In striatocapsular hemorrhage, dysphagia is a common and so far underrecognized symptom. FEES results indicate predominant impairment of oral motor control. Swallowing impairment is not related to other clinical deficits, stroke severity or lesion characteristics. Thus, detailed dysphagia assessment is indicated in all cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysphagia is an important symptom of acute stroke, with a reported incidence ranging between 29 [5] and 81% [44] depending on the diagnostic criteria, timing and method of assessment, and stroke features in the respective patient population [43]. Due to malnutrition [11, 12] and aspiration, disturbed swallowing is associated with the development of chest infection, prolonged hospital stay, increased mortality and poor long-term outcome (i.e., dependency and nursing home admission) [30, 40, 43, 52, 53]. It has been shown that early detection of swallowing impairment guiding decisions about feeding strategy and leading towards timely treatment is associated with a more favorable outcome in dysphagic stroke patients [8, 18, 22, 29]. Therefore, current research focuses on investigating the relationship between stroke characteristics and dysphagia features in order to identify predictors for impaired swallowing and to distinguish those patients who have a special need for further evaluation or intervention. However, presumably due to its higher incidence, many studies have been focused on ischemic strokes only [39, 54]. Others included a small number of hemorrhagic stroke cases, but did not analyze them as a separate group regarding swallowing disturbance patterns [28, 40, 41, 49]. There is only one study, by Smithard et al. (2007) [53], that shows that patients classified as “not being safe to swallow” were more likely to have had an intracranial hemorrhage of any location including subarachnoid hemorrhage than an ischemic infarction.
The striatocapsular area is of particular interest as it is the most frequently affected site of spontaneous intracerebral hemorrhage caused by the main risk factor, hypertension [4, 9, 17, 50]. Although this area is supplied by a variety of arteries, it is still referred to as a single clinical entity and also known as basal ganglionic hemorrhage [9].
Thus, the purpose of this study was to describe the characteristics of dysphagia in patients with intracerebral hemorrhage of the striatocapsular area using Fiberoptic Endoscopic Evaluation of Swallowing (FEES), which has proved to be an excellent bedside method for a quick, safe and precise dysphagia assessment in the first days after stroke [16, 36, 38, 59–61].
Patients and methods
Subjects
Thirty consecutive first-ever stroke patients meeting the inclusion criteria were enrolled in this study. Patients were eligible to be included if they had an isolated striatocapsular hemorrhage diagnosed by CT scan on admission. The striatocapsular area was defined as an area including the caudate nucleus, putamen, globus pallidus, anterior and posterior limbs of the internal capsule and subinsular area [9, 24]. Lesion side and the presence of ventricular rupture were determined. Hematoma size was calculated using the ABC/2 method where A is the greatest hemorrhage diameter by CT, B is the diameter 90° to A, and C is the approximate number of CT slices with hemorrhage multiplied by the slice thickness [23, 33]. Exclusion criteria were symptom onset more than 24 h before admittance to our stroke unit, preexisting swallowing dysfunction, a severely reduced state of consciousness and endotracheal intubation on admission. Stroke severity was measured on admission using the National Institutes of Health Stroke Scale (NIH-SS) [7].
All patients were evaluated by FEES within 72 h after admission. Stroke symptoms, later need for endotracheal intubation, occurrence of pneumonia and feeding status by the time of discharge from our hospital were documented. The diagnosis of pneumonia was based on the presence of three or more of the following features during hospital stay: fever (>38°C), productive cough with purulent sputum, abnormal respiratory examination [tachypnea (>22/min), tachycardia, inspiratory crackles, bronchial breathing], abnormal chest radiograph, arterial hypoxemia (PO2 < 9.3 kPa), or isolation of a relevant pathogen and use of antibiotics [40]. Informed consent was obtained from all subjects, or their next of kin, in case the patient’s communication was impaired.
