Abstract
This literature review explores a wide range of themes addressing the links between swallowing and consciousness. Signs of consciousness are historically based on the principle of differentiating reflexive from volitional behaviors. We show that the sequencing of the components of swallowing falls on a continuum of voluntary to reflex behaviors and we describe several types of volitional and non-volitional swallowing tasks. The frequency, speed of initiation of the swallowing reflex, efficacy of the pharyngeal phase of swallowing and coordination between respiration and swallowing are influenced by the level of consciousness during non-pathological modifications of consciousness such as sleep and general anesthesia. In patients with severe brain injury, the level of consciousness is associated with several components related to swallowing, such as the possibility of extubation, risk of pneumonia, type of feeding or components directly related to swallowing such as oral or pharyngeal abnormalities. Based on our theoretical and empirical analysis, the efficacy of the oral phase and the ability to receive exclusive oral feeding seem to be the most robust signs of consciousness related to swallowing in patients with disorders of consciousness. Components of the pharyngeal phase (in terms of abilities of saliva management) and evoked cough may be influenced by consciousness, but further studies are necessary to determine if they constitute signs of consciousness as such or only cortically mediated behaviors. This review also highlights the critical lack of tools and techniques to assess and treat dysphagia in patients with disorders of consciousness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In healthy individuals, swallowing is such an automated sensorimotor mechanism that, apart from episodes of food “going down the wrong way” due to distraction, no one consciously experiences their swallowing. An exception exists with mindfulness and we can consciously experience our swallowing if we decide to voluntarily pay attention to it. Depending on the disease, the prevalence of dysphagia can be very high in neurological populations [1,2,3,4,5] and different components of the swallowing sequence can be affected. In acquired brain injury, we can reasonably assume that, the more severe the brain injury, the more severe the dysphagia [6, 7]. The severity of brain injury is classically defined, among other things, according to the Glasgow Coma Scale (GCS) [8], on admission and coma duration [9]. The question of what factors (e.g., lesion localization and volume, type of brain injury, consciousness) most affect the severity of dysphagia following brain injury has not yet been completely elucidated.
Consciousness is a complex phenomenon. In the field of clinical science, researchers define consciousness based on two components: wakefulness (arousal) and awareness (subjective experience) [10]. Consciousness allows us to be aware of objects and events, inside and outside our body [10, 11]. Wakefulness and awareness are generally correlated. Although healthy people are aware when they are awake, during coma and in most cases during general anesthesia, patients are neither awake nor aware. Modifications of consciousness can be pathological (disorders of consciousness) or non-pathological (sleep or anesthesia).
Disorders of consciousness (DoC) represent different states along a continuum from coma (no arousal and no awareness) to being conscious and awake (preserved arousal and awareness). Between the two extremes, unresponsive wakefulness syndrome (UWS, previously termed vegetative state) is defined by recovery of arousal in the absence of any sign of awareness [12], whereas minimally conscious state (MCS) refers to preserved arousal and reproducible but inconsistent signs of consciousness [13]. The MCS entity can be subdivided into minimally conscious state MINUS (MCS−) and PLUS (MCS +) based on the presence (MCS +) or absence (MCS−) of behaviors indicating at least partial preservation of language abilities [14, 15]. When patients recover the ability to functionally communicate or to use two objects appropriately, we consider that they are emerging from the minimally conscious state (EMCS) [13]. Patients with locked-in syndrome (LIS) have woken from their coma and are fully conscious but are unable to show behavioral signs of consciousness except by eye movements [16]. UWS and MCS are usually transitional states between coma and higher levels of consciousness. However, some patients present prolonged, chronic DoC.
Misdiagnosis can have serious medical and ethical consequences for patients and their families. Indeed, functional outcomes and prognoses are better for MCS than UWS [17, 18]. Moreover, response to treatment seems to be better in patients in MCS [19]. Regarding pain management, noxious stimuli seem to elicit a larger cerebral response in patients in MCS than with UWS, suggesting that patients in MCS may be more likely to feel pain than those with UWS [20,21,22]. Finally, level of consciousness influences end-of-life decisions [23, 24].
Recent guidelines for the diagnosis of patients with DoC recommend that one use valid, reliable standardized neurobehavioral assessments of consciousness [25,26,27]. The Coma Recovery Scale–Revised (CRS-R) is the reference standard for the clinical bedside evaluation of consciousness [28], as it fulfills all the Aspen Neurobehavioral Workgroup criteria [29]. The diagnostic criteria for consciousness in the CRS-R are classified in six categories (auditory, visual, motor, oromotor/verbal, communication, arousal). Beyond behaviors assessed using the CRS-R, some authors have identified other criteria linked to level of consciousness [30, 31] and other possible signs of consciousness [32,33,34,35].
Level of consciousness has an impact on a variety of abilities such as language [36], motor function [37], sphincter function [38] and feeding [39]. Most patients with DoC are fed by enteral feeding tube [40, 41]. However, the true impact of consciousness on swallowing abilities remains poorly understood. It is relatively clear to therapists working in dysphagia rehabilitation that level of consciousness influences swallowing abilities. However, the links between swallowing and consciousness have not yet been examined to any great extent.
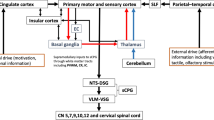
Because of the scarcity of studies directly related to swallowing and consciousness, in this review we chose to explore a wide range of themes addressing the links between swallowing and consciousness rather than focusing on one topic or answering one specific question (see Fig. 1).
Section 1: Swallowing from the Perspective of Volition
The approach historically used to determine whether or not a patient is conscious consists in the comparison of reflexive and voluntary behaviors [13]. However, the difference between conscious and reflexive behaviors remains ambiguous [42]. In fact, there are no empirical characteristics that allow us to reliably distinguish reflexive behaviors from conscious behaviors [42].
Prochazka et al. [43] demonstrated that the distinction between voluntary and reflex differs depending on the approach. The Prochazka/Loeb/Rothwell position [43] describes voluntary behaviors as those that proceed under conscious control (Loeb) and that we can interrupt, influence (Rothwell) and suppress at will (Prochazka) and reflex behaviors as those that are automatic and hard to suppress (Prochazka) and that cannot be modified voluntarily. Some researchers [44, 45] also agree that all voluntary behaviors contain automatic processes contributing to their rapidity and flexibility. Moreover, two types of reflexes are involved in swallowing: somatic and autonomic reflexes (see below) [46]. Somatic reflexes implicate striated/skeletal muscles, and autonomic reflexes target smooth muscles.
Based on these characteristics (see Table 1), we will analyze the different components of swallowing and try to distinguish voluntary from reflex behavior.
Components of Swallowing
Swallowing is divided into three phases (oral, pharyngeal, esophageal), each one comprising several components. The oral phase is classically described as the voluntary phase of swallowing while the pharyngeal and esophageal phases are the reflexive phases [47]. To confirm this assumption, we will now discuss the different components in each phase of swallowing in light of the characteristics of voluntary versus reflex behaviors (Fig. 2).
Oral Phase
Although we chew and transport food without consciously controlling each orofacial movement, the oral phase is the only phase of swallowing that can be entirely interrupted and consciously controlled. In that respect, the modifiable and suppressible character of the oral phase categorizes this phase as voluntary behavior. In addition, several studies have demonstrated that consciously controlling the oral phase modifies its sequencing [48,49,50]. Indeed, the chewing sequence can be significantly lengthened (almost twice as long) with volition (e.g., chewing with a conscious effort, or a specific number of chews) than without volition (i.e., eating normally). These data emphasize the role of automatic processes in natural feeding conditions. In other words, most of the time, the various lip and tongue movements occur without volition but rather as semiautomatic periodic or rhythmic movements, which explains why, in “controlled” conditions, the oral phase lasts longer.
The notion of semiautomatic periodic or rhythmic movements is not recent [51]. Many studies, especially those by Sessle’s team [52,53,54,55], have explored the neural control of orofacial movements in primates using intracortical microstimulations. They indicated that the primary motor cortex dedicated to the orofacial area is involved in voluntary movements but also in the control of semiautomatic movements, such as tongue and mastication movements. Studies of oral reflexes also showed that diffuse stimuli to the palate in decerebrated and anesthetized cats elicited rhythmic tongue activity [56]. Moreover, in the field of epilepsy, one study showed that electrical stimulation of the right inferior frontal gyrus (fronto-opercular cortex) leads to oroalimentary automatisms (lip movements, chewing) [57].
