Abstract
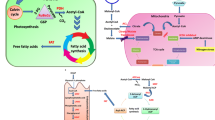
This study has shown that a co-culture of an oleaginous yeast Rhodotorula glutinis TISTR 5159 and a microalga Chlorella vulgaris var. vulgaris TISTR 8261 enhanced biomass and lipid production from glycerol. It is possible that the microalga may function as an oxygen producer in the co-culture and enhance the growth of yeast. The use of 3% pure glycerol as a carbon source and urea as a nitrogen source with a molar carbon-to-nitrogen (C/N) ratio of 32 gave the highest biomass and lipid production. These produced a 5.7-fold and 3.8-fold of biomass and lipid, respectively, compared to the initial unoptimized condition. The co-culture system was also applied to convert crude glycerol, a by-product from a biodiesel plant, to biomass and lipid. The lipid produced from the crude glycerol by the co-culture was mainly composed of palmitic acid (C16:0) 40.52% and oleic acid (C18:1) 21.30%, which was a plant oil-like fatty acid composition. This suggests that it has a high potential to be used as a biodiesel feedstock.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Microbial lipids can be produced by oleaginous microorganisms. Among these oleaginous microorganisms, yeast has an advantage over bacteria, molds and algae because it is unicellular, has a relatively high growth rate and accumulates lipid rapidly in discrete lipid bodies. The lipid produced can be used as a feedstock for biodiesel production (Saenge et al. 2010). Compared to other vegetable oils and animal fats, the production of yeast lipid has many advantages: a short life cycle, requires little labor to grow, is easy to scale up and is relatively independent of special requirements for place, season and climate (Li and Wang 1997).
Since crude glycerol is the main by-product of the conversion of oils into biodiesel, comprising approximately 10% by mass of the oils fed to the process (Dasari et al. 2005), the use of crude glycerol as a source of biomass and lipid production by oleaginous yeast would be of considerable benefit in terms of an economical and environmental method providing biodiesel feedstock. Therefore, there has been much research on the biological conversion of crude glycerol to biomass and lipid using oleaginous yeasts like Yarrowia lipolytica (Papanikolaou and Aggelis 2002; Papanikolaou et al. 2008; Makri et al. 2010), Cryptococcus curvatus (Liang et al. 2010) and Rhodotorula glutinis (Saenge et al. 2010). In addition to using a single culture of oleaginous yeast, it is possible that the biomass and lipid production could be improved using a co-culture system of yeast with a microalga. In a previous study, it was found that a co-culture of an oleaginous yeast R. glutinis and a microalga Chlorella vulgaris could enhance lipid production using effluent from a seafood processing plant and molasses from a sugar cane plant (Cheirsilp et al. 2011). This is because the microalga would act as an oxygen generator for the yeast while the yeast could provide CO2 to the microalga, and together they would carry out an improved production of lipid.
The aim of this study was to convert crude glycerol to a biodiesel feedstock by the co-culture system of yeast and microalga. This system has the potential for not only developing a new energy source but also protecting the aquatic environments from glycerol waste and reducing the global anthropogenic greenhouse effect. In order to minimize unknown variables that could result from the use of crude glycerol, the biomass and lipid production from pure glycerol by a single culture and a co-culture of yeast with microalga were first tested. The medium compositions including the nitrogen source, carbon-to-nitrogen (C/N) ratio, and glycerol concentration were optimized. Then, the production of biomass and lipid from crude glycerol was examined. In addition, the fatty acid composition of the microbial lipid extracted from the biomass of yeast and microalga grown with crude glycerol was determined.
Materials and methods
Microorganisms
Rhodotorula glutinis TISTR 5159 and Chlorella vulgaris var. vulgaris TISTR 8261 obtained from the Thailand Institute of Scientific and Technological Research were used for the production of lipid. The yeast R. glutinis was maintained on a yeast and malt extract (YM) medium agar slant. One liter of the YM medium contained 10 g glucose, 5 g peptone, 3 g yeast extract, 3 g malt extract, and 15 g agar. The pH was adjusted to 6.0. A plate culture was incubated at 30°C for 24 h. The cells were then transferred to a 250-mL flask containing 50 mL of culture medium. The flask was incubated at 30°C and 140 rpm for 24 h for a seed culture.