Equipment
Equipment consisted of a 3.1-mm-diameter flexible fiberoptic rhinolaryngoscope (ENF-P4, Olympus, Hamburg, Germany), light source (Endovision Telecam, SL PAL 20212020, Storz, Tuttlingen, Germany), camera (Endovision Telecam, SL PAL 20212030, Storz), color monitor (DVM 14M2MDE, Sony, Tokyo, Japan) and video recorder (SVO9500MDP, Sony). All examinations were recorded and stored on hard disc for later review.
Dysphagia assessment
All subjects were endoscopically assessed by a trained neurologist together with a speech language pathologist in accordance with our protocol for fiberoptic endoscopic assessment of dysphagia in acute stroke patients, which we had previously developed and evaluated [16, 59, 60]. Following this protocol each examined patient was classified according to our six-point fiberoptic endoscopic dysphagia severity scale (FEDSS) that allows a quick deduction of clinical consequences. The detailed protocol is described elsewhere [16, 59]. In brief, the examination starts with rating the severity of oropharyngeal secretions. In case saliva pooling with penetration or aspiration was found, severe dysphagia was suspected, and a score of 6 was given. Patients who were able to handle their saliva without penetration or aspiration received a teaspoon of puree consistency next. Those who showed penetration or aspiration without sufficient protective reflex (i.e., coughing or swallowing) on at least one of three attempts were again diagnosed with severe dysphagia (score 5). If sufficient protective reflexes were present, score 4 was attributed. Patients managing puree consistency without any aspiration events were exposed to a teaspoon of colored water. Its penetration or aspiration without sufficient protective reflex kept the patient on his former score, while the presence of protective reflexes led to score 3. If patients were able to swallow liquids three times without penetration or aspiration, a small piece of white bread was given to them at the last step. Here, penetration or aspiration or massive residues (>50% of bolus size) in the valleculae or piriform sinus were taken as evidence of severe difficulty with this food consistency, resulting in score 2. If none of these findings were observed on three consecutive trials, a score of 1 was given. Any score above 1 on the FEDSS was classified as dysphagia.
Each of the six scores is accompanied by specific recommendations concerning protective and rehabilitative measures. For example, scores 4–6 are all indicative of severe dysphagia that forbids an oral diet (score 6: no oral food; endotracheal intubation maybe necessary in case of respiratory distress; score 5: nil by mouth; score 4: small amount of pureed food during swallowing therapy only). On the other hand, scores 1–3 allow for an early oral feeding with a specifically adapted diet (score 3: puree consistency only, score 2: soft solid food, score 1: normal diet) [16].
According to the FEES standard protocol proposed by Langmore [35, 37], salient parameters of swallowing function such as premature spillage and delayed swallowing reflex were also examined. As described in detail elsewhere [35, 58], the swallowing reflex was rated as delayed if both the norms of spillage frequency to the pyriforms and the normal average dwell time in the pyriforms (i.e., 1.5 s) were exceeded. A disturbed oral bolus control was determined when food and/or liquid boluses spilled over the base of tongue into the hypopharynx during the oral swallowing stage, long before the swallow was initiated (test of oral containment).
Statistical analysis
Descriptive statistics were used to quantify patient characteristics and salient videoendoscopic findings. Binary logistic regression analysis was applied to test for significant associations among stroke symptoms, presence of ventricular rupture and lesion side with occurrence of penetration/aspiration and dysphagia in general. Spearman correlation analyses were performed to investigate the relation among age, NIH-SS, hematoma volume and FEDSS. The analyses were carried out using PASW Statistics 18.0.
Results
Demographic and clinical features as well as stroke characteristics of the 30 patients enrolled in the study are presented in Table 1. The average NIH-SS of our study population was 11 points, indicating a prominent neurological deficit in most of the included patients. The mean hematoma volume was 14.71 ml, and ventricular rupture occurred in five cases. One patient was managed surgically (ventricular drain). Four patients required endotracheal intubation after FEES because of insufficient protective reflexes (n = 1), respiratory failure (n = 2) and need for neurosurgical intervention (n = 1). Pneumonia was diagnosed in nine cases (30.0%).