The oral phase of swallowing can be classified as a voluntary behavior but, like any another motor activity, it includes some automatic processes. As described by Humbert and German [58], during feeding, the different components of the oral phase can moved along the continuum of low to high voluntary control depending on the degree of attention dedicated specifically to them. The pattern-generating circuits for chewing and licking are located in the brainstem but receive direct cortical inputs [59].
Pharyngeal Phase
The triggering of what is commonly called the “swallowing reflex” heralds the end of the oral phase and the beginning of the pharyngeal phase. This reflex is a somatic reflex because it involves striated/skeletal muscles.
In “natural” conditions, the swallowing reflex occurs in response to saliva accumulation or to the presence of liquid or food in the oropharyngeal space (i.e., area of the soft palate, faucial pillars, pharyngeal surface of the epiglottis, dorsal pharyngeal wall). Indeed, when a sensory input (presence of saliva or a liquid or solid bolus) reaches a certain threshold, it triggers the swallowing reflex, which elicits the start of the sequence leading to protection of the airway and transportation of the bolus to the esophagus [60]. The timing of the initiation of the swallowing reflex is influenced by the waking state (see Sect. 2), type of bolus (shortest with liquids) [61] and cognitive functions [62,63,64].
Although the swallowing reflex is usually triggered without conscious perception, it can be evoked voluntarily. Moreover, the swallowing reflex can be artificially initiated in humans by air pulses [65] or electrical stimulations [66, 67] of the pharyngeal area. Whereas the execution of the oral phase can be stopped at any time, the swallowing reflex is hard to suppress for a long time during feeding or at rest.
If we refer to the definitions of Prochazka et al. [43], the swallowing reflex can be triggered voluntarily but is usually automatic and is hard to suppress. It is thus on the borderline between a voluntary behavior and a reflex. Moreover, we can postulate that the transition between a voluntary behavior and a somatic reflex takes place somewhere between the beginning of the stage II oral phase transport and the triggering of the swallowing reflex.
On the other hand, the proceedings occurring after the trigger of the swallowing reflex (pharyngeal phase) cannot be suppressed voluntarily, unlike the oral phase and, to a lesser extent, the triggering of the swallowing reflex. However, some studies have shown that the pharyngeal phase can be influenced voluntarily to some extent; for example, patients can learn maneuvers that change swallowing physiology and help to reduce aspirations (e.g., Mendelsohn maneuver or effortful swallow) [58, 68, 69]. The process of the pharyngeal phase is mainly a somatic reflex.
Esophageal Phase
The opening of the upper esophageal sphincter (UES) marks the end of the pharyngeal phase and the start of the esophageal phase. The UES is also called the inferior pharyngeal sphincter [68]. Muscles involved in the upper third of the esophagus (mainly the UES) are striated muscles under the control of vagal cholinergic motoneurons in the nucleus ambiguus of the brainstem (partly with the vagus cranial nerve X). In the lower two-thirds of the esophagus, which is composed of smooth muscles, neural control switches to the autonomic/vegetative (enteric) nervous system through motoneurons situated in the ganglia [68, 70,71,72].
The esophageal phase cannot be voluntarily triggered or suppressed. The only influence on the esophageal phase is a passive or active effect on the UES. In fact, Shaker et al. [73] showed that head-raising exercises improve the UES, among other things. The mechanism at play is a passive stretch of the UES and/or an improvement of pharynx propulsion, which facilitates the opening of the UES. More recently, Winiker et al. demonstrated that volitional modulation of the pressure in the region of the UES (active effect) is also possible in healthy subjects after training using visual biofeedback exercises [74]. For these reasons and given anatomical considerations [75], we can assume that the transition between a somatic and an autonomic reflex (and consequently between striated and smooth muscles) takes place somewhere in the upper third of the esophagus.
Swallowing Tasks
In the last 20 years, several researchers have explored the different stages of swallowing in healthy participants and in patients with dysphagia. Swallowing has been studied in several conditions (saliva, liquid or food swallowing) and, in addition to the voluntary or reflexive nature of each component of swallowing, some authors also distinguish swallowing tasks depending on the influence of volition.
Ertekin et al. [76] distinguished between reflexive swallows (water introduced to the back of the tongue with a syringe), nasopharyngeal swallows (water introduced through a canula at the level of the uvula), spontaneous swallows (accumulation of saliva in the mouth that triggered spontaneous swallowing) and voluntary swallows (1–3 mL of water swallowed voluntarily) in an electrophysiological study. They showed, among other things, that the time interval between the onset of submental EMG and the onset of upward deflection of the larynx was significantly shorter for reflexive, nasopharyngeal and spontaneous swallows than for voluntary swallows. Kern et al. [77] compared reflexive (rapid injection of water into the pharynx) and voluntary swallows (cued to swallow saliva volitionally once every 30 s by a tactile cue) in a neuroimaging study. While reflexive swallowing was associated with bilateral activity concentrated in the primary sensory/motor regions, volitional swallowing was represented bilaterally in the insula, prefrontal, cingulate, and parieto-occipital regions in addition to the primary sensory/motor cortex.
One decade later, Ertekin [78] dedicated a literature review to a comparison of spontaneous swallowing and voluntary swallowing. He described spontaneous swallowing as a “type of protective reflex action that occurs to ensure safety of the upper airway tract against any escape of food particles or saliva, or as an emotion-related reflex activity occurring during stressful conditions” (2011, p. 184). Spontaneous swallowing occurs without awareness while one is awake or asleep. The oral phase is bypassed in most cases, although there may be partial excitation. Spontaneous swallowing is also sometimes called “reflexive swallowing” or “non-nutritive swallowing.” On the other hand, he described voluntary swallowing (also called “conscious swallowing”) as sequential eating or drinking voluntarily initiated or facilitated by the cerebral cortex during the awake and aware state [78].
In Table 2, we describe several types of volitional (VOST) and non-volitional swallowing tasks (NVOST) related to the concept of reflexive (RS), spontaneous (SS) and voluntary swallowing (VS). Because of the potentially different brain activations and different physiological mechanisms at play during nutritive compared to non-nutritive swallowing, we also make a distinction between these two types of swallowing tasks. Reflexive swallowing refers to triggering of the swallowing reflex by an external stimulus (tactile or with the injection of a bolus). In this case, the participation of the oral phase is diminished but not completely bypassed considering the involvement of the tongue in any swallowing process. Non-nutritive spontaneous swallowing refers to the management of saliva and secretions that are produced spontaneously by all healthy humans, while nutritive spontaneous swallowing is associated with eating and drinking. Volitional swallowing tasks refer to tasks occurring further to an internal or external request.
Section 2: Non-pathological Modifications of Consciousness: Sleep and Anesthesia
Investigating swallowing in non-pathological modifications of consciousness such as sleep or anesthesia allows to explore swallowing without the ambiguity of conscious control. Indeed, sleep and anesthesia are associated with reduced consciousness and lack of volition, enabling volitional versus non-volitional swallowing to be distinguished.
Sleep is classically divided into three stages of nonrapid eye movement (NREM) sleep (N1, N2 and N3) and rapid eye movement (REM) sleep. As described by Sanders et al. [79], in NREM sleep individuals are generally considered unconscious, disconnected and not responsive, but people recall dreams after being woken from NREM sleep in 23 to 74% of cases. In contrast, during REM sleep, individuals are sometimes considered conscious (in approximately 80% of REM sleep awakenings) and report vivid dreams, but they do not experience their environment. They are disconnected and not responsive.
During sleep and in the case of DoC, the absence of consciousness does not lead to a complete absence of swallowing. Several studies have explored spontaneous saliva swallowing in healthy adults during sleep [80,81,82,83,84,85,86,87]. During sleep, swallowing is episodic, absent for long periods and influenced by sleep stage [85,86,87]. The deeper the sleep stage, the lower the mean swallowing frequency. Swallowing occurs almost in association with movement arousals in both REM and NREM sleep [82, 86, 87]. Some authors reported no [86] or very few [87] swallows during deep sleep (NREM stage N3). Regarding the efficacy of the pharyngeal phase, healthy adults have lower velopharyngeal and hypopharyngeal swallowing pressures when asleep [80]. In their study, Kelly et al. [81] showed that breathing-swallowing coordination differed between volitional (saliva swallowing on command) and non-volitional swallowing (spontaneous saliva swallowing without cuing) conditions but not between their two non-volitional conditions (spontaneous saliva swallowing during waking and sleep). Moreover, during a functional test (instillation of water in the pharynx), more aspirations after swallowing were observed during sleep than during wakefulness, as well as more repetitive swallowing and coughing after swallowing [83].
