Abstract
Microbial biomass which mostly generated from the microbial processes of bacteria, yeasts, and microalgae is an important resource. Recent concerns in microbial biomass production field, especially microbial lipid production for biofuel, have been focused towards the mixed culture of microalgae and yeast. To more comprehensive understanding of the mixed culture for microbial biomass, mono Chlorella pyrenoidosa, mono Yarrowia lipolytica and the mixed culture were investigated in the present work. Results showed that the mixed culture achieved significantly faster cell propagation of microalga and yeast, smaller individual cell size of yeast and higher relative chlorophyll content of microalga. The mixed culture facilitated the assimilation of carbon and nitrogen and drove the carbon flow to carbohydrate. Besides higher lipid yield (0.77 g/L), higher yields of carbohydrates (1.82 g/L), protein (1.99 g/L) and heating value (114.64 kJ/L) indicated the microbial biomass harvested from the mixed culture have more potential utilization in renewable energy, feedstuff, and chemical industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial biomass which is mostly generated from the microbial processes of bacteria, yeasts and microalgae contains a large amount of valuable components for food, feed, fertilizer and biofuels production. Recently, microalgae have attracted wider attention for its valuable application on fuel, food and pharmaceuticals products. Chlorella genus is a widely available microalga and has many commercial applications including food supplements, feed additives, biofuel feedstocks, etc. [1]. Yeast is considered as potential microbial resources for its relatively shorter doubling time, less affected by the environmental stress compared to other microbes. Yarrowia lipolytica is a typical species of yeast which is classified as GRAS (generally recognized as safe) for human [2]. Furthermore, Y. lipolytica displays a broad carbon spectrum (glycerol, glucose and hydrophobic substrates etc.) and is known as an extracellular protein-secreting species makes it plays an important role in the process of biosynthesis, biodegradation and biotransformation [3,4,5].
The mixed culture of microorganism has been used in many bioprocesses including waste degradation, remediation and biomass generation for bioactive compounds production [6]. The recent energy crisis has triggered significant attention on the microbial lipids which are used as feedstock for biodiesel production. Recent concerns in the microbial lipid production field for biofuel production have been focused towards the mixed culture of microalgae and yeast. A number of microalgae and yeast species in various combinations have been studied and the enhanced lipid production in the mixed culture was confirmed [7,8,9,10]. For example, 40–50% increases of total lipid were obtained in the mixed culture of Rhodotorula glutinis and Scenedesmus obliquus in a 5-L photobioreactor compared to mono-cultures alone [11]. In addition to lipid, microbial biomass also contains large amounts of carbohydrates and proteins. Carbohydrates are feedstock for alternative biofuels (bioethanol) and value-added chemicals via catalytic pyrolysis [12]. Single-cell proteins (SCPs) are important protein supplement in human foods or animal feeds. Hence, study of other associated main components (carbohydrates and protein) assimilation in cells is essential for better understanding of the advantages of the mixed culture. In research of synergistic effects between microalga and yeast in the mixed culture, O2/CO2 balance, substrate exchange, dissolved oxygen, pH adjustment, and the release of trace elements have been expounded to clarify the symbiotic relationship which may exist between yeast and microalga, and it is beneficial in terms of increasing biomass and lipid yield [10, 11, 13]. However, the effects of individual microalgae and yeast cell in the mixed culture system have not been studied well and the related information is not available.
In this study, to expand the perspective of the mixed culture for microbial biomass production and discuss the potential biomass utilization, the nitrogen and organic carbon removal, biomass growth, cell characteristics of C. pyrenoidosa and Y. lipolytica, biochemical profile of biomass including carbohydrate and protein content, and the higher heating value (HHV) of biomass were compared among three systems (mono C. pyrenoidosa, mono Y. lipolytica and mixed culture of C. pyrenoidosa and Y. lipolytica). This research will provide wider insight for the mixed culture of microalga and yeast, and promote the establishment of a full-component utilization scheme for microbial biomass.