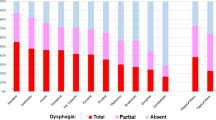
The period of time from admission to FEES performance was less than 48 h on average. The procedure was tolerated well by all patients. Frequencies of salient videoendoscopic findings and the resulting FEDSS score are displayed in Table 2. The overall incidence of dysphagia (i.e., FEDSS > 1) was 76.7% (n = 23). The mean FEDSS score was 3.1 ± 1.5. Except for one patient who showed pooling of secretions and silent aspiration of saliva, at least one food consistency could be tested in all subjects. One third of the patients showed penetration of puree consistency, while aspiration without sufficient protective reflex only happened in one case so that the examination was stopped at this point. With liquids, penetration occurred in almost two-thirds of the 28 remaining patients, and 39.3% of these showed aspiration as well. The majority of subjects (n = 23, 76.7%) were found with leakage to the valleculae, even reaching into the pirifom sinus in 16 cases. In two patients, isolated leakage to the valleculae was observed, which does not count for the FEDSS score. Thus, a score of 1 was given. Penetration and severe residue were uncommon in the ten patients receiving soft solid food, and aspiration was not noticed. According to their respective FEDSS score, 14 patients were put on “nil by mouth” and enteral tube feeding was started; 8 of these patients had small amounts of pureed food during swallowing therapy only. Six subjects were able to manage pureed food, and in three patients soft solid food could be given. Seven subjects demonstrated no relevant signs of dysphagia, and dietary adaptations were not necessary.
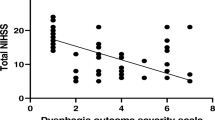
Presence of ventricular rupture, lesion side and stroke symptoms did not predict dysphagia in general or the occurrence of penetration/aspiration in particular. Patients’ age inversely correlated with hematoma volume (p < 0.05) and NIH-SS on admission (p < 0.01), whereas NIH-SS and hematoma volume showed a strong positive correlation (p < 0.01) (see Table 3). None of these three parameters significantly related to FEDSS score.
By the time of discharge from our hospital, four patients (13.3%) were nourished via a percutaneous endoscopic gastrostomy (PEG) tube, and two patients (6.7%) were still on nasogastric tube feeding. Pureed food was managed by eight subjects (26.7%), and six subjects (20.0%) were on soft solid food. Ten patients (33.3%) were on a normal diet. Regarding the 23 patients with dysphagia on admission, 30.4% (n = 7) gained two points on the FEDSS, and 21.7% (n = 5) improved at least for one point. In 43.5% (n = 10), dysphagia severity remained unchanged, and a single subject changed one point for the worse. Mean duration of hospitalization was 12.9 ± 5.3 days. There were no in-hospital deaths.
Discussion
Our results show that clinically significant dysphagia is a common symptom in striatocapsular hemorrhage with an incidence that is among the highest reported so far for a specific lesion location [41, 43]. Only in brainstem stroke was dysphagia found in more than 80% of cases [44]. Subjects had predominant impairment of the oral swallowing phase with premature leakage as a main finding. The mean FEDSS score of our study population was within the range of the results of former studies (2.29 ± 1.57 [59] to 3.96 ± 1.40 [16]) in which a mixed stroke patient population (i.e., hemorrhagic and ischemic) had been involved. This is striking as the basal ganglia and internal capsule are not brain areas typically linked to swallowing at first sight and up to now dysphagia was not recognized as a typical symptom in striatocapsular hemorrhage [51]. Several studies using different swallowing tasks and imaging methods demonstrated activation in this area during deglutition [26, 46, 47, 55, 63], whereas others did not [27, 42]. Clinical studies show increasing evidence for the relevance of subcortical lesions as the cause of dysphagia [10, 14, 39, 48, 62]. The basal ganglia functionally connect the cerebral cortex and the thalamus with one likely function being gating of sensory input to achieve motor control in deglutition [31, 55]. It seems that damage of the basal ganglia and internal capsule in our patients mainly led to disturbed oral motor control causing premature leakage in the majority of subjects. In accordance with our results Steinhagen et al. (2009) [54] comparing clinical and videoendoscopic dysphagia patterns with predefined lesion locations found that infarctions of basal ganglia and the internal capsule were associated with buccofacial apraxia, also resulting in oral phase dysphagia. Han et al. (2005) [28] reported that patients with recurrent cortico-subcortical stroke exhibited a significantly higher rate of abnormalities in the oral phase, including leakage, than their counterparts with brainstem stroke, which is known to cause mainly pharyngeal phase dysphagia [3, 34, 56]. Moreover, Cola et al. (2010) [10] stated that swallowing deficits involving oral control and transfer might be a marker of subcortical neural axis involvement. Thus, there is growing evidence that impairment of oral motor control is a characteristic symptom of striatocapsular lesions.