The microalga C. vulgaris was grown in a modified Chu 13 medium (Largeau et al. 1980). One liter of modified Chu 13 medium contained 0.2 g KNO3, 0.04 g K2HPO4, 0.1 g MgSO4·7H2O, 0.054 g CaCl2·2H2O, 0.01 g Fe citrate, 0.1 g citric acid, 0.036 g NaHCO3 and 1 mL of micro-elements. Each 1 L of micro-elements contained of 2.85 g H3BO3, 1.8 g MnCl2·4H2O, 0.02 g ZnSO4·7H2O, 0.08 g CuSO4·5H2O, 0.08 g CoCl2·6H2O and 0.05 g Na2MoO4·2H2O. The pH was adjusted to 6.7 before sterilization. Pre-culture was carried out in a 250-mL flask containing 50 mL of culture medium. The flask was incubated at 30°C and 140 rpm under a 3.0 klux light intensity with a 16:8 h light:dark cycle for 72 h for a seed culture.
Culture media for production of biomass and lipid
Glycerol-based medium was used for biomass and lipid production. It contained 1% of pure glycerol as a carbon source and ammonium sulfate as a nitrogen source with a molar C/N ratio of 16. The pH was adjusted to 6.0. Where noted, pure glycerol was replaced with crude glycerol in the same amount of glycerol content. Crude glycerol was obtained from the Prince of Songkla University biodiesel plant (Songkhla, Thailand) of purity 50%, with impurities composed mainly of potassium and sodium salts (4–5%), methanol (1–3%), non-glycerol organic matter (1.6–7.5%) and water (36%).
Single culture and co-culture
Flasks containing 90 mL of culture medium sterilized by autoclave were inoculated with a 10% seed culture of yeast or microalga or a mixture of both. The initial cell counts of yeast and microalga were 1.0 × 106 and 3.0 × 105 cells/mL, respectively. The cultures were incubated for 5 days at 30°C and 140 rpm under a 3.0 klux light intensity with a 16:8 h light:dark cycle.
Analytical methods
Individual cell counts of yeast and microalga were determined with a haemocytometer (Cai et al. 2007). Biomass was harvested by centrifugation at 7,500 rpm for 15 min. The pellets were then washed with acetone and distilled water twice and dried at 60°C in a hot air oven to get constant weight. Extraction of lipid from biomass was performed according to the modified procedure of Folch et al. (1957). Lipid was extracted with a mixture of chloroform:methanol (2:1, v/v) for 1 h. The extracted lipid was then centrifuged to obtain a clear supernatant and the solvent was removed by evaporation under vacuum.
The method for producing fatty acid methyl esters (FAME) from the extracted lipid involved hydrolysis of the lipid followed by esterification (Jham et al. 1982). The fatty acid composition in the FAME was analyzed using a HP6850 Gas Chromatography equipped with a cross-linked capillary FFAP column (length 30 m, 0.32 mm I.D, 0.25 μm film thickness) and a flame ionization detector. Operating conditions were as follows: inlet temperature 290°C; oven temperature initial 210°C hold 12 min, then ramp to 250°C at 20°C/min, hold for 8 min; detector temperature 300°C. Fatty acids were identified by comparing their retention times with known pure standards.
The glycerol concentration was determined following the method of Kosugi et al. (1994). The C/N ratio was calculated based on the molar basis of the carbon content from glycerol and the nitrogen content from the nitrogen source. The formula used for the conversion of glycerol concentration from % (w/v) to M carbon was (glycerol) (%) × 0.333 = M carbon.
Results and discussion
Biomass and lipid production from pure glycerol by single culture and co-culture of yeast and microalga
The single culture and co-culture of yeast R. glutinis and microalga C. vulgaris using 1% pure glycerol as a carbon source and ammonium sulfate as a nitrogen source with the molar C/N ratio of 16 are compared in Fig. 1. The single culture of yeast grew faster than the single culture of microalga. The biomass of yeast increased continuously until the end of cultivation while the numbers of yeast cell increased until day 2 of cultivation. In the co-culture, during 2 days of cultivation the numbers of yeast cell as well as its biomass increased faster than those in the single culture. The microalga grew slowly either in the single culture or in the co-culture. It should be noted that the numbers of microalga cells in the co-culture were less than in the single culture. This could be because the light may have scarcely penetrated through the high concentration of yeast cells in the co-culture.