Patients with neurological impairments (cerebral atrophy or lacunar infarct) demonstrated a delayed response between the delivery of water in the pharynx and the triggering of swallowing when asleep, compared to when awake, while the healthy group showed no significant difference between wakefulness and sleep [88]. In Parkinson’s disease patients, the mean duration of sleep decreases while the number of spontaneous saliva swallowing increases compared to healthy subjects [89]. Moreover, patients present more multiple swallows than healthy subjects.
Anesthesia can also be considered as a way of exploring consciousness but cannot be considered simply as an “absence of consciousness” [90]. Different consciousness states can be observed during general anesthesia, depending on the anesthetic agent and dose: (1) a complete absence of subjective experience (unconsciousness); (2) conscious experience without perception of the environment (disconnected consciousness, as in dreaming); or (3) episodes of oriented consciousness with awareness of the environment (connected consciousness) [90].
Some authors [91,92,93,94,95] have shown that general anesthetics (e.g., propofol, sevoflurane, ketamine, midazolam), which generally cause some form of unconsciousness [79], can alter swallowing. Thus, during general anesthesia, the frequency of spontaneous saliva swallowing decreases and the number of pathological swallows (characterized by inspiration or followed by an inspiration) increases [91]. Moreover, studies analyzing the efficacy of swallowing after the injection of a liquid at the back of the tongue or the pharynx during anesthesia showed that the latency between the injection and the initiation of the swallowing reflex [92, 94, 96], and the number of aspirations [93] increase while laryngeal reflexes are depressed [95]. Moreover, coordination between respiration and swallowing can change with deep sedation or during the recovery period from general anesthesia [97, 98].
All this information shows that the frequency, speed of initiation of the swallowing reflex, efficacy of the pharyngeal phase of swallowing (mainly the number of aspirations) and coordination between respiration and swallowing are influenced by the level of consciousness during sleep and general anesthesia.
In the next section, we will see how consciousness affects swallowing in patients with brain injuries.
Section 3: Links Between Consciousness and Swallowing in Brain-Injured Patients
The prevalence of dysphagia after severe brain injury is very high [41], mainly due to the large number of brain areas dedicated to swallowing (see above), any of which can be severely damaged by a brain injury. A large majority of patients with DoC require artificially delivered hydration and nutrition, mainly through a gastrostomy feeding tube [41, 99]. The aim of this section is to examine the extent, variety and characteristics of swallowing disabilities in patients with acquired brain injury (ABI), and identify which swallowing components are related to consciousness. To better understand these links, we reviewed and synthetized studies analyzing swallowing in relation to consciousness level (see supp mat 1 for search strategy and selected criteria). We found 18 studies that describe a link between consciousness and swallowing abilities (see Table 3 for characteristics and detailed results of the studies and Table 4 for a summary). Nine studies explored swallowing abilities for all etiologies [39,40,41, 100,101,102,103,104,105], while nine focused solely on traumatic brain injury (TBI) [7, 106,107,108,109,110,111,112,113].
Regarding the scale used to assess the level of consciousness, twelve studies reported the results of swallowing in patients diagnosed with the Rancho Los Amigos (RLA) Scale [114], four with the Coma Recovery Scale—Revised (CRS-R) [28], one with the Sensory Modality Assessment and Rehabilitation Technique (SMART) [115], one with the Wessex Head Injury Matrix (WHIM) [116] and one with the Full Outline of UnResponsiveness (FOUR) [117] (see supp mat 2 for description of scales).
The current literature shows some links between swallowing and consciousness in patients with ABI. However, the heterogeneity of the swallowing-related components described, the level of consciousness considered, the various study designs and the lack of clear diagnoses of DoC in a large majority of studies mean that we must be cautious when interpreting the results. In patients with severe brain injury, the level of consciousness is associated with several components related to swallowing, such as the possibility of extubation, risk of pneumonia, type of feeding or components directly related to swallowing such as oral or pharyngeal abnormalities.
Only four studies analyzed swallowing-related components specifically in patients with DoC diagnosed with a validated repeated behavioral scale [40, 41, 102, 105]. Both oral and pharyngeal phases of swallowing can be impaired in patients with DoC.
We identify a strong link between the oral phase of swallowing and level of consciousness [40, 41, 105]. Indeed, we did not detect an effective oral phase of swallowing (lip prehension, tongue propulsion and no post-swallowing oral stasis) in any of the patients with UWS [40, 41], and in only a small minority of those in MCS [40, 41, 105]. This also helped to explain why no patients with UWS were able to achieve full oral feeding and why only a small proportion of the patients in MCS could safely resume full oral feeding with easy-to-swallow food [40, 41]. Despite the ability of some patients in MCS to resume oral feeding, a higher level of consciousness (i.e., EMCS) is probably necessary to allow a full return to ordinary oral feeding. Interestingly, in the study of Wang et al. [105], mouth opening was observed in only one UWS patient in their cohort and this patient recovers a MCS state of consciousness 6 months later. An effective oral phase should be considered as a sign of consciousness and, consequently, it should be taken into account in diagnosing DoC.
There also seems to be a difference between patients with UWS and MCS regarding pharyngeal components of swallowing. Patients with UWS and MCS differed in their spontaneous saliva management [41]. Indeed, patients with UWS had more pharyngo-laryngeal secretions and saliva aspiration and a larger proportion present extubation failure and still had a tracheostomy in place at the time of the evaluation [41, 102]. These results suggest that there is a link between the pharyngeal phase of swallowing and level of consciousness in this cohort. However, at this point, we are not able to identify whether the mechanism involved is a decrease in the frequency of spontaneous swallowing or a lack of efficacy of the pharyngeal phase as such, especially pharyngeal propulsion.
The cough reflex (“evoked cough” if we refer to Eccles’s classification [118]) was another component that was more evident in MCS than with UWS [41]. This result support the fact that evoked cough is not solely a brainstem-mediated reflex response but is a sensorimotor behavior under cortical influence [119, 120]. Indeed, the impact of level of consciousness on the existence of the cough response may be linked to the scope of the underlying cortical damage.
Section 4: Evaluation and Treatment of Swallowing in Patients with DoC
Assessment of Swallowing
Determining the efficacy of swallowing in patients with DoC is difficult and challenging because they may not respond to commands (UWS, MCS–) and their responses may fluctuate. Most such patients are fed by enteral nutrition because of severe dysphagia [41]. Understanding swallowing disorders in this population will help clinicians determine the nature and judge the efficacy of the therapy to be applied. Moreover, a better understanding of the pathophysiology of swallowing in patients with DoC will also contribute to our understanding of the links between consciousness and swallowing.
Classically, we distinguish between clinical bedside assessments and objective swallowing assessment (e.g., FEES and VFSS).
A series of screening protocols or bedside assessments have been developed in the last 20 years to explore swallowing [121]. However, most of them require the patient to participate actively (respond to commands) and therefore are not suitable for assessing swallowing in patients with DoC.
Three behavioral assessments developed for patients with DoC include a swallowing subscale or item: the Disorders of Consciousness Scale (DOCS) [122, 123], the Comprehensive Assessment Measure for the Minimally Responsive Individual (CAMMRI) [124] and the CRS-R [28].
One of the eight DOCS subscales is called “Taste & Swallowing” [122, 123]. It evaluates patient response to pre-swallowing stimulation (when we explain that we will apply the stimulation) and the ability to swallow within 15 to 20 s of a stimulation. The taste stimulation consists in touching the lips and gums with a cotton swab soaked in orange juice and observing the patient’s reactions (no response, generalized response or localized response). This item has the advantage of avoiding a functional swallowing test, which can expose the patient to a high risk of inhalation.
The CAMMRI includes a 7-item dysphagia rating scale ranging from “profound dysphasia” to “functional swallowing” [124]. It consists of a checklist that requires clinicians to evaluate oral motor impairment, pharyngeal phase of swallowing, cough reflex, secretion management, risk of aspiration and type of feeding. To be objective, this scale requires a FEES or VFSS to be performed. The CAMMRI also has an oral/facial sensitivity subtest that assesses reaction to firm and soft touch on the face and inside the mouth [124].