Materials and methods
Strains and culture medium
The microalga C. pyrenoidosa 15–2070 was obtained from Carolina Biological Supply Company, USA. The yeast Y. lipolytica GIM2.197 was obtained from Guangdong Microbial Culture Collection Center, China. The seed media of microalga and yeast were BG11 and YPD, respectively. The medium used for the mono- and mixed cultures was described as follows: 1-L medium containing glycerol 7 g, yeast extract 2 g, NH4HCO3 0.12 g, citric acid 6.0 mg, ferric ammonium citrate 6.0 mg, EDTA 1.0 mg, NaNO3 1.5 g, K2HPO4·2H2O 0.051 g, MgSO4·7H2O 0.075 g, CaCl2 0.024 g, Na2CO3 0.02 g, A5 trace mineral solution 1.0 mL. The composition of A5 was: H3BO4 2.86 g/L, MnCl2·4H2O 1.81 g/L, ZnSO4·7H2O 0.222 g/L, Na2MoO4·2H2O 0.391 g/L, CuSO4·5H2O 0.079 g/L, Co(NO3)2·6H2O 0.049 g/L. All media were sterilized at 121 °C for 20 min before use, NH4HCO3 was added into the sterilized medium after filtration by a 0.2-μm filter unit (Millex-GP, Millipore).
Seed culture and experimental set-up
The yeast Y. lipolytica was cultivated in YPD medium at 28 °C and 150 rpm for 24 h. The microalga C. pyrenoidosa was cultivated at 28 °C and 150 rpm for 4 days under illumination at 45 ± 3 μ mol photons/m2/s with 2% CO2 supplemented from the bottom of the flasks. For preparing the high cell density inoculums, the seed cultures were centrifuged at 4000 rpm for 5 min at 25 ± 1 °C, and the precipitate was diluted suitably using sterilized water.
The mono- and mixed cultures were performed in shaker using 250-mL flasks with 75-mL working volume and cultivated at 28 ± 1 °C, and 170 rpm with the light supply of 45 ± 3 μmol photons/m2/s. The initial cell counts of microalga and yeast after inoculation were 7.5 × 106 and 2.0 × 105 cells/mL, respectively. Flasks containing three kinds of culture were taken out from shaker at 24 h, 48 h, 72 h, 120 h and 168 h and the samples were used for analyses. All the experiments were carried out in triplicate.
Analytical methods
Measurement of biomass and pH
Total 3 mL of culture was collected and centrifuged (Centrifuge 5804 R, Eppendorf AG, Hamburg, Germany) at 6000 rpm for 4 min. Cells were washed twice by distilled water and dried at 60 °C for biomass dry weight determination. A pH meter (Five Easy Plus, Mettler-Toledo, Australia) was used to determine the pH of the culture.
Single-cell analysis by flow cytometry
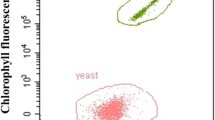
1 mL of culture was centrifuged at 6000 rpm for 5 min. Cells were washed with distilled water and diluted to 1 × 106 cells/mL for analysis of cell counting, individual cell size and fluorescent intensity of chlorophyll by flow cytometry (Beckman CytoFLEX, Germany). Cell population of microalga and yeast in the mixed culture was distinguished via algal chlorophyll auto-fluorescence. The data were analyzed and performed using FlowJo Version 8 software (TreeStar, Ashland, CA, USA).
The mean specific growth rate (μMean) was calculated using cell concentration data from flow cytometry using Eq. 1.
where C168 and C0 are the cells concentration at time t168 and t0, respectively. The μMean is expressed in d−1. Individual cell size can be described by forward scatter (FSC) measurement, specifically the integral (“area”) of a FSC pulse (FSC-A) [14]. The change of cell size was calculated using FSC-A value from flow cytometry (Eq. 2).
where Ftn is the FSC-A value at 24 h, 48 h, 72 h, 120 h and 168 h, respectively; Ft0 is the FSC-A value at 0 h.
Red fluorescence (670 nm) is linearly correlated with the chlorophyll content of living microalga cells [15]. The mean of integral fluorescent signal of PC5.5 channel (PC5.5-A) which with 488 nm Laser and 690/50 Filter was used to characterize relative chlorophyll content of microalgae in the mono- and mixed cultures.