At day 5 of cultivation, the biomass of yeast in the single culture became a little higher than that in the co-culture. This may have resulted from the faster depletion of the nutrients in the co-culture than that in the single culture. It should be noted that the glycerol did not deplete during the cultivation. The glycerol consumption by the co-culture was 76.7%, which was higher than that of the single cultures of yeast (64.8%) and microalga (72.1%). It has been reported that glycerol could be used as a carbon source for C. vulgaris under mixotrophic condition (Liang et al. 2009). In this study, the light and dark period was set at 16:8 h, bearing in mind further applications in outdoor photobioreactors with natural light:dark cycles.
During 4 days of cultivation, as the biomass in the co-culture was higher than that in the single cultures, so was the lipid production. The slight decrease in lipid production after 3 days of cultivation by the co-culture could be due to the internal degradation of storage lipids by microorganisms. It has been reported that it is common for oleaginous microorganisms to reserve lipid during growth, especially in any nitrogen starvation phase, then degrade it under carbon starvation conditions (Papanikolaou et al. 2004; Fakas et al. 2007). However, since a remarkable amount of glycerol remained in the medium after 3 days of cultivation (3.43 g/L), the lipid turnover in this yeast might not be due to the depletion of glycerol. It is possible that the decrease in glycerol concentration might reduce its uptake rate and, when the uptake rate of the extra-cellular carbon source is reduced, the lipid turnover occurs. The lipid turnover of yeast Rhodotorula sp. was also observed in the study of Chatzifragkou et al. (2011) when the glycerol concentration reduced to a level lower than would be as “threshold” for glycerol conversion to lipid.
The contributions to the higher productivity in the co-culture were obviously different between the two species. Since the yeast dominated in the co-culture in terms of number of cells, it is, therefore, reasonable to postulate that the yeast benefited more from the mutualism. Thus, the improvement in the biomass in the co-culture mainly resulted from the promoted growth of the yeast. It is possible that the microalga may function as an oxygen producer in the co-culture and enhance the growth of yeast. Xue et al. (2010) found that the dissolved oxygen in the culture of yeast R. glutinis greatly increased when microalga Spirulina platensis was added into the culture. In addition, the relationship was symbiotic, as the yeast produced CO2 that could be used by the microalga. In the co-culture, both the two metabolic reactions of CO2 release and uptake were combined and were complementary. Since the production of lipid from microorganisms is subjected to the constraints of high operation costs, even a small improvement in the culture techniques could result in substantial savings in their biomass production.
Optimizing the medium for the co-culture of yeast and microalga
Since the productivity of biomass and lipid in the co-culture of yeast and microalga was higher than that in the single culture of each, further experiments to optimize the medium composition were carried out. The co-cultures of yeast and microalga using 1% pure glycerol as a carbon source and various nitrogen sources with the molar C/N ratio of 16 are shown in Fig. 2. To economically produce lipid, several cheap inorganic nitrogen sources, such as ammonium sulfate, ammonium chloride, ammonium nitrate and a relatively cheap organic nitrogen source, urea, were tested. The addition of ammonium chloride, ammonium nitrate and urea increased the numbers of yeast cells compared to the use of ammonium sulfate. The highest value of yeast cells was obtained when using urea as a nitrogen source. It was found that ammonium nitrate and urea could enhance the growth of microalga. However, the numbers of microalga cell decreased after 2 days of cultivation when using ammonium nitrate as a nitrogen source. Therefore, urea was considered to be the most suitable nitrogen source because it enhanced the growth of both strains and gave the highest values of biomass and lipid. In the study of Shia et al. (2000), they also found that urea was the most suitable nitrogen source for the heterotrophic growth of Chlorella protothecoides.