The CRS-R includes baseline observations of spontaneous behaviors including sticking out the tongue and opening and closing the mouth. On the motor function scale, in the “automatic motor response” item, if the patient does not show episodes of automatic motor behaviors, the examiner can propose to test mouth-opening ability when a spoon is presented. However, this item is proposed only if the examiner judges that the patient presents an inability to move their limbs and is not able to perform a wave sign. Moreover, the item tests the ability to inhibit the automatic motor behavior of opening the mouth when a spoon is presented because we ask the patient not to move at all.
Bicego et al. [125] developed an observation chart based on the Facial Oral Tract Therapy (FOTT) tool. The FOTT is a rehabilitation approach that can be used with patients with DoC as it does not require active participation [110]. This tool contains a series of items related to head and body posture, orofacial area (e.g., lip and jaw position, aperture of the jaw, appearance of the lips, tongue and cheeks), oral and perioral sensitivity, saliva swallowing, respiration, and cough and orofacial reflexes. They also proposed a bolus swallowing test. Although it is appropriate for patients with DoC, this tool is only available in French and has not been validated with a cohort of patients with DoC.
Similarly, we recently published a protocol study that aims to validate the SWallowing Assessment in Disorders of Consciousness (SWADOC). This bedside assessment has been developed to assess components related to swallowing in patients with DoC [126]. The SWADOC was inspired by Bicego et al. [125] assessment. It includes both qualitative and quantitative items. Items are grouped into 11 categories: (1) Arousal; (2) Resting position of the head, eyes, mandibles and lips; (3) External facial stimulations; (4) Initiation of mouth opening; (5) Mouth cavity observations; (6) Initiation of the saliva swallowing reflex; (7) Stimulation of the saliva swallowing reflex; (8) Lip prehension, tongue propulsion and reactions to 5 mL functional test; (9) Respiration; (10) Voice, speech, language; and (11) Tonicity and sensitivity profiles. A subsection of the SWADOC, the “SWADOC-scored”, includes only eight quantitative items (four items related to the oral phase and four to the pharyngeal phase). Items of the SWADOC-scored must be scored as one of the four severity levels indicated for each item (scores from 0 to 3). The SWADOC-scored allows one to calculate three performance scores: the oral phase subscore, the pharyngeal phase subscore and the total swallowing score (maximum 24). Concurrent validity is assessed with the Facial Oral Tract Therapy Swallowing Assessment of Saliva (FOTT-SAS) [127]. This scale has seven questions: if items 1 to 4 are answered “Yes” and items 5 to 7 are answered “No,” oral intake should be initiated (see Table 5).
Clinical bedside assessments are essential in day-to-day clinical work to gain an initial idea of a patient’s swallowing capacity, guide therapy and track progress. However, they remain subjective because hypotheses are made based on external signs of dysphagia (e.g., cough, voice changing). To objectively determine the efficacy of the pharyngeal phase of swallowing, an objective swallowing assessment is mandatory (FEES or VFSS). Such swallowing assessments, performed by experienced clinicians, constitute the gold standard tools to assess dysphagia in patients at high risk of inhalation [128, 129]. They allow the mechanisms at play during swallowing to be analyzed more precisely and possible silent aspiration to be detected. The high prevalence of silent aspiration in patients with DoC [41] makes the combination of a bedside clinical assessment with an objective swallowing assessment essential.
Objective swallowing assessments can be challenging to do with patients with DoC. In Mackay et al. [7] study, one of the inclusion criteria to perform a VFSS was a level IV RLA score (corresponding approximately to EMCS). Moreover, a VFSS was performed only if patients were able to show automatic or volitional responses to presentation of food or a spoon (i.e., mouth opening). In contrast, Brady et al. [100] showed that FEES and VFSS are feasible in patients at levels II and III. In another study O’Neil-Pirozzi et al. [108] with acute tracheostomized patients with severe DoC following TBI, the authors argued that “these patients may be poor candidates when: (i) swallows are not observed spontaneously and cannot be elicited using digital stimulation to the laryngeal area; (ii) a profound bite reflex is present; and/or iii) the patient cannot tolerate an upright position for a minimum of 15 min” (p. 396). We also showed recently that an objective swallowing assessment can be successfully completed in patients with DoC but that a functional swallowing test (food or liquid testing) can be difficult if patients have severe trismus (lockjaw) or completely lack an oral phase of swallowing [41]. Together, this information suggests that four criteria are necessary when performing a functional swallowing test (liquid or solid food testing) with an objective swallowing assessment (FEES or VFSS) in patients with DoC: (1) semi-seated position for a minimum of 15 min; (2) mouth opening (automatic response to presentation of food or spoon or active opening without severe hypertonia of the jaw muscles); (3) at least minimal tongue propulsion; and (4) swallows are observed spontaneously or can be elicited using stimulation to the pharyngo-laryngeal area.
Treatment of Orofacial Area and Swallowing
Swallowing has not been studied much in patients with DoC, and swallowing treatment is even less studied. In 2010, the National Italian Consensus Conference drew up recommendations on rehabilitation programs for patients with severe ABI in the intensive hospital phase [130]. These recommendations include some indications concerning swallowing (see Table 6).
Other researchers have given some directions on how to manage swallowing in patients with DoC, such as using a nonfeeding program [106, 108]. A nonfeeding program consists in stroking, stretching, applying firm pressure or providing thermal and taste stimulations to desensitize inappropriate orofacial responses and facilitate more normal swallowing and intraoral responses. Recently, Jakobsen et al. [131] proposed a nonfeeding protocol of stimulation based on three specific preselected FOTT stimulation techniques (stroking of the gums and facilitation of tongue and hyoid movements) to non-tracheotomised patients with acute neurogenic dysphagia. They found a tendency to improvement of specific swallowing parameters (frequency of swallowing, elevation of larynx and speed of laryngeal elevation) in the intervention group [131]. However, for now, this is the only published study examining the effect of these techniques on dysphagia in a population with neurological disease.
Brady et al. [39, 132] suggest that, if patients do not demonstrate aspiration in an objective swallowing assessment, therapeutic feedings can be used. Therapeutic feedings consist of giving small amounts of food to stimulate the oral and pharyngeal phases of swallowing and provide a positive experience for the patient.
A modified Delphi study requested speech language therapists’ (SLT) opinions about best practices to assess and treat patients with DoC [133]. For the first time, an expert panel of 36 SLTs reached a consensus on 67 statements covering assessment, management and service delivery for patients in prolonged DoC. This study constitutes the starting point for developing SLT guidelines when working with patients with DoC. In Table 7, we report the statements related to the assessment or treatment of dysphagia and the percentage of agreement.
The Delphi study addressed the use of the FOTT [134] as part of the SLT intervention for patients with DoC but reported that only a small percentage of speech therapists are trained in its use. Moreover, only half of the participants agreed that SLTs should use the FOTT with patients in prolonged DoC. The authors also emphasized the lack of English language papers on that topic and the study design’s limitations [135, 136]. Recently, a practice-oriented book on the FOTT was published that allows clinicians to learn more about this approach [137].
Discussion/Conclusion
As we described in the introduction, identifying signs of consciousness is essential regarding functional and survival prognosis [17, 18, 138], pain management [22] and end-of-life decisions [24]. The identification of behavioral signs of consciousness is historically based on the principle of differentiating reflexive from volitional behaviors, with the idea that unconscious patients show only purely reflexive behaviors while conscious patients show volitional behaviors [13]. However, some ambiguity still exists between conscious and reflexive behaviors [42]. In fact, there are no empirical characteristics that allow one to reliably distinguish reflexive behaviors from conscious behaviors [42].
Based on the characteristics of swallowing components in each phase of swallowing, we tried to distinguish voluntary from reflexive components of swallowing. Our classification is based on the characteristics of voluntary behavior and somatic and autonomic reflexes. We postulated that the triggering of the swallowing reflex constitutes the borderline between voluntary and reflexive behaviors. Components that occur before the initiation of the swallowing reflex (oral phase components) can be considered as voluntary while components that happen afterward (pharyngeal and esophageal components) can be considered reflexive. The opening of the UES constitutes the border between somatic reflexes (pharyngeal phase) and autonomic reflexes (esophageal phase).
In light of this information and based on the results of experimental studies, we will discuss the conscious or unconscious nature of each phase of swallowing.
Although they contain automatic processes, oral phase components can be interrupted, influenced and suppressed, placing them in the category of “voluntary behaviors.” Based on our two retrospective studies in patients with DoC [40, 41], the efficacy of the oral phase seems to be the most robust sign of consciousness. Indeed, until now, no typical patients with UWS are described in the literature as having a complex oral phase of swallowing enabling the preparation and mastication of solid food. Therefore, oral phase components can be considered conscious components.