Medium composition detection
Total nitrogen (TN), and ammonium (NH3-N) were determined using a Hach DR2700 Spectrophotometer (Hach Co., USA) and Hach reagents (CAT No.2714100, and 2606945, respectively) following the manufacturer’s procedure. NO3− was determined using a Metrohm ion chromatograph (883 Compact IC Pro, Switzerland) equipped with a Metrosep A Supp4-250 analytical column. The total organic carbon (TOC) was determined using an elemental analyzer (Vario TOC, Elementar, Hanau, Germany). A modified colorimetric method based upon cupric–glycerol complex was used to determine the glycerol concentration [16]. Briefly, 0.3 mL of 15% copper sulfate solution and 3.5 mL of 5% sodium hydroxide solution were reacted to generate copper hydroxide suspension. Then, 0.4 mL of either glycerol standard solution or sample supernatant was added into above-mentioned copper hydroxide suspension. After vigorous vortex for 1 min, the well-mixed solutions were centrifuged at 5,000 rpm for 3 min, and clear supernatants were collected and measured for absorbance at 630 nm.
Biomass composition and HHV analysis
Carbohydrates content was analyzed by phenol–sulfuric acid method [17]. Total lipid content in the dry biomass was determined by solvent extraction [18]. About 5 mg of freeze-dried biomass was used for elemental analysis using vario EL cube (Elementar, Germany). Crude protein content was determined and expressed as N% × 6.25 [19]. HHV was calculated using well-established correlations (Eq. 3) given by [20], based on the elemental composition.
where C, N, and H represent carbon, nitrogen, and hydrogen contents of material, respectively, expressed in mass percentages.
Statistical analysis
All the experiments were conducted in triplicate and the data were presented with the mean and standard deviation unless the specifically states otherwise. Statistical analyses were performed using the Data Analysis Tool Pak available on Microsoft Excel. Two-factor analysis of variance without replication analysis was used to estimate the statistical significant differences.
Results and discussion
Removal of nitrogen and organic carbon in mono- and mixed cultures
Nitrogen is an essential nutrient and required for the growth of all organisms. In nature, most of the microorganisms have developed their own mechanisms for uptake and assimilation of mineral either forms of nitrogen (such as ammonium and nitrate) or organic forms of nitrogen [21]. To have a comprehensive knowledge of the removal and uptake of different forms of nitrogen in the mono- and mixed cultures, TN (495.00 mg/L) which was mainly composed of NH3–N (35.17 mg/L), NO3–N (278.53 mg/L) and organic nitrogen was introduced in the culture system. It can be noticed that the mixed culture enhanced the removal of nitrogen (Fig. 1). The TN removal rate of the mixed culture (66.33%) was higher than that of mono-culture of yeast (31.99%) and microalga (17.85%) at the end of cultivation (Fig. 1a). The result was consistent with previous studies, which reported that the removal efficiencies for TN for the mixed culture (51.18%) were higher than those for the mono-culture of microalgae (29.28%) and yeast (46.31%) [9]. Although 100% NH3–N removal was achieved in the mixed culture and the mono yeast culture during the first 24 h, there was a sharp NH3–N concentration increase after 72 h in the mono yeast culture. The mono microalgae culture exhibited continuous removal abilities of NH3–N and 68.15% removal rate was reached at the end of cultivation (Fig. 1b). Continuous removal of NO3–N was observed in the mixed culture and 42.07% removal rate was achieved at the end of culture, while no removal was recorded in the mono microalgae culture and the mono yeast culture (Fig. 1c).