Presence of dysphagia in general as well as penetration and aspiration in particular were not predicted by other clinical symptoms. This may partly be due to the fact that the small patient population was quite homogenous regarding their clinical deficits, and some symptoms like facial palsy were present in almost every subject. Pneumonia incidence of 30% in our study population was within the upper range of previously reported findings in acute stroke [15, 28, 52, 59]. More than 43% of patients aspirated. In support of our results other authors found that subcortical and especially basal ganglionic infarctions are significantly associated with occurrence of aspiration [1, 14] and poststroke infection [57]. Moreover, Minnerup et al. (2010) [45] reported a positive correlation between intracerebral hemorrhage and incidence of pneumonia and infections in general.
There is an ongoing debate whether dysphagia severity and deficits in a particular stage of swallowing are related to lesion side. Concerning subcortical stroke, prior research has suggested left hemispheric infarction to be associated with a higher degree of dysphagia [10], although the sample size in this study was small, and the power is therefore limited. We did not find any association between lesion side and dysphagia. Ventricular rupture and lesion volume are generally associated with a worse outcome [6, 13, 17]. However, in our study these factors were not related to the FEDSS score. This may be due to the small sample size and a selection bias: subjects with large hematoma and extensive ventricular rupture were more likely to be intubated on admission and therefore excluded from the trial. The same is possibly true for the reciprocal association between age and NIH-SS or hematoma volume. The older the patients, the more likely they were to be excluded because of a severely reduced state of consciousness [2]. In a recent study by Kiphuth et al. [32], neurocritical care patients with spontaneous intracerebral hemorrhage were investigated searching for predictors of later percutaneous endoscopic gastrostomy (PEG) tube placement. Their results partly go along with ours because besides lobar hemorrhages with increasing volume, there was no other hematoma site, and especially not hematoma volume, which was associated with PEG tube placement.
So far we have little information on the natural history of recovery of swallowing deficits after purely subcortical or hemorrhagic stroke. We show for the first time data of the clinical course of dysphagia and its resulting nutritional adaptations in the acute phase after striatocapsular hemorrhage. Cola (2010) [10] observed that, despite receiving swallowing treatment, all of their four dysphagic patients suffering from subcortical infarction remained on an unaltered diet throughout hospitalization. Others hypothesized that dysphagia resulting from acute disconnection between the cortex and brain stem is likely to improve quickly as compared to dysphagia resulting from damage to important primary areas for swallowing control [25]. This seems to be true for our study population: within 2 weeks more than half of our initially dysphagic patients (n = 23) gained at least one point on the FEDSS, indicating substantial short-term recovery. By the time of discharge 74% were on an oral diet; only 26% were still on tube feeding. These results are in line with another bigger study tracking clinical improvement in a mixed stroke population (i.e., ischemic and hemorrhagic, cortical and subcortical) [21]. However, it is still unknown whether ischemia and hemorrhage disturb swallowing to the same extent. Overall comparison of both types of stroke showed no differences with respect to incidence of dysphagia [19] and outcome parameters like malnutrition [20] or survival [30]. But to address this matter suitably it would be essential to compare dysphagia characteristics in both conditions with matched lesion location.