The triggering of the swallowing reflex can be initiated voluntarily but usually occurs below conscious control. Non-pathological consciousness studies have taught us that sleep and anesthesia tend to decrease the frequency of spontaneous saliva swallowing. Until now, there have been no data about the frequency of saliva swallowing in patients with DoC. However, we highlighted the link between spontaneous saliva swallowing and level of consciousness by highlighting the higher proportion of extubation failure, tracheostomies, pharyngo-laryngeal secretions and saliva aspiration in patients with UWS than in MCS [41, 102]. To identify which mechanism (the frequency of triggering of the swallowing reflex or the efficacy of the pharyngeal phase) is more influenced by consciousness, it would be interesting to explore the frequency of spontaneous swallowing in patients with different levels of consciousness. Based on existing data, we can postulate that the frequency of the swallowing reflex may be influenced by consciousness.
Previously, there were no data about the esophageal phase of swallowing in patients with DoC. Based on our theoretical assumptions, we postulate that the esophageal components of swallowing in the upper third of the esophagus can be influenced by the level of consciousness (but this still needs to be demonstrated) while the components of the lower two-thirds part of the esophagus are unconscious processes.
According to the literature and the main findings of our studies, the presence of oral phase components (mainly mouth opening, lip prehension and lingual propulsion) and the ability to receive exclusive oral feeding can be considered as signs of consciousness. Indeed, these components seem to be present only in patients with (E)MCS [41], with UWS patients that will recover a MCS state of consciousness [105] or in patients with MCS-like patterns of brain activity on neuroimaging tools [40, 41]. Several other components related to swallowing (see Table 8) can be considered to be linked to the level of consciousness (cortically mediated state) without constituting signs of consciousness as such, based on current data. Further prospective studies will help refine our understanding of these associations and determine which swallowing behaviors suggest consciousness in patients with DoC.
Finally, we reviewed current knowledge of the assessment and treatment of dysphagia in patients with DoC. In day-to-day practice, clinicians need to appraise and measure swallowing-related capacities in patients with DoC. However, the majority of existing tools are not adapted to these patients. Indeed, they require active participation by the patient or involve a functional test with a significant amount of liquid or solid food, exposing the patient to a high risk of aspiration. To address this problem, we developed a new tool—the SWADOC—and proposed a validation study.
Moreover, an objective swallowing examination performed by an otorhinolaryngologist is feasible and relevant for patients with DoC regardless of their level of consciousness and whether it is done to discuss the utility of maintaining a tracheostomy, document the utility of botulinum toxin to improve saliva management, or assess the feasibility of therapeutic feeding [39, 41, 101, 108].
Even though evidence regarding the benefits of stimulation is still scanty, there is growing evidence that patients with DOC need intensive rehabilitative interventions [139, 140]. These kinds of care can benefit patients who make functional progress but also those who do not, by reducing later acute care hospital readmissions and enhancing comfort [139].
The research field on the links between swallowing and consciousness deserves our attention, and there is an urgent need for clinical guidelines focusing on assessment and treatment of dysphagia in patients with DoC.
References
Arnold M, Liesirova K, Broeg-Morvay A, et al. Dysphagia in acute atroke: incidence, burden and impact on clinical outcome. PLoS ONE. 2016;11: e0148424. https://doi.org/10.1371/journal.pone.0148424.
Dunn K, Rumbach A. Incidence and risk factors for dysphagia following non-traumatic subarachnoid hemorrhage: a retrospective cohort study. Dysphagia. 2019;34:229–39. https://doi.org/10.1007/s00455-018-9934-3.
Guan X-L, Wang H, Huang H-S, et al. Prevalence of dysphagia in multiple sclerosis: a systematic review and meta-analysis. Neurol Sci. 2015;36:671–81. https://doi.org/10.1007/s10072-015-2067-7.
Kalf JG, de Swart BJM, Bloem BR, et al. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18:311–5. https://doi.org/10.1016/j.parkreldis.2011.11.006.
Michel A, Vérin E, Gbaguidi X, et al. Oropharyngeal dysphagia in community-dwelling older patients with dementia: Prevalence and relationship with geriatric parameters. J Am Med Dir Assoc. 2018;19:770–4. https://doi.org/10.1016/j.jamda.2018.04.011.
Formisano R, Voogt RD, Buzzi MG, et al. Time interval of oral feeding recovery as a prognostic factor in severe traumatic brain injury. Brain Inj. 2004;18:103–9. https://doi.org/10.1080/0269905031000149470.
Mackay LE, Morgan AS, Bernstein BA. Swallowing disorders in severe brain injury: Risk factors affecting return to oral intake. Arch Phys Med Rehabil. 1999;80:365–71. https://doi.org/10.1016/S0003-9993(99)90271-X.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2.
Asikainen I, Kaste M, Sarna S. Predicting late outcome for patients with traumatic brain injury referred to a rehabilitation programme: a study of 508 Finnish patients 5 years or more after injury. Brain Inj. 1998;12:95–107. https://doi.org/10.1080/026990598122737.
Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn Sci. 2005;9:556–9. https://doi.org/10.1016/j.tics.2005.10.010.
Damasio A, Meyer K. Consciousness: AN overview of the phenomenon and of its possible neural basis. In: The Neurology of Consciousness. Elsevier; 2009. p. 3–14. https://doi.org/10.1016/B978-0-12-374168-4.00001-0
Laureys S, Celesia GG, Lavrijsen J, et al. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med. 2010;1:68. https://doi.org/10.1186/1741-7015-8-68.
Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–53.
Bruno M-A, Vanhaudenhuyse A, Thibaut A, et al. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: recent advances in our understanding of disorders of consciousness. J Neurol. 2011;258:1373–84. https://doi.org/10.1007/s00415-011-6114-x.
Thibaut A, Bodien YG, Laureys S, et al. Minimally conscious state “plus”: diagnostic criteria and relation to functional recovery. J Neurol. 2020;267:1245–54. https://doi.org/10.1007/s00415-019-09628-y.
Gosseries O, Bruno M-A, Vanhaudenhuyse A, et al. Consciousness in the Locked-in Syndrome. In: The Neurology of Consciousness. Elsevier; 2009. p. 191–203. https://doi.org/10.1016/B978-0-12-374168-4.00015-0
Luaute J, Maucort-Boulch D, Tell L, et al. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology. 2010;75:246–52. https://doi.org/10.1212/WNL.0b013e3181e8e8df.
Noé E, Olaya J, Navarro MD, et al. Behavioral recovery in disorders of consciousness: a prospective study with the spanish version of the coma recovery scale-revised. Arch Phys Med Rehabil. 2012;93:428-433.e12. https://doi.org/10.1016/j.apmr.2011.08.048.
Thibaut A, Bruno M-A, Ledoux D, et al. tDCS in patients with disorders of consciousness: sham-controlled randomized double-blind study. Neurology. 2014;82:1112–8. https://doi.org/10.1212/WNL.0000000000000260.
Boly M, Faymonville M-E, Schnakers C, et al. Perception of pain in the minimally conscious state with PET activation: an observational study. Lancet Neurol. 2008;7:1013–20. https://doi.org/10.1016/S1474-4422(08)70219-9.
Demertzi A, Racine E, Bruno M-A, et al. Pain perception in disorders of consciousness: neuroscience, clinical care, and ethics in dialogue. Neuroethics. 2013;6:37–50. https://doi.org/10.1007/s12152-011-9149-x.
Demertzi A, Schnakers C, Ledoux D, et al. Different beliefs about pain perception in the vegetative and minimally conscious states: a European survey of medical and paramedical professionals. Prog Brain Res 2009; 329–38.
Bernat J. Ethical issues in the management of patients with impaired consciousness. In: Handbook of clinical neurology. Paris: Elsevier; 2008. p. 369–82.
Demertzi A, Ledoux D, Bruno M-A, et al. Attitudes towards end-of-life issues in disorders of consciousness: a European survey. J Neurol. 2011;258:1058–65. https://doi.org/10.1007/s00415-010-5882-z.
Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: disorders of consciousness. Arch Phys Med Rehabil. 2018;99:1699–709. https://doi.org/10.1016/j.apmr.2018.07.001.
Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: disorders of consciousness. Neurology. 2018;91:450–60. https://doi.org/10.1212/WNL.0000000000005926.
Kondziella D, Bender A, Diserens K, et al. European academy of neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020;27:741–56. https://doi.org/10.1111/ene.14151.
Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–9. https://doi.org/10.1016/j.apmr.2004.02.033.
Seel RT, Sherer M, Whyte J, et al. Assessment scales for disorders of consciousness: evidence-based recommendations for clinical practice and research. Arch Phys Med Rehabil. 2010;91:1795–813. https://doi.org/10.1016/j.apmr.2010.07.218.
Chatelle C, Hauger SL, Martial C, et al. Assessment of nociception and pain in participants in an unresponsive or minimally conscious state after acquired brain injury: the relation between the coma recovery scale-revised and the nociception coma scale-revised. Arch Phys Med Rehabil. 2018;99:1755–62. https://doi.org/10.1016/j.apmr.2018.03.009.
van Ommen HJ, Thibaut A, Vanhaudenhuyse A, et al. Resistance to eye opening in patients with disorders of consciousness. J Neurol. 2018;265:1376–80. https://doi.org/10.1007/s00415-018-8849-0.
Arzi A, Rozenkrantz L, Gorodisky L, et al. Olfactory sniffing signals consciousness in unresponsive patients with brain injuries. Nature. 2020;581:428–33. https://doi.org/10.1038/s41586-020-2245-5.
Carrière M, Cassol H, Aubinet C, et al. Auditory localization should be considered as a sign of minimally conscious state based on multimodal findings. Brain Commun 2020; 2. https://doi.org/10.1093/braincomms/fcaa195.
Hermann B, Salah AB, Perlbarg V, et al. Habituation of auditory startle reflex is a new sign of minimally conscious state. Brain. 2020;143:2154–72. https://doi.org/10.1093/brain/awaa159.
Lancioni GE, Bosco A, Olivetti Belardinelli M, et al. Assessing learning as a possible sign of consciousness in post-coma persons with minimal responsiveness. Front Hum Neurosci 2014; 8. https://doi.org/10.3389/fnhum.2014.00025
Aubinet C, Murphy L, Bahri MA, et al. Brain, Behavior, and Cognitive Interplay in Disorders of Consciousness: A Multiple Case Study. Front Neurol 2018;9. doi:https://doi.org/10.3389/fneur.2018.00665
Thibaut A, Chatelle C, Wannez S, et al. Spasticity in disorders of consciousness: a behavioral study. Eur J Phys Rehabil Med. 2015;51:389–97.
Foxx-Orenstein A, Kolakowsky-Hayner S, Marwitz JH, et al. Incidence, risk factors, and outcomes of fecal incontinence after acute brain injury: findings from the traumatic brain injury model systems national database. Arch Phys Med Rehabil. 2003;84:231–7. https://doi.org/10.1053/apmr.2003.50095.
Brady SL, Darragh M, Escobar NG, et al. Persons with disorders of consciousness: are oral feedings safe/effective? Brain Inj. 2006;20:1329–34. https://doi.org/10.1080/02699050601111435.
Mélotte E, Maudoux A, Delhalle S, et al. Is oral feeding compatible with an unresponsive wakefulness syndrome? J Neurol. 2018;265:954–61. https://doi.org/10.1007/s00415-018-8794-y.
Mélotte E, Maudoux A, Delhalle S, et al. Swallowing in patients with disorders of consciousness: a cohort study. Ann Phys Rehabil Med. 2020;64. https://doi.org/10.1016/j.rehab.2020.04.008.
Fischer DB, Truog RD. What is a reflex?: A guide for understanding disorders of consciousness. Neurology. 2015;85:543–8. https://doi.org/10.1212/WNL.0000000000001748.
Prochazka A, Clarac F, Loeb GE, et al. What do reflex and voluntary mean? Modern views on an ancient debate. Exp Brain Res. 2000;130:417–32. https://doi.org/10.1007/s002219900250.
D’Ostilio K, Garraux G. Brain mechanisms underlying automatic and unconscious control of motor action. Front Hum Neurosci 2012; 6. https://doi.org/10.3389/fnhum.2012.00265
Sumner P, Husain M. At the edge of consciousness: automatic motor activation and voluntary control. Neuroscientist. 2008;14:474–86. https://doi.org/10.1177/1073858408314435.
Miller AJ. Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med. 2002;13:409–25. https://doi.org/10.1177/154411130201300505.
Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol. 2003;114:2226–44. https://doi.org/10.1016/S1388-2457(03)00237-2.
Palmer JB, Hiiemae KM, Matsuo K, et al. Volitional control of food transport and bolus formation during feeding. Physiol Behav. 2007;91:66–70. https://doi.org/10.1016/j.physbeh.2007.01.018.
Furuya J, Hara A, Nomura T, et al. Volitional chewing with a conscious effort alters and facilitates swallowing during feeding sequence. J Oral Rehabil. 2014;41:191–8. https://doi.org/10.1111/joor.12140.
Ashiga H, Takei E, Magara J, et al. Effect of attention on chewing and swallowing behaviors in healthy humans. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-42422-4.
Dubner R, Sessle BJ, Storey AT. The neural basis of oral and facial function. New York: Plenum; 1978.
Sessle BJ, Adachi K, Avivi-Arber L, et al. Neuroplasticity of face primary motor cortex control of orofacial movements. Arch Oral Biol. 2007;52:334–7. https://doi.org/10.1016/j.archoralbio.2006.11.002.
Sessle BJ, Yao D, Nishiura H, et al. Properties and plasticity of the primate somatosensory and motor cortex related to orofacial sensorimotor function. Clin Exp Pharmacol Physiol. 2005;32:109–14. https://doi.org/10.1111/j.1440-1681.2005.04137.x.
Yao D, Yamamura K, Narita N, et al. Neuronal activity patterns in primate primary motor cortex related to trained or semiautomatic jaw and tongue movements. J Neurophysiol. 2002;87:2531–41. https://doi.org/10.1152/jn.2002.87.5.2531.
Martin RE, Murray GM, Kemppainen P, et al. Functional properties of neurons in the primate tongue primary motor cortex during swallowing. J Neurophysiol. 1997;78:1516–30. https://doi.org/10.1152/jn.1997.78.3.1516.
Miller AJ. Deglutition. Physiol Rev. 1982;62:129–84.
Maestro I, Carreño M, Donaire A, et al. Oroalimentary automatisms induced by electrical stimulation of the fronto-opercular cortex in a patient without automotor seizures. Epilepsy Behav. 2008;13:410–2. https://doi.org/10.1016/j.yebeh.2008.03.013.
Humbert IA, German RZ. New directions for understanding neural control in swallowing: the potential and promise of motor learning. Dysphagia. 2013;28:1–10. https://doi.org/10.1007/s00455-012-9432-y.
Moore JD, Kleinfeld D, Wang F. How the brainstem controls orofacial behaviors comprised of rhythmic actions. Trends Neurosci. 2014;37:370–80. https://doi.org/10.1016/j.tins.2014.05.001.
Nishino T. The swallowing reflex and its significance as an airway defensive reflex. Front Physiol. 2013. https://doi.org/10.3389/fphys.2012.00489.
Palmer JB, Rudin NJ, Lara G, et al. Coordination of mastication and swallowing. Dysphagia. 1992;7:187–200. https://doi.org/10.1007/BF02493469.
Dodderi T, Philip NE, Mutum K. Effects of a dual swallow-attention task on swallow and cognitive performance measures. Percept Mot Skills. 2018;125:109–25. https://doi.org/10.1177/0031512517742283.
Dodderi T, Larisa V. Influence of attention resource allocation on sequential swallow in healthy young adults. Int J Brain Cogn Sci. 2016;5:1–6.
Troche MS, Okun MS, Rosenbek JC, et al. Attentional resource allocation and swallowing safety in Parkinson’s disease: a dual task study. Parkinsonism Relat Disord. 2014;20:439–43. https://doi.org/10.1016/j.parkreldis.2013.12.011.
Theurer JA, Czachorowski KA, Martin LP, et al. Effects of oropharyngeal air-pulse stimulation on swallowing in healthy older adults. Dysphagia. 2009;24:302–13. https://doi.org/10.1007/s00455-009-9207-2.
Takatsuji H, Zakir HMD, Mostafeezur RMD, et al. Induction of the swallowing reflex by electrical stimulation of the posterior oropharyngeal region in awake humans. Dysphagia. 2012;27:473–80. https://doi.org/10.1007/s00455-012-9393-1.
Tsukano H, Taniguchi H, Hori K, et al. Individual-dependent effects of pharyngeal electrical stimulation on swallowing in healthy humans. Physiol Behav. 2012;106:218–23. https://doi.org/10.1016/j.physbeh.2012.02.007.