Biological uptake and accumulation are considered to be a major way of nitrogen removal as nitrogen is an essential element for various biological substance syntheses (e.g., protein, nucleic acid, and phospholipid) [22]. In addition to biological removal, ammonia stripping is another important way of nitrogen removal [23]. Ammonium ion–ammonia equilibrium in aqueous phase is governed by pH and temperature, and free ammonia at pH 9.25 can be stripped out of solution by aeration [24]. Since aeration and temperature in our experiment were both constant, the differences of ammonia stripping among the three systems were attributed to pH variation. Apart from the later stage of the mixed culture, pH of three culture systems was below 9.25 (Figure S1). Obviously, nitrogen removal in this work relies mainly on biological uptake and accumulation and the rapid growth of yeast in the mono yeast culture and the mixed culture contributed to removal of NH3–N in the early stage. It was reported that ammonia release is a mechanism of protection from yeast cell death under limited nutrient conditions [25]. This may be the reason of the NH3–N concentration increase in mono yeast culture after 72 h; at this time point, the total organic carbon almost exhausted (Fig. 2a). NH3–N concentration increase was not been observed at the later stage of the mixed culture may be due to the released NH3–N was stripped out of solution which was caused by high pH.
Unlike Chlorella, which can utilize ammonia and nitrate nitrogen for growth, Y. lipolytica cannot utilize NO3–N for growth [26]. No removal of NO3–N in the mono yeast culture was logical. For microalgae, ammonium is more preferred than nitrate since a redox reaction is not involved [23]. It is widely documented that nitrate consumption does not occur until the ammonium is almost completely consumed [27]. Hence, the presence of ammonia nitrogen stopped NO3–N assimilation completely in the mono microalga culture.
Glycerol can be used as a good carbon source by Y. lipolytica. When both glycerol and glucose were contained in the culture medium, glycerol was used firstly by yeast [4]. In this study, glycerol (initial concentration 7 g/L) was introduced into culture system as a carbon source. The profile of TOC was basically consistent with glycerol (Fig. 2). The mixed culture displayed obvious advantage of glycerol uptake in the early stage of culture. However, with the culture proceeding, the advantage disappeared. The mono yeast culture and the mixed culture exhibited remarkable uptake rate (94.72% and 93.48%), which was higher than that of the mono microalga culture (just 9.94%). Although C. pyrenoidosa could grew in mixotrophic condition in the presence of glycerol [28], its glycerol utilization capacity obviously inferior to Y. lipolytica.
Biomass growth and cell characteristics of C. pyrenoidosa and Y. lipolytica
As shown in Fig. 3a, the mixed culture displayed significant advantage (p < 0.01) in biomass production than mono microalga and mono yeast culture during the whole process. The highest biomass concentration (5.77 g/L) and mean biomass productivity (0.76 g/L/d) were achieved by mixed culture (Table 1). This result is consistent with the previous studies which reported mixed culture of microalgae and yeast can enhance the biomass production [6, 13, 23]. The highest biomass obtained in this study was higher than that from the mixed culture of R. glutinis and Chlorella vulgaris (4.63 g/L) [6] and that from the mixed culture of Y. lipolytica and C. vulgaris (1.62 g/L) [23]. It is widely documented that there was symbiotic relationship including O2/CO2 exchange, pH adjustment and substance exchange between microalga and yeast in the mixed culture, further providing higher biomass production compared with the mono-culture [6, 13].
Biomass concentration (a) and the respective growth curves of microalga and yeast cell (b) in the mono- and the mixed cultures. ** represent the significant difference between the mixed culture and mono-cultures (p < 0.01), asterisks and hashes represent the significant difference of cell concentration between the mixed- and mono-cultures of corresponding microalgae and yeast (p < 0.05), respectively
In this study, flow cytometry (FCM) was used for cell counting. The Chlorophyll fluorescence of microalgae can enable to count the microalgae and yeast separately by setting an FCM gate [29]. As shown in Fig. 3b, cell concentration of microalgae and yeast in the mixed culture was significantly higher than that in the corresponding mono-culture. Specifically, the mean specific growth rate of microalga (0.30 day−1) and yeast (0.99 day−1) in the mixed culture was significantly higher than that of the mono-culture of microalga (0.15 day−1) and yeast (0.88 day−1), respectively (Table 1). This indicated that synergistic effect may exist in the mixed culture which was beneficial to cell propagation of microalga and yeast. Interestingly, the maximal biomass in the mixed culture and the mono-culture of yeast was basically reached before 48 h, while yeast cell concentration sharply increased after 48 h. In order to comprehensive understanding the enhanced biomass production in the mixed culture, individual cell sizes of microalgae and yeast, relative Chlorophyll content of microalga cell were investigated.