Conclusion
Dysphagia mainly due to impaired oral motor control is a common and so far underrecognized symptom in striatocapsular hemorrhage. Infectious complications frequently arise. Since clinical parameters were not related to the incidence and severity of swallowing dysfunction, every patient must be considered at risk for dysphagia. We therefore recommend that all patients with striatocapsular hemorrhage should receive detailed swallowing assessment within the first 3 days after symptom onset. There is evidence from our data that dysphagia in this patient group has a substantial potential for short-term improvement. Whether the type of stroke, lesion location or size is related to specific videoendoscopic dysphagia characteristics is still debated. Further studies in patients with a predefined type of intracerebral hemorrhage (e.g., lobar, pontine) are needed to clarify this issue.
References
Alberts MJ, Horner J, Gray L, Brazer SR (1992) Aspiration after stroke: lesion analysis by brain MRI. Dysphagia 7:170–173
Arboix A, Vall-Llosera A, Garcia-Eroles L, Massons J, Oliveres M, Targa C (2002) Clinical features and functional outcome of intracerebral hemorrhage in patients aged 85 and older. J Am Geriatr Soc 50:449–454
Aydogdu I, Ertekin C, Tarlaci S, Turman B, Kiylioglu N, Secil Y (2001) Dysphagia in lateral medullary infarction (Wallenberg’s syndrome): an acute disconnection syndrome in premotor neurons related to swallowing activity? Stroke 32:2081–2087
Badjatia N, Rosand J (2005) Intracerebral hemorrhage. Neurologist 11:311–324
Barer DH (1989) The natural history and functional consequences of dysphagia after hemispheric stroke. J Neurol Neurosurg Psychiatry 52:236–241
Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G (1993) Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 24:987–993
Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V (1989) Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20:864–870
Carnaby G, Hankey GJ, Pizzi J (2006) Behavioural intervention for dysphagia in acute stroke: a randomised controlled trial. Lancet Neurol 5:31–37
Chung CS, Caplan LR, Yamamoto Y, Chang HM, Lee SJ, Song HJ, Lee HS, Shin HK, Yoo KM (2000) Striatocapsular haemorrhage. Brain 123(Pt 9):1850–1862
Cola MG, Daniels SK, Corey DM, Lemen LC, Romero M, Foundas AL (2010) Relevance of subcortical stroke in dysphagia. Stroke 41:482–486
Davalos A, Ricart W, Gonzalez-Huix F, Soler S, Marrugat J, Molins A, Suner R, Genis D (1996) Effect of malnutrition after acute stroke on clinical outcome. Stroke 27:1028–1032
Dennis M (2000) Nutrition after stroke. Br Med Bull 56:466–475
Dennis MS (2003) Outcome after brain haemorrhage. Cerebrovasc Dis 16(Suppl 1):9–13
Ding R, Logemann JA (2000) Pneumonia in stroke patients: a retrospective study. Dysphagia 15:51–57
Dziewas R, Ritter M, Schilling M, Konrad C, Oelenberg S, Nabavi DG, Stogbauer F, Ringelstein EB, Ludemann P (2004) Pneumonia in acute stroke patients fed by nasogastric tube. J Neurol Neurosurg Psychiatry 75:852–856
Dziewas R, Warnecke T, Olenberg S, Teismann I, Zimmermann J, Kramer C, Ritter M, Ringelstein EB, Schabitz WR (2008) Towards a basic endoscopic assessment of swallowing in acute stroke—development and evaluation of a simple dysphagia score. Cerebrovasc Dis 26:41–47
Elijovich L, Patel PV, Hemphill JC 3rd (2008) Intracerebral hemorrhage. Semin Neurol 28:657–667
Elmstahl S, Bulow M, Ekberg O, Petersson M, Tegner H (1999) Treatment of dysphagia improves nutritional conditions in stroke patients. Dysphagia 14:61–66
Falsetti P, Acciai C, Palilla R, Bosi M, Carpinteri F, Zingarelli A, Pedace C, Lenzi L (2009) Oropharyngeal dysphagia after stroke: incidence, diagnosis, and clinical predictors in patients admitted to a neurorehabilitation unit. J Stroke Cerebrovasc Dis 18:329–335