Shaker R, editor. Principles of deglutition: a multidisciplinary text for swallowing and its disorders. New York: Springer; 2013.
Wheeler-Hegland KM, Rosenbek JC, Sapienza CM. Submental sEMG and hyoid movement during mendelsohn maneuver, effortful swallow, and expiratory muscle strength training. J Speech Lang Hear Res. 2008;51:1072. https://doi.org/10.1044/1092-4388(2008/07-0016).
Broussard DL, Altschuler SM. Brainstem viscerotopic organization of afferents and efferents involved in the control of swallowing. Am J Med. 2000;108:79–86. https://doi.org/10.1016/S0002-9343(99)00343-5.
Musgrove RE, Chiu W-H, Goldberg JA. Vagal motoneurons in Parkinson’s disease. In: Genetics, neurology, behavior, and diet in Parkinson’s Disease. Elsevier; 2020. p. 327–43. https://doi.org/10.1016/B978-0-12-815950-7.00021-7
Richards W, Sugarbaker D. Neuronal control of esophageal function. Chest Surg Clin N Am. 1995;5:157–71.
Shaker R, Easterling C, Kern M, et al. Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology. 2002;122:1314–21. https://doi.org/10.1053/gast.2002.32999.
Winiker K, Gozdzikowska K, Guiu Hernandez E, et al. Potential for behavioural pressure modulation at the upper oesophageal sphincter in healthy swallowing. Dysphagia Published Online First. 2021. https://doi.org/10.1007/s00455-021-10324-1
Meyer G, Austin R, Brady C, et al. Muscle anatomy of the human esophagus. J Clin Gastroenterol. 1986;8:131–4. https://doi.org/10.1097/00004836-198604000-00005.
Ertekin C, Kiylioglu N, Tarlaci S, et al. Voluntary and reflex influences on the initiation of swallowing reflex in man. Dysphagia. 2001;16:40–7. https://doi.org/10.1007/s004550000041.
Kern MK, Jaradeh S, Arndorfer RC, et al. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol-Gastrointest Liver Physiol. 2001;280:354–60. https://doi.org/10.1152/ajpgi.2001.280.3.G354.
Ertekin C. Voluntary versus spontaneous swallowing in man. Dysphagia. 2011;26:183–92. https://doi.org/10.1007/s00455-010-9319-8.
Sanders RD, Tononi G, Laureys S, et al. Unresponsiveness ≠ Unconsciousness. Anesthesiology. 2012;116:946–59. https://doi.org/10.1097/ALN.0b013e318249d0a7.
Guiu Hernandez E, Gozdzikowska K, Jones RD, et al. Pharyngeal swallowing during wake and sleep. Dysphagia Published Online First. 2019. https://doi.org/10.1007/s00455-019-09989-6
Kelly BN, Huckabee M-L, Jones RD, et al. The influence of volition on breathing-swallowing coordination in healthy adults. Behav Neurosci. 2007;121:1174–9. https://doi.org/10.1037/0735-7044.121.6.1174.
Lichter I, Muir R. The pattern of swallowing during sleep. Electroencephalogr Clin Neurophysiol 1975; 38.
Okuno K, Nohara K, Takai E, et al. Sleep stage coordination of respiration and swallowing: a preliminary study. Dysphagia. 2016;31:579–86. https://doi.org/10.1007/s00455-016-9719-5.
Pohl D, Arevalo F, Singh E, et al. Swallowing activity assessed by ambulatory impedance-pH monitoring predicts awake and asleep periods at night. Dig Dis Sci. 2013;58:1049–53. https://doi.org/10.1007/s10620-012-2474-z.
Sato K, Umeno H, Chitose S-I, et al. Deglutition and respiratory patterns during sleep in younger adults. Acta Otolaryngol (Stockh). 2011;131:190–6. https://doi.org/10.3109/00016489.2010.522595.
Sato K, Chitose S, Sato K, et al. Deglutition and respiratory patterns during sleep in the aged. Acta Otolaryngol. 2016;136:1278–84. https://doi.org/10.1080/00016489.2016.1203991.
Sato K, Nakashima T. Human adult deglutition during sleep. Ann Otol Rhinol Laryngol. 2006;115:334–9. https://doi.org/10.1177/000348940611500503.
Pinto A, Yanai M, Nakagawa T, et al. Swallowing reflex in the night. Lancet Lond Engl. 1994;344:820–1.
Uludag IF, Tiftikcioglu BI, Ertekin C. Spontaneous swallowing during all-night sleep in patients with parkinson disease in comparison with healthy control subjects. Sleep. 2016;39:847–54. https://doi.org/10.5665/sleep.5640.
Bonhomme V, Staquet C, Montupil J, et al. General anesthesia: a probe to explore consciousness. Front Syst Neurosci. 2019. https://doi.org/10.3389/fnsys.2019.00036.
D’Angelo OM, Diaz-Gil D, Nunn D, et al. Anesthesia and increased hypercarbic drive impair the coordination between breathing and swallowing. Anesthesiology. 2014;121:1175–83. https://doi.org/10.1097/ALN.0000000000000462.
D’Honneur G, Rimaniol JM, el Sayed A, et al. Midazolam/propofol but not propofol alone reversibly depress the swallowing reflex. Acta Anaesthesiol Scand. 1994;38:244–7.
Gemma M, Pasin L, Oriani A, et al. Swallowing impairment during propofol target-controlled infusion. Anesth Analg. 2016;122:48–54. https://doi.org/10.1213/ANE.0000000000000796.
Rimaniol JM, D’Honneur G, Duvaldestin P. Recovery of the swallowing reflex after propofol anesthesia. Anesth Analg. 1994;79:856–9. https://doi.org/10.1213/00000539-199411000-00007.
Taylor PA, Towey RM, Rappoport AS. Further work on the depression of laryngeal reflexes during ketamine anaesthesia using a standard challenge technique. Br J Anaesth. 1972;44:1163.
Nishino T, Takisawa K, Yokokawa N, et al. Depression of the swallowing reflex during sedation and/or relative analgesia produced by inhalation of 50% nitrous oxide in oxygen. Anesthesiology. 1987;67:995–8.
Cedborg AIH, Sundman E, Boden K, et al. Effects of morphine and midazolam on pharyngeal function, airway protection, and coordination of breathing and swallowing in healthy adults. Anesthesiology. 2015;122:1253–67.
Nishino T, Hiraga K. Coordination of swallowing and respiration in unconscious subjects. J Appl Physiol. 1991;70:988–93.
Royal College of Physicians. The vegetative state: guidance on diagnosis and management. Clin Med. 2003;3:249–54. https://doi.org/10.7861/clinmedicine.3-3-249.
Brady SL, Pape TLB, Darragh M, et al. Feasibility of instrumental swallowing assessments in patients with prolonged disordered consciousness while undergoing inpatient rehabilitation. J Head Trauma Rehabil. 2009;24:384–91.
Bremare A, Rapin A, Veber B, et al. Swallowing disorders in severe brain injury in the arousal phase. Dysphagia. 2016;31:511–20. https://doi.org/10.1007/s00455-016-9707-9.
Godet T, Chabanne R, Marin J, et al. Extubation failure in brain-injured patients: risk factors and development of a prediction score in a preliminary prospective cohort study. Anesthesiology. 2017;126:104–14. https://doi.org/10.1097/ALN.0000000000001379.
Kjaersgaard A, Nielsen LH, Sjölund BH. Factors affecting return to oral intake in inpatient rehabilitation after acquired brain injury. Brain Inj. 2015;29:1094–104. https://doi.org/10.3109/02699052.2015.1022883.
Millwood J, MacKenzie S, Munday R, et al. A report from an investigation of abnormal oral reflexes, lip trauma and awareness levels in patients with profound brain damage. J Disabil Oral Health. 2005;6:72–8.
Wang J, Wang J, Hu X, et al. The initiation of swallowing can indicate the prognosis of disorders of consciousness: a self-controlled study. Front Neurol. 2019. https://doi.org/10.3389/fneur.2019.01184.
Winstein CJ. Frequency, progression, and outcome in adults following head injury. Phys Ther. 1983;63:8.
Mackay LE, Morgan AS, Bernstein BA. Factors affecting oral feeding with severe traumatic brain injury. J Head Trauma Rehabil. 1999;14:435–47.