Effect of nitrogen source on the cell counts of yeast and microalga, biomass and lipid production in the co-cultures using 1% pure glycerol as a carbon source and various nitrogen sources at the molar C/N ratio of 16. The nitrogen sources were ammonium sulfate, ammonium chloride, ammonium nitrate and urea
A nutrient imbalance in the culture medium is known to trigger lipid accumulation in oleaginous microorganisms. Lipid production requires a medium with an excess of carbon source and limited other nutrients, usually nitrogen. Thus, oleaginous potential is critically affected by the C/N ratio of the culture. At a low C/N, the carbon flux is distributed to allow cellular proliferation. At a high C/N ratio, when cells run out of nitrogen, they cannot multiply and excess carbon substrate is assimilated continuously to produce storage lipid. To determine the effect of C/N ratio, 1% pure glycerol was used as a carbon source and urea was used as a nitrogen source with various C/N ratios of 16, 24 and 32 (Fig. 3). The numbers of yeast cells increased significantly when the C/N ratio increased from 16 to 24. There was no significant difference in the numbers of microalga cells with various C/N ratios. The highest biomass and lipid production was obtained at a molar C/N ratio of 32. It should be noted that the biomass was at a maximum at day 2 of cultivation while that for lipid production was at day 3. As the culture growth progresses, a change in the C/N ratio is expected with relatively lower levels of nitrogen. The conditions for lipid production might therefore be more favorable during the later stage of culture growth. According to Angerbauer et al. (2008), the lipid production by Lipomyces starkeyi was enhanced when the C/N ratio was increased. A high C/N ratio at 25 was also used for the lipid production from glycerol by C. curvatus (Meesters et al. 1996). In the study of Illman et al. (2000), they also reported that the lipid production by microalga C. vulgaris increased significantly when it was grown in low nitrogen medium, while the difference in final dry weights was small.
In order to further increase the biomass and lipid production, the concentration of pure glycerol was increased from 1 to 2, 3 and 4% while using urea as the nitrogen source and maintaining the molar C/N ratio at 32. In addition, to reduce the cost of lipid production, pure glycerol was replaced with crude glycerol, a by-product from a biodiesel plant, at the same glycerol content. The results of biomass, lipid production and lipid yield are shown in Fig. 4. The biomass and, consequently, lipid production increased with increasing concentration of glycerol. However, there was little increase of biomass when the pure glycerol was increased from 3 to 4% and this led to a lower lipid yield. In the study of Meesters et al. (1996), when the concentration of pure glycerol was increased to higher than 32 g/L (3.2%), there was no further increase in the growth of C. curvatus and its growth was inhibited at a glycerol concentration of 64 g/L (6.4%). In this study, the biomass and lipid production using crude glycerol were lower than those using pure glycerol. This could be due to the presence of impurities in the crude glycerol. Liang et al. (2010) also reported that C. curvatus was inhibited when grown with crude compared with pure glycerol. However, it has been reported that Y. lipolytica could grow with crude glycerol just as well as with pure glycerol (Papanikolaou and Aggelis 2002). This could be due the higher purity of glycerol (65–85%) used in their study compared to that used in this study (50%).
The lipid yields of the co-culture with pure glycerol in this study were 0.05–0.12 g lipid/g glycerol, while those with crude glycerol were only 0.03–0.06 g lipid/g glycerol. In a recent investigation, the maximum lipid yield from glycerol by yeast that could be met in optimized culture conditions was 0.20–0.22 g/g (Papanikolaou and Aggelis 2009). The lower lipid yield in this study is presumably due to the relatively poor regulation of the enzymes involved in the primary metabolic steps of glycerol assimilation (Fakas et al. 2009). In the study of Chatzifragkou et al. (2011), a number of yeast strains were screened in relation to their ability to convert raw glycerol into lipid. Although strains belonging to the genus Rhodotorula have been reported to accumulate significant lipid quantities, in their study noticeable differences related with glycerol uptake, biomass production and lipid accumulation were observed between the two Rhodotorula strains studied. One Rhodotorula strain accumulated lipid up to 22% based on its biomass while the other accumulated only 3.4%. Therefore, the oleaginous character has a strongly strain-dependent capability. Apart from using glycerol as a substrate, Rhodotorula spp. have been revealed to be capable of producing high lipid quantities during growth on various natural substrates (Li et al. 2010; Zhao et al. 2010) and industrial effluent (Saenge et al. 2011).