Finestone HM, Greene-Finestone LS, Wilson ES, Teasell RW (1995) Malnutrition in stroke patients on the rehabilitation service and at follow-up: prevalence and predictors. Arch Phys Med Rehabil 76:310–316
Finestone HM, Woodbury MG, Foley NC, Teasell RW, Greene-Finestone LS (2002) Tracking clinical improvement of swallowing disorders after stroke. J Stroke Cerebrovasc Dis 11:23–27
Foley N, Teasell R, Salter K, Kruger E, Martino R (2008) Dysphagia treatment post stroke: a systematic review of randomised controlled trials. Age Ageing 37:258–264
Gebel JM, Sila CA, Sloan MA, Granger CB, Weisenberger JP, Green CL, Topol EJ, Mahaffey KW (1998) Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 trial. Stroke 29:1799–1801
Ghika JA, Bogousslavsky J, Regli F (1990) Deep perforators from the carotid system. Template of the vascular territories. Arch Neurol 47:1097–1100
Gonzalez-Fernandez M, Kleinman JT, Ky PK, Palmer JB, Hillis AE (2008) Supratentorial regions of acute ischemia associated with clinically important swallowing disorders: a pilot study. Stroke 39:3022–3028
Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE (1999) Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol 277:G219–G225
Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG (1999) Identification of the cerebral loci processing human swallowing with H2(15)O PET activation. J Neurophysiol 81:1917–1926
Han DS, Chang YC, Lu CH, Wang TG (2005) Comparison of disordered swallowing patterns in patients with recurrent cortical/subcortical stroke and first-time brainstem stroke. J Rehabil Med 37:189–191
Hinchey JA, Shephard T, Furie K, Smith D, Wang D, Tonn S, Stroke Practice Improvement Network Investigators (2005) Formal dysphagia screening protocols prevent pneumonia. Stroke 36:1972–1976
Ickenstein GW, Stein J, Ambrosi D, Goldstein R, Horn M, Bogdahn U (2005) Predictors of survival after severe dysphagic stroke. J Neurol 252:1510–1516
Kaji R (2001) Basal ganglia as a sensory gating devise for motor control. J Med Invest 48:142–146
Kiphuth IC, Kuramatsu JB, Lucking H, Kloska S, Schwab S, Huttner HB (2011) Predictive factors for percutaneous endoscopic gastrostomy in patients with spontaneous intracranial hemorrhage. Eur Neurol 65:32–38
Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J (1996) The ABCs of measuring intracerebral hemorrhage volumes. Stroke 27:1304–1305
Kwon M, Lee JH, Kim JS (2005) Dysphagia in unilateral medullary infarction: lateral vs medial lesions. Neurology 65:714–718
Langmore SE (2001) Endoscopic Evaluation and Treatment of Swallowing Disorders. Thieme, New York/Stuttgart
Langmore SE (2003) Evaluation of oropharyngeal dysphagia: which diagnostic tool is superior? Curr Opin Otolaryngol Head Neck Surg 11:485–489
Langmore SE, Schatz K, Olsen N (1988) Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia 2:216–219
Lim SH, Lieu PK, Phua SY, Seshadri R, Venketasubramanian N, Lee SH, Choo PW (2001) Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia 16:1–6
Logemann JA, Shanahan T, Rademaker AW, Kahrilas PJ, Lazar R, Halper A (1993) Oropharyngeal swallowing after stroke in the left basal ganglion/internal capsule. Dysphagia 8:230–234
Mann G, Hankey GJ, Cameron D (1999) Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke 30:744–748
Mann G, Hankey GJ, Cameron D (2000) Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovasc Dis 10:380–386
Martin RE, Goodyear BG, Gati JS, Menon RS (2001) Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 85:938–950
Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R (2005) Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 36:2756–2763