As shown in Fig. 4a, the individual cell size of yeast was becoming smaller along with the culture proceeding and it was significantly smaller in the mixed culture. The cell growth is actually including cell division (reproduction) and cell development which related to cell size. Budded Y. lipolytica cells divide asymmetrically into larger parents and smaller daughters, and need enough resources to maintain average size over the generations [30]. During nitrogen starvation, cells of the yeast Saccharomyces cerevisiae increased threefold in number without net biomass growth [31]. Hence, smaller average individual cell size of yeast cell along with the culture proceeding may be caused by the gradual nutrient starvation. Furthermore, the increase of dissolved oxygen concentration can cause the reduction of yeast cell size [32]. Hence, higher O2 concentration caused by the photosynthesis of microalgae in the mixed culture could lead to smaller individual cell size of yeast in the mixed culture.
In terms of C. pyrenoidosa, the individual cell size after inoculation both in the mono C. pyrenoidosa culture and mixed culture has an increase in 30–50%. Microscopic photographs also show the same results (Figure S2). Comparing with BG11 which was used as seed media, media used for the mono- and mixed cultures provide more adequate nutrition. This may increase the individual cell size of C. pyrenoidosa after inoculation as the average individual cell size depends on surrounding environment [33]. When we focused on the comparison of the mono-culture and mixed culture, microalga is with significantly bigger individual cell size than mono C. pyrenoidosa culture at 24 h. It has been reported that microalgae with a bigger individual cell size when growth at high CO2 [34]. Hence, higher CO2 concentration caused by the aerobic respiration of yeast in the mixed culture might cause this result. After 24 h, the weaken growth of yeast and the continuous photosynthetic autotrophic growth of algae could result in a decrease of CO2 concentration [13], which caused the change of microalga cell size in the mixed culture not obvious comparing with mono C. pyrenoidosa culture. It was reported that individual cell size of algae was also affected by light intensity, being bigger at low light intensity [35]. Hence, the attenuation of light intensity caused by the increased yeast cell concentration might contribute to the large cell size of microalgae in the mixed culture. Furthermore, light intensity is also one of the factors having effect on the chlorophyll content. It was reported that the increase of total chlorophyll content is one way of physiologically adapting to reduced irradiance in terrestrial plants and algae [36]. Hence, the attenuation of light intensity in the mixed culture might result in the increase of the chlorophyll content per microalgae cell (Fig. 4b).
Biomass biochemical profile of mono- and mixed cultures
In many microorganisms, multiple forms of storage carbon products can be found, such as carbohydrates and lipids. As biosynthesis of the multiple forms of storage compounds may require the same carbon precursors and reducing power, thus understanding the change rule of carbon storage is the key issue of biomass enhancement in the mixed culture (Fig. 5a, b). Total carbohydrates content of the mixed culture is higher than those of the mono microalga culture and the mono yeast culture. Total carbohydrate contents were uptrend in the mixed culture (17.18–31.72%), the mono microalga culture (16.18–19.2%) and the mono yeast culture (14.14–24.68%). Contrary to carbohydrate contents, the total lipid contents after 72 h were downtrend in the mixed culture and the mono-culture. Total lipid content of the mixed culture was higher than that of the mono yeast culture and lower than that of the mono microalga culture. It was concluded that assimilated carbon was mainly flow to the carbohydrates, and this effect has been strengthened in the mixed culture. Lipids and carbohydrates likely compete with each other for carbon units, and accumulation of storage lipids either occurs in parallel or lags behind the accumulation of storage carbohydrates [37]. Carbohydrates are most likely preferred as reserve metabolites under conditions of short-term nutritional stress, as observed in plants [38]. The biochemical analyses showed that the crude protein content in the mono microalga fluctuates from 50.28 to 54.58%, while there was downtrend along with the culture proceeding in the mono yeast (48.67–29.13%) and the mixed culture (44.06–34.65%) (Fig. 5c). During nitrogen starvation, little protein was accumulated [31]. Hence, the deplete of the available nitrogen in the medium may be the main reason of the decrease in the protein content of the cells in the mono yeast culture and the mixed culture. In addition, the ammonia release at the later stage of culture further aggravated the decrease of protein content in the mono yeast culture [25]. The crude protein content of the mixed culture was lower than that of mono-cultures before 48 h, and then higher than that of the mono yeast culture and lower than that of the mono microalga culture. The profile of HHV, which is a major indicator of biomass quality in energy properties, is given in Fig. 5d. The HHV value of the mono microalga culture was uptrend and the mono yeast culture was downtrend, while the mixed culture displayed uptrend till 72 h and then decreased. HHV value of the mixed culture was higher than that of the mono microalga culture and mono yeast culture till 72 h, and then higher than that of the mono-culture of yeast and lower than that of microalga.