After optimization of the co-culture, the biomass and lipid production were enhanced by 5.7-fold and 3.8-fold, respectively, compared to the unoptimized condition. In addition, since a higher glycerol concentration gave a lower lipid yield, perhaps the use of the fed-batch fermentation technique would further improve the biomass and lipid production by these two microorganisms. Moreover, since it was reported that the lipid production by Y. lipolytica was enhanced in highly aerated cultures (Papanikolaou and Aggelis 2002), the co-culture system would be an alternative method to further improve the lipid production by this strain.
Fatty acid composition of microbial lipid produced from crude glycerol
Table 1 shows the fatty acid composition of lipid extracted from yeast and microalga growing with crude glycerol. The fatty acid chain lengths were in the range of C12:0 to C24:0. The main fatty acids were long-chain fatty acids with 16 and 18 carbon atoms including palmitic acid as the predominant fatty acid (40.52%) followed by oleic acid (21.30%) and stearic acid (17.15%). Since the lipid from the single culture of R. glutinis grown with crude glycerol (Saenge et al. 2010) and other oleaginous yeasts such as Rhodosporidium toruloides Y4 (Li et al. 2007) and Rhodotorula mucilaginosa TJY15a (Li et al. 2010) contained oleic acid as the predominant fatty acid, the high content of palmitic acid could be derived from the presence of microalgal lipid. It has been reported that the fatty acids found in Chlorella vulgaris were mainly palmitic acid (47–63%) and oleic acid (10–37%), depending on the culture conditions (Converti et al. 2009). Petkov and Garcia (2007) also found that palmitic acid was the main fatty acid in C. vulgaris (26%) followed by linoleic acid (24%), linolenic acid (20%) and oleic acid (16%). The fatty acid compositional profile in this study is similar to that of plant oil which contains mainly palmitic and oleic acids. This indicates that the microbial lipid from this co-culture ha potential as a biodiesel feedstock. Although the high content of saturated fatty acid (palmitic acid) is evidence of lower fuel properties at low temperatures, it would provide better oxidative stability.
Conclusion
Improved biomass and lipid production from glycerol were achieved using a co-culture of oleaginous yeast R. glutinis with microalga C. vulgaris. Urea was the best source of cheap nitrogen and lipid production required a relatively high C/N ratio. By optimizing the medium composition, the biomass and lipid production were enhanced by 5.7-fold and 3.8-fold, respectively. In addition, the co-culture was used to convert crude glycerol to lipid. The fatty acid composition of the lipid produced from crude glycerol was similar to that of plant oil which made it suitable for biodiesel production. Although the lipid production in this study was not as high as in previously reported work, the co-culture system could be applied to other oleaginous yeasts that could grow and give a higher biomass when grown on crude glycerol.
References
Angerbauer C, Siebenhofer M, Mittelbach M, Guebitz GM (2008) Conversion of sewage sludge into lipids by Lipomyces starkeyi for biodiesel production. Bioresour Technol 99:3051–3056
Cai SQ, Hu C, Du S (2007) Comparisons of growth and biochemical composition between mixed culture of alga and yeast and monocultures. J Biosci Bioeng 104(5):391–397
Chatzifragkou A, Makri A, Belka A, Bellou S, Mavrou M, Mastoridou M, Mystrioti P, Onjaro G, Aggelis G, Papanikolaou S (2011) Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 36:1097–1108
Cheirsilp B, Suwannarat W, Niyomdecha R (2011) Mixed culture of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for lipid production from industrial wastes and its use as biodiesel feedstock. New Biotechnol 28(4):362–368
Converti A, Casazza AA, Ortiz EY, Perego P, Borghi MD (2009) Effect of temperature and nitrogen concentration on the growth and lipids content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process: Proc Intensification 48(6):1146–1151
Dasari MA, Kiatsimkul PP, Sutterlin WR, Suppes GJ (2005) Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl Catal A: Gen 281:225–231
