Abstract
This paper describes the association of two bioreactors: one photoautotrophic and the other heterotrophic, connected by the gas phase and allowing an exchange of O2 and CO2 gases between them, benefiting from a symbiotic effect. The association of two bioreactors was proposed with the aim of improving the microalgae oil productivity for biodiesel production. The outlet gas flow from the autotrophic (O2 enriched) bioreactor was used as the inlet gas flow for the heterotrophic bioreactor. In parallel, the outlet gas flow from another heterotrophic (CO2 enriched) bioreactor was used as the inlet gas flow for the autotrophic bioreactor. Aside from using the air supplied from the auto- and hetero-trophic bioreactors as controls, one mixotrophic bioreactor was also studied and used as a model, for its claimed advantage of CO2 and organic carbon being simultaneously assimilated. The microalga Chlorella protothecoides was chosen as a model due to its ability to grow under different nutritional modes (auto, hetero, and mixotrophic), and its ability to attain a high biomass productivity and lipid content, suitable for biodiesel production. The comparison between heterotrophic, autotrophic, and mixotrophic Chlorella protothecoides growth for lipid production revealed that heterotrophic growth achieved the highest biomass productivity and lipid content (>22%), and furthermore showed that these lipids had the most suitable fatty acid profile in order to produce high quality biodiesel. Both associations showed a higher biomass productivity (10–20%), when comparing the two separately operated bioreactors (controls) which occurred on the fourth day. A more remarkable result would have been seen if in actuality the two bioreactors had been inter-connected in a closed loop. The biomass productivity gain would have been 30% and the lipid productivity gain would have been 100%, as seen by comparing the productivities of the symbiotic assemblage with the sum of the two bioreactors operating separately (controls). These results show an advantage of the symbiotic bioreactors association towards a cost-effective microalgal biodiesel production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae could be seen as a suitable alternative feedstock for the next generation of biodiesel production. Certain species contain high amounts of oil, which can be extracted, processed, and refined into biodiesel, using the currently available technology for processing vegetable oils.
Currently, biodiesel is produced from plant and animal oils only, and not from microalgae [3] but this is likely to change. Microalgae have faster growth rates than plants, allowing the use of non-arable land and non-potable water, using far less water and avoiding food crop displacement; their production is not seasonal and they can be harvested daily [12]. The oil yield from algae is estimated to be 5.8 l m−2, which is nine times higher than the best oil yield crop (palm 0.595 l m−2) [7, 8]. However, microalgae as a feedstock for biodiesel are currently more expensive than traditional agricultural crops and further R&D is required to reach the market. Two of the key factors in microalgal production are low productivity and high labor and harvesting costs [6]. The use of fully controlled photobioreactors and fermenters allows a reduction in labor costs. An operating cell concentration of 0.5 g/l is widely reported in open ponds, whereas cell concentrations above 5 g/l have been reported in a closed thin photobioreactor [25]. Several hundred tons of microalgae have been produced annually at a cost of over 10 €/kg [4]. In terms of scale, productivities and costs, microalgae biomass production technology must advance in magnitude to be considered as an alternative fuel source [4]. Several technical and physiological difficulties associated with the supply and distribution of light and carbon dioxide to the photosynthetic mode of cultivation have been described [16]. High light intensities combined with low biomass concentrations could be detrimental to photosynthetic cultures due to photo-inhibition and photo-oxidation. Low light intensities and/or high biomass concentrations enhance endogenous biomass consumption by respiration. Both situations are common in outdoor mass cultures, inducing low productivities.
Carbon dioxide may be supplied by blowing air into the photobioreactor, but the carbon dioxide content in the air is only 0.04%, making dense cultures C-limited, as microalgae need 2–5% of carbon dioxide for their optimum growth. Soeder [24] estimated US$2,000 for pure carbon dioxide to produce 1 ton of dry algal biomass. Flue gas can be used as a cheap carbon dioxide source, but SO2, NO x and VOC may cause microalgae growth inhibition [15]. However, to remove the toxic pollutants in flue gas is quite expensive.
Some microalgae assimilate and thus utilize organic carbon as an energy source for growth in the dark (heterotrophy) or combined with CO2 uptake under light (mixotrophy), offering the possibility of increasing cell concentration (up to 40 g/l) and productivity [16]. It is widely observed that the maximum specific growth rate of algae increases in the following order: heterotrophy, autotrophy, and mixotrophy. Mixotrophic cultures exhibit high cell densities and specific growth rates and thus, higher biomass output rates [17].
Heterotrophic cultures are denser (up to 100 g/l) and, according to Lee [16], projected costs of producing algae in industrial fermenters seems to be approximately ten times lower when compared to the conventional autotrophic cultivation mode. On the other hand, dense heterotrophic cultures are often oxygen-limited. The aeration conditions are of crucial importance for cell growth as the specific growth rate decreases when the cells grow under restricted supply oxygen conditions. Moreover, oxygen transfer is likely to be a limiting factor during a commercial-scale high-cell-density cultivation of heterotrophic microalgae, leading to a decrease in process productivity. In such conditions, to maintain aerobic conditions, a very high stirrer speed has to be maintained during much of the process, resulting in power input increase and increased costs. Cell proliferation of microalgae can be negatively affected by mechanical agitation due to severe shear stress, which is the way generally used to improve mass transfer in submerged fermentations [13].
Another solution was suggested by Pack [22], who claimed a method for producing economically biomass of microalgae and fish, by establishing a symbiotic relation between the above two organisms in a culture pond exposed to sunlight or artificial illumination.