Yield is a key factor for whether one system is suitable for practical and commercial applications. As shown in Table 2, the mixed culture achieved higher yield of protein, carbohydrates, lipid and calorific value. The lipid yield of the mixed culture (0.77 g/L) which was 3.85 times of the mono yeast culture and 4.53 times of mono microalga culture was obtained in the current study. Similar result was also detected in the mixed culture of C.pyrenoidosa and R. glutinis, in which the maximum lipid productivity in the mixed culture was significantly higher than that of mono microalgae and yeast cultures, measuring 134.05 and 247.94%, respectively [39]. Furthermore, the protein yield of the mixed culture (1.99 g/L) which was 2.52 times of the mono yeast culture and 3.21 times of mono microalga culture; the carbohydrates yield of the mixed culture (1.82 g/L) which was 2.72 times of the mono yeast culture and 7.91 times of mono microalga culture; HHV yield of the mixed culture (114.64 kJ/L) which was 2.35 times of the mono yeast culture and 4.70 times of mono microalga culture were also recorded in the current study.
Potential applications expansion
Most of the studies related to the mixed culture of microalgae and yeast focused on enhancement of lipid production [9, 11, 13]. Excepting lipid, carbohydrates and protein contents are very high in microbial biomass. Carbohydrates are feedstock for alternative biofuels (bioethanol) and value-added chemicals via catalytic pyrolysis [12]. Single-cell proteins (SCPs) are important protein supplement in human foods or animal feeds. Hence, comprehensive and reasonable utilization of biomass components seems more reasonable. Furthermore, thermochemical conversion, which usually can utilize the entire biomass feedstock for biofuel production, is considered as a simpler route to produce biofuels [40]. The technology can be a complement to chemical and biochemical methods for the maximal utilization of microbial biomass [40]. The results of this study suggested that the promising advance of the mixed culture of microalgae and yeast should not be confined to lipid production. It can be extended to enhance the protein and carbohydrates production which can be helpful for food/feeding industry and chemical industry. Furthermore, the mixed culture system has better capacity to provide gaseous, liquid fuel, solid fuel and prepare chemicals if the harvested microbial biomass be utilized by thermochemical conversion.
Conclusion
Besides the higher yield of biomass and lipid, the mixed culture of C. pyrenoidosa and Y. lipolytica can achieve the higher yields of carbohydrates, protein, and HHV. Aside from benefiting to cell propagation of microalga and yeast, the mixed culture also makes individual cell size of yeast smaller and relative chlorophyll content of microalga higher. Furthermore, the mixed culture could strengthen the carbon flow to carbohydrates biosynthesis. To comprehensively understand the synergistic effect of the mixed culture, the differential transcriptome analysis of microalga and yeast cells in the mono- and mixed cultures is in progress.