Fakas S, Galiotou-Panayotou M, Papanikolaou S, Komaitis M, Aggelis G (2007) Compositional shifts in lipid fractions during lipid turnover in Cunninghamella echinulata. Enzyme Microb Technol 40:1321–1327
Fakas S, Makri A, Bellou S, Aggelis G (2009) Pathways to aerobic glycerol catabolism and their regulation. In: Aggelis G (ed) Microbial conversions of raw glycerol. Nova Science, New York, pp 9–18
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Illman AM, Scragg AH, Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb Technol 27:631–635
Jham GN, Teles FFF, Campos LG (1982) Use of aqueous HCl/MeOH as esterification reagent for analysis of fatty acids derived from soybean lipids. J Am Oil Chem Soc 59(3):132–133
Kosugi Y, Igusa H, Tomizuka N (1994) Continual conversion of free fatty acid in rice bran oil to triacylglycerol by immobilized lipase. J Jpn Oil Chem Soc 71:445–448
Largeau C, Casadevall E, Berkaloff C, Dhamelincourt P (1980) Sites of accumulation and composition of hydrocarbons in Botryococcus braunii. Phytochemistry 19:1043–1051
Li Q, Wang MY (1997) Use food industry waste to produce microbial oil. Sci Technol Food Ind 6:65–69
Li Y, Zhao Z, Bai F (2007) High–density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed–batch culture. Enzyme Microb Technol 41:312–317
Li M, Liu GL, Chi Z, Chi ZM (2010) Single cell oil production from hydrolysate of cassava starch by marine-derived yeast Rhodotorula mucilaginosa TJY15a. Biomass Bioenergy 34:101–107
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Liang Y, Cui Y, Trushenski J, Blackburn JW (2010) Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour Technol 101:7581–7586
Makri A, Fakas S, Aggelis G (2010) Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour Technol 101:2351–2358
Meesters PAEP, Huijberts GNM, Eggink G (1996) High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl Microbiol Biotechnol 45:575–579
Papanikolaou S, Aggelis G (2002) Lipid production by Yarrowia lipolytica growing on industrial glycerol in a single-stage continuous culture. Bioresour Technol 82:43–49
Papanikolaou S, Aggelis G (2009) Biotechnological valorisation of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica. Lipid Technol 21:83–87
Papanikolaou S, Sarantou S, Komaitis M, Aggelis G (2004) Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple-limited media. J Appl Microbiol 97:867–875
Papanikolaou S, Fakas S, Fick M, Chevalot I, Galiotou-Panayotou M, Komaitis M, Marc I, Aggelis G (2008) Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: Production of 1,3-propanediol, citric acid and single cell oil. Biomass Bioenergy 32:60–71
Petkov G, Garcia G (2007) Which are fatty acids of the green alga Chlorella? Biochem Syst Ecol 35(5):281–285
Saenge C, Cheirsilp B, Suksaroge TT, Bourtoom T (2010) Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem 46(1):210–218
Saenge C, Cheirsilp B, Suksaroge T, Bourtoom T (2011) Efficient concomitant production of lipids and carotenoids by oleaginous red yeast Rhodotorula glutinis cultured in palm oil mill effluent and application of lipids for biodiesel production. Biotechnol Bioprocess Eng 16(1):23–33
Shia XM, Zhang XW, Chen F (2000) Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzyme Microb Technol 27:312–318
Xue F, Miao J, Zhang X, Tan T (2010) A new strategy for lipids production by mix cultivation of Spirulina platensis and Rhodotorula glutinis. Appl Biochem Biotechnol 160(2):498–503
Zhao CH, Zhang T, Li M, Chi ZM (2010) Single cell oil production from hydrolysates of inulin and extract of tubers of Jerusalem artichoke by Rhodotorula mucilaginosa TJY15a. Process Biochem 45:1121–1126
Acknowledgements
The authors would like to thank Prince of Songkla University and the Thai government for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheirsilp, B., Kitcha, S. & Torpee, S. Co-culture of an oleaginous yeast Rhodotorula glutinis and a microalga Chlorella vulgaris for biomass and lipid production using pure and crude glycerol as a sole carbon source. Ann Microbiol 62, 987–993 (2012). https://doi.org/10.1007/s13213-011-0338-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0338-y