This symbiotic approach could be extended to any microalgae capable of growth under hetero and autotrophic nutritional modes, particularly, microalgae belonging to Chlorella Genus, such as Chlorella protothecoides, which have been recognized as a good lipid producer for biodiesel [19, 20].
According to the present work, extra dissolved carbon dioxide required for the growth of photoautotrophic microalgae is supplied through respiration of heterotrophic microalgae. The use of CO2 from microalgal respiration as carbon source for autotrophic microalgal biomass production has particular significance in three aspects: (1) low-cost C source; (2) C source of universal supply; (3) toxicity-free C source. This is in sharp contrast to the produced microalgae using flue gases as carbon dioxide source: any use besides oil for biodiesel, such as carotenoids for the feed, food and pharmaceutical markets, may face too strong politico-social resistance. Furthermore, part of the oxygen needed for the respiration of heterotrophic microalgae is supplied through photosynthesis of photoautotrophic microalgae.
The aim of this work is to demonstrate the symbiotic effect of the association of two bioreactors, a photoautotrophic and a heterotrophic one.
Materials and methods
Microalgal strain
The microalgal strain used in this study was Chlorella protothecoides strain 25 from the UTEX Collection (Texas University of Austin, USA).
Medium
Heterotrophic and mixotrophic Chlorella were cultivated in a simple organic medium containing per liter: 18.2 g glucose, 2 g yeast extract and 3 g Red Sea salt. The pH was adjusted to 7.2 by adding NaOH. This medium decreases production costs and gives demonstrably equivalent results to a microalgal standard medium (unpublished results). To prevent contaminations, a mixture of three antibiotics was added: chloramphenicol 5 mg/l, penicillin-G 62 mg/l, and streptomycin 100 mg/l previously filter sterilized. The use of antibiotics did not affect Chlorella growth rate and maximal biomass density (unpublished results).
Autotrophic Chlorella was cultivated in an inorganic medium containing per liter: 1.25 g KNO3, 1.25 g KH2PO4, 1 g MgSO4·7H2O, 0.11 g CaCl2·2H2O, 0.5 g NaHCO3, 0.1 mg FeEDTA·3H2O, and 10-ml trace elements solution [26]. The trace elements solution contained per liter: 286 mg H3BO3, 154 mg MnSO4·H2O, 22 mg ZnSO4, 5 mg CuSO4, 6 mg Na2MoO4·2H2O and 8 mg CoSO4·6H2O.
All media were sterilized before inoculation.
Cultivation
The glass bubble column bioreactor is often used for microalgae autotrophic cultivation and was easily adapted for heterotrophic and mixotrophic cultivation.
Five of these bioreactors were used to grow Chlorella in three different modes: two autotrophic, two heterotrophic and one mixotrophic growth.
Four of them were associated in pairs by connecting the gas phase from the first to the second: one group was an autotrophic bioreactor (a) that had its gas exit connected to the gas inlet of an heterotrophic bioreactor (H); another group was an heterotrophic bioreactor (h) that had its gas exit connected to the gas inlet of an autotrophic bioreactor (A). The fifth bioreactor was standing alone. Atmospheric air (compressed) was supplied to the first bioreactor in each group association and to the mixotrophic bioreactor (Fig. 1).
We designate the bioreactors that were receiving atmospheric air as control bioreactors (a, h, mixo) and the ones receiving air enriched with CO2 or O2 as symbiotic bioreactors (A, H, respectively).
Each bubble column bioreactor was filled with 810 ml of the respective medium and 90 ml of inoculum. Two sorts of inocula were used: one obtained from a 3-day growth of heterotrophic Chlorella incubated in Erlenmeyer at 28°C and 180 rpm; another obtained from a 5-day growth of autotrophic Chlorella incubated in bubble column at 28°C and 180 rpm. All cultures were axenic. Microscopic observations were done daily during the growth to check for possible contamination.
The five bubble columns were incubated under low light conditions (150 μE m−2 s−1). However, the ones containing heterotrophic Chlorella (h) were covered with aluminium foil to avoid light reaching the culture. The incubation temperature ranged from 25 to 28°C. Air inflow was 750 ml/min.
Microalgae were harvested at the end of 7 days by centrifugation at 5,000 rpm for 10 min at 5°C. The pellet was first freeze-dried and then grounded to powder in a mortar prior to analysis.
Analytical methods
Growth analysis
The growth of Chlorella was monitored daily by determination of dry weight (DW) after filtering 10–15 ml culture medium with a cellulose nitrate filter and drying 24 h at 100°C and by measuring the optical density at 540 nm [2]. Both measures were made in duplicate.
Determination of fatty acid composition
Fatty acid composition of Chlorella after 7 days growth under different cultivation modes was analyzed by gas chromatography. The fatty acids were transesterified by the method of Lepage and Roy [18] with modifications prior to GC analysis. To a sample of Chlorella powder (100 mg) 2 ml of a mixture of methanol/acetyl chloride (95:5 v/v) and 0.2 ml of internal standard solution from heptadecanoic acid in petroleum benzin 60–80°C (5 mg/ml, Sigma) were added.
The mixture was sealed in a light-protected Teflon-lined vial under nitrogen atmosphere and heated at 80°C for 1 h. The vial contents were allowed to cool down to room temperature, diluted with 1 ml of water and 2 ml of n-heptane and let stay for 15 min. The upper layer was recovered, then dried over anhydrous Na2SO4 filtered and collected in a vial, obtaining the fatty acid methyl esters. These were analyzed by gas chromatography in a Varian 3800 chromatograph (Palo Alto, California, USA), equipped with a flame ionization detector (FID). Separation was carried out in a 0.32 mm × 30 