References
Guccione A, Biondi N, Sampietro G, Rodolfi L, Bassi N, Tredici MR (2014) Chlorella for protein and biofuels: from strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol Biofuels 7:84
Ledesma-Amaro R, Nicaud JM (2016) Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog Lipid Res 61:40–50
Rywinska A, Juszczyk P, Wojtatowicz M, Robak M, Lazar Z, Tomaszewska L, Rymowicz W (2013) Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass Bioenerg 48:148–166
Rywinska A, Rymowicz W, Zarowska B, Skrzypinski A (2010) Comparison of citric acid production from glycerol and glucose by different strains of Yarrowia lipolytica. World J Microbiol Biotechnol 26(7):1217–1224
Barth G, Gaillardin C (1997) Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol Rev 19(4):219–237
Cheirsilp B, Suwannarat W, Niyomdecha R (2011) Mixed culture of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for lipid production from industrial wastes and its use as biodiesel feedstock. N Biotechnol 28(4):362–368
Cheirsilp B, Kitcha S, Torpee S (2012) Co-culture of an oleaginous yeast Rhodotorula glutinis and a microalga Chlorella vulgaris for biomass and lipid production using pure and crude glycerol as a sole carbon source. Ann Microbiol 62(3):987–993
Xue F, Miao J, Zhang X, Tan T (2010) A new strategy for lipid production by mix cultivation of Spirulina platensis and Rhodotorula glutinis. Appl Biochem Biotechnol 160(2):498–503
Ling J, Nip S, Cheok WL, de Toledo RA, Shim H (2014) Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour Technol 173:132–139
Santos CA, Ferreira ME, da Silva TL, Gouveia L, Novais JM, Reis A (2011) A symbiotic gas exchange between bioreactors enhances microalgal biomass and lipid productivities: taking advantage of complementary nutritional modes. J Ind Microbiol Biotechnol 38(8):909–917
Yen HW, Chen PW, Chen LJ (2015) The synergistic effects for the co-cultivation of oleaginous yeast-Rhodotorula glutinis and microalgae-Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour Technol 184:148–152
Budhi S, Mukarakate C, Iisa K, Pylypenko S, Ciesielski PN, Yung MM, Donohoe BS, Katahira R, Nimlos MR, Trewyn BG (2015) Molybdenum incorporated mesoporous silica catalyst for production of biofuels and value-added chemicals via catalytic fast pyrolysis. Green Chem 17(5):3035–3046
Zhang Z, Ji H, Gong G, Zhang X, Tan T (2014) Synergistic effects of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for enhancement of biomass and lipid yields. Bioresour Technol 164:93–99
Tzur A, Moore JK, Jorgensen P, Shapiro HM, Kirschner MW (2011) Optimizing optical flow cytometry for cell volume-based sorting and analysis. PloS One 6:1
Borcier E, Morvezen R, Boudry P, Miner P, Charrier G, Laroche J, Hegaret H (2017) Effects of bioactive extracellular compounds and paralytic shellfish toxins produced by Alexandrium minutum on growth and behaviour of juvenile great scallops Pecten maximus. Aquat Toxicol 184:142–154
Spagnolo F (1953) Spectrophotometric determination of glycerol as sodium-cupri-glycerol complex. AnaCh 25(10):1566–1568
Nielsen SS (2010) Phenol-sulfuric acid method for total carbohydrates. Springer, New York
Qin L, Shu Q, Wang Z, Shang C, Zhu S, Xu J, Li R, Zhu L, Yuan Z (2014) Cultivation of Chlorella vulgaris in dairy wastewater pretreated by UV irradiation and sodium hypochlorite. Appl Biochem Biotechnol 172(2):1121–1130
Higgs RJ, Chase LE, Ross DA, Van Amburgh ME (2015) Updating the Cornell Net Carbohydrate and Protein System feed library and analyzing model sensitivity to feed inputs. J Dairy Sci 98(9):6340–6360
Friedl A, Padouvas E, Rotter H, Varmuza K (2005) Prediction of heating values of biomass fuel from elemental composition. Anal Chim Acta 544(1–2):191–198
Geisseler D, Horwath WR, Joergensen RG, Ludwig B (2010) Pathways of nitrogen utilization by soil microorganisms—a review. Soil Biol Biochem 42(12):2058–2067