Meng NH, Wang TG, Lien IN (2000) Dysphagia in patients with brainstem stroke: incidence and outcome. Am J Phys Med Rehabil 79:170–175
Minnerup J, Wersching H, Brokinkel B, Dziewas R, Heuschmann PU, Nabavi DG, Ringelstein EB, Schabitz WR, Ritter MA (2010) The impact of lesion location and lesion size on poststroke infection frequency. J Neurol Neurosurg Psychiatry 81:198–202
Mosier K, Bereznaya I (2001) Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res 140:280–289
Mosier K, Patel R, Liu WC, Kalnin A, Maldjian J, Baredes S (1999) Cortical representation of swallowing in normal adults: functional implications. Laryngoscope 109:1417–1423
Perlman AL, Booth BM, Grayhack JP (1994) Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia 9:90–95
Power ML, Hamdy S, Goulermas JY, Tyrrell PJ, Turnbull I, Thompson DG (2009) Predicting aspiration after hemispheric stroke from timing measures of oropharyngeal bolus flow and laryngeal closure. Dysphagia 24:257–264
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF (2001) Spontaneous intracerebral hemorrhage. N Engl J Med 344:1450–1460
Ropper AH, Samuels MA (2009) Adams and Victor’s Principles of Neurology. McGraw Hill Medical, New York
Smithard DG, O’Neill PA, Parks C, Morris J (1996) Complications and outcome after acute stroke. Does dysphagia matter? Stroke 27:1200–1204
Smithard DG, Smeeton NC, Wolfe CD (2007) Long-term outcome after stroke: does dysphagia matter? Age Ageing 36:90–94
Steinhagen V, Grossmann A, Benecke R, Walter U (2009) Swallowing disturbance pattern relates to brain lesion location in acute stroke patients. Stroke 40:1903–1906
Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H (2003) Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia 18:71–77
Teasell R, Foley N, Fisher J, Finestone H (2002) The incidence, management, and complications of dysphagia in patients with medullary strokes admitted to a rehabilitation unit. Dysphagia 17:115–120
Walter U, Knoblich R, Steinhagen V, Donat M, Benecke R, Kloth A (2007) Predictors of pneumonia in acute stroke patients admitted to a neurological intensive care unit. J Neurol 254:1323–1329
Warnecke T, Oelenberg S, Teismann I, Hamacher C, Lohmann H, Ringelstein EB, Dziewas R (2010) Endoscopic characteristics and levodopa responsiveness of swallowing function in progressive supranuclear palsy. Mov Disord 25:1239–1245
Warnecke T, Ritter MA, Kroger B, Oelenberg S, Teismann I, Heuschmann PU, Ringelstein EB, Nabavi DG, Dziewas R (2009) Fiberoptic endoscopic Dysphagia severity scale predicts outcome after acute stroke. Cerebrovasc Dis 28:283–289
Warnecke T, Teismann I, Oelenberg S, Hamacher C, Ringelstein EB, Schabitz WR, Dziewas R (2009) The safety of fiberoptic endoscopic evaluation of swallowing in acute stroke patients. Stroke 40:482–486
Warnecke T, Teismann I, Oelenberg S, Hamacher C, Ringelstein EB, Schabitz WR, Dziewas R (2009) Towards a basic endoscopic evaluation of swallowing in acute stroke—identification of salient findings by the inexperienced examiner. BMC Med Educ 9:13
Wong EH, Pullicino PM, Benedict R (2001) Deep cerebral infarcts extending to the subinsular region. Stroke 32:2272–2277
Zald DH, Pardo JV (1999) The functional neuroanatomy of voluntary swallowing. Ann Neurol 46:281–286
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sonja Suntrup and Tobias Warnecke contributed equally to this work.
Rights and permissions
About this article
Cite this article
Suntrup, S., Warnecke, T., Kemmling, A. et al. Dysphagia in patients with acute striatocapsular hemorrhage. J Neurol 259, 93–99 (2012). https://doi.org/10.1007/s00415-011-6129-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6129-3