O’Neil-Pirozzi TM, Jack Momose K, Mello J, et al. Feasibility of swallowing interventions for tracheostomized individuals with severely disordered consciousness following traumatic brain injury. Brain Inj. 2003;17:389–99. https://doi.org/10.1080/0269905031000070251.
Terré R, Mearin F. Prospective evaluation of oro-pharyngeal dysphagia after severe traumatic brain injury. Brain Inj. 2007;21:1411–7. https://doi.org/10.1080/02699050701785096.
Hansen TS, Engberg AW, Larsen K. Functional oral intake and time to reach unrestricted dieting for patients with traumatic brain injury. Arch Phys Med Rehabil. 2008;89:1556–62. https://doi.org/10.1016/j.apmr.2007.11.063.
Hansen TS, Larsen K, Engberg AW. The association of functional oral intake and pneumonia in patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2008;89:2114–20. https://doi.org/10.1016/j.apmr.2008.04.013.
Terré R, Mearin F. Evolution of tracheal aspiration in severe traumatic brain injury-related oropharyngeal dysphagia: 1-year longitudinal follow-up study. Neurogastroenterol Motil. 2009;21:361–9. https://doi.org/10.1111/j.1365-2982.2008.01208.x.
Mandaville A, Ray A, Robertson H, et al. A retrospective review of swallow dysfunction in patients with severe traumatic brain injury. Dysphagia. 2014;29:310–8. https://doi.org/10.1007/s00455-013-9509-2.
Hagen C, Malkmus D, Durham P. Rehabilitation of the head injured adult: Comprehensive physical management. Levels of cognitive functioning. 1979.
Gill-Thwaites H, Munday R. The Sensory Modality Assessment and Rehabilitation Technique (SMART): a comprehensive and integrated assessment and treatment protocol for the vegetative state and minimally responsive patient. Neuropsychol Rehabil. 1999;9:305–20. https://doi.org/10.1080/096020199389392.
Shiel A, Horn S, Wilson B, et al. The Wessex Head Injury Matrix (WHIM) main scale: a preliminary report on a scale to assess and monitor patient recovery after severe head injury. Clin Rehabil. 2000;14:408–16.
Wijdicks E, Bamlet W, Maramattom B, et al. Validation of a new coma scale: the FOUR score. Ann Neurol. 2005;58:585–93.
Eccles R. Central mechanisms IV: conscious control of cough and the placebo effect. In: Hand Exp Pharmacol. Springer-Verlag Berlin Heidelberg; 2009. p. 241–62.
Mazzone SB, Cole LJ, Ando A, et al. Investigation of the Neural Control of Cough and Cough Suppression in Humans Using Functional Brain Imaging. J Neurosci. 2011;31:2948–58.
Hegland KW, Bolser DC, Davenport PW. Volitional control of reflex cough. J Appl Physiol. 2012;113:39–46. https://doi.org/10.1152/japplphysiol.01299.2011.
Martino R, Flowers HL, Shaw SM, et al. A systematic review of current clinical and instrumental swallowing assessment methods. Curr Phys Med Rehabil Rep. 2013;1:267–79. https://doi.org/10.1007/s40141-013-0033-y.
Pape T, Lundgren S, Guernon A, et al. Disorders of Consciousness Scale (DOCS): administration manual. Washington(DC): U.S.: 2011.
Pape TL-B, Heinemann AW, Kelly JP, et al. A measure of neurobehavioral functioning after coma. Part I: theory, reliability, and validity of the disorders of consciousness scale. J Rehabil Res Dev. 2005;42:1. https://doi.org/10.1682/JRRD.2004.03.0032.
Gollega A, Meghji C, Renton S, et al. Multidisciplinary assessment measure for individuals with disorders of consciousness. Brain Inj. 2015;29:1460–6. https://doi.org/10.3109/02699052.2015.1071426.
Bicego A, Lejoly K, Maudoux A, et al. Déglutition et états de conscience altérée. Rev Neurol. 2014;170:630–41. https://doi.org/10.1016/j.neurol.2014.04.004.
Mélotte E, Belorgeot M, Herr R, et al. The development and validation of the SWADOC: a study protocol for a multicenter prospective cohort study. Front Neurol. 2021. https://doi.org/10.3389/fneur.2021.662634.
Mortensen J, Jensen D, Kjaersgaard A. A validation study of the facial-oral tract therapy swallowing assessment of saliva. Clin Rehabil. 2016;30:410–5. https://doi.org/10.1177/0269215515584381.
Carnaby-Mann G, Lenius K. The bedside examination in dysphagia. Phys Med Rehabil Clin N Am. 2008;19:747–68.
Swan K, Cordier R, Brown T, et al. Psychometric properties of visuoperceptual measures of videofluoroscopic and fibre-endoscopic evaluations of swallowing: a systematic review. Dysphagia. 2019;34:2–33. https://doi.org/10.1007/s00455-018-9918-3.
De Tanti A, Zampolini M, Pregno S, et al. Recommendations for clinical practice and research in severe brain injury in intensive rehabilitation: the Italian Consensus Conference. Eur J Phys Rehabil Med. 2015;51:89–103.
Jakobsen D, Poulsen I, Schultheiss C, et al. The effect of intensified nonverbal facilitation of swallowing on dysphagia after severe acquired brain injury: a randomised controlled pilot study. NeuroRehabilitation. 2019;45:525–36. https://doi.org/10.3233/NRE-192901.
Brady SL, Pape TL-B. Swallowing evaluation and treatment for individuals with disordered consciousness. ASHA Lead. 2011;16:12–4.
Roberts H, Greenwood N. Speech and language therapy best practice for patients in prolonged disorders of consciousness: a modified Delphi study. Int J Lang Commun Disord. 2019;54:841–54. https://doi.org/10.1111/1460-6984.12489.
Hansen TS, Jakobsen D. A decision-algorithm defining the rehabilitation approach: ‘Facial oral tract therapy’®. Disabil Rehabil. 2010;32:1447–60. https://doi.org/10.3109/09638280903556482.
Seidl RO, Nusser-Müller-Busch R, Hollweg W, et al. Pilot study of a neurophysiological dysphagia therapy for neurological patients. Clin Rehabil. 2007;21:686–97. https://doi.org/10.1177/0269215507076393.
Konradi J, Lerch A, Cataldo M, et al. Direct effects of Facio-Oral Tract Therapy on swallowing frequency of non-tracheotomised patients with acute neurogenic dysphagia. SAGE Open Med. 2015;3:205031211557895. https://doi.org/10.1177/2050312115578958.
Nusser-Müller-Busch R, Gampp Lehmann K, editors. Facial-Oral Tract Therapy (F.O.T.T.): For eating, swallowing, nonverbal communication and speech. Cham: Springer International Publishing; 2021. https://doi.org/10.1007/978-3-030-51637-6
Giacino J, Kalmar K. The vegetative and minimally conscious states: a comparison of clinical features and functional outcome. J Head Trauma Rehabil. 1997;12:36–51.
Whyte J, Nakase-Richardson R. Disorders of consciousness: outcomes, comorbidities, and care needs. Arch Phys Med Rehabil. 2013;94:1851–4. https://doi.org/10.1016/j.apmr.2013.07.003.
Willems M, Sattin D, Vingerhoets A, et al. Longitudinal changes in functioning and disability in patients with disorders of consciousness: the importance of environmental factors. Int J Environ Res Public Health. 2015;12:3707–30. https://doi.org/10.3390/ijerph120403707.
Acknowledgements
The study was supported by the University and University Hospital of Liège, Belgian National Funds for Scientific Research (F.R.S-FNRS); Leon Fredericq Foundation (CNRF funds); European Union’s Horizon 2020 Framework Program for Research and Innovation [Human Brain Project SGA3, Grant Number 945539]; the BIAL Foundation; AstraZeneca Foundation; Generet fund and King Baudouin foundation; James McDonnell Foundation; Mind Science Foundation; IAP research network P7/06 of the Belgian Government (Belgian Science Policy); Public Utility Foundation – Université Européenne du Travail; and Fondazione Europea di Ricerca Biomedica. O.G. is research associate and S.L. is research director at the F.R.S-FNRS.
Author information
Authors and Affiliations
Contributions
EM reviewed the literature and drafted the article with the help of AM and OG. All authors reviewed it critically for important intellectual content and gave final approval of the revised manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mélotte, E., Maudoux, A., Panda, R. et al. Links Between Swallowing and Consciousness: A Narrative Review. Dysphagia 38, 42–64 (2023). https://doi.org/10.1007/s00455-022-10452-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-022-10452-2