Qin L, Wei D, Wang Z, Alam MA (2018) Advantage Assessment of mixed culture of Chlorella vulgaris and Yarrowia lipolytica for treatment of liquid digestate of yeast industry and cogeneration of biofuel feedstock. Appl Biochem Biotechnol 187(3):856–869
Qin L, Liu L, Wang Z, Chen W, Wei D (2018) Efficient resource recycling from liquid digestate by microalgae-yeast mixed culture and the assessment of key gene transcription related to nitrogen assimilation in microalgae. Bioresour Technol 264:90–97
Markou G, Georgakakis D (2011) Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewaters: a review. ApEn 88(10):3389–3401
Vachova L, Palkova Z (2005) Physiological regulation of yeast cell death in multicellular colonies is triggered by ammonia. J Cell Biol 169(5):711–717
Ramanna L, Guldhe A, Rawat I, Bux F (2014) The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour Technol 168:127–135
Addy MM, Kabir F, Zhang R, Lu Q, Deng X, Current D, Griffith R, Ma Y, Zhou W, Chen P, Ruan R (2017) Co-cultivation of microalgae in aquaponic systems. Bioresour Technol 245:27–34
Rai MP, Nigam S, Sharma R (2013) Response of growth and fatty acid compositions of Chlorella pyrenoidosa under mixotrophic cultivation with acetate and glycerol for bioenergy application. Biomass Bioenerg 58:251–257
Collier JL (2010) Flow cytometry and the single cell in phycology. J Phycol 36(4):628–644
Jorgensen P, Tyers M (2004) How cells coordinate growth and division. Curr Biol 14(23):R1014–R1027
Johnston GC, Singer RA, Mcfarlane S (1977) Growth and cell division during nitrogen starvation of the yeast Saccharomyces cerevisiae. J Bacteriol 132(2):723
Lopes M, Gomes N, Goncalves C, Coelho MAZ, Mota M, Belo I (2008) Yarrowia lipolytica lipase production enhanced by increased air pressure. Lett Appl Microbiol 46(2):255–260
Lee K, Lee CG (2001) Effect of light/dark cycles on wastewater treatments by microalgae. Biotechnol Bioproc Eng 6(3):194–199
Giordano M, Bowes G (1997) Gas exchange and C allocation in Dunaliella salina cells in response to the N source and CO2 concentration used for growth. Plant Physiol 115(3):1049–1056
Singh SP, Singh P (2015) Effect of temperature and light on the growth of algae species: a review. Renew Sustain Energy Rev 50:431–444
Benvenuti G, Bosma R, Cuaresma M, Janssen M, Barbosa MJ, Wijffels RH (2015) Selecting microalgae with high lipid productivity and photosynthetic activity under nitrogen starvation. J Appl Phycol 27(4):1–7
Shtaida N, Khozingoldberg I, Boussiba S (2015) The role of pyruvate hub enzymes in supplying carbon precursors for fatty acid synthesis in photosynthetic microalgae. PsynR 125(3):407–422
Streb S, Zeeman SC (2012) Starch metabolism in Arabidopsis. Arabidopsis Book 10(e0160):e0160
Lu L, Chen J, Lim PE, Dong W (2018) Dual-species cultivation of microalgae and yeast for enhanced biomass and microbial lipid production. J Appl Phycol 30(6):1–11
Vardon DR, Sharma BK, Blazina GV, Rajagopalan K, Strathmann TJ (2012) Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Bioresour Technol 109:178–187
Acknowledgements
This work was supported by the Sciences and Technology of Guangzhou [Grant no. 201704030084], the National Natural Science Foundation of China [Grant no. 21606230], the Natural Science Foundation for research team of Guangdong Province [Grant no. 2016A030312007], and the National Key Research and Development Program-China [Grant no. 2016YFB0601004]. The authors are grateful to Mohammad Asraful Alam for his kind help in English editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qin, L., Liu, L., Wang, Z. et al. The mixed culture of microalgae Chlorella pyrenoidosa and yeast Yarrowia lipolytica for microbial biomass production. Bioprocess Biosyst Eng 42, 1409–1419 (2019). https://doi.org/10.1007/s00449-019-02138-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02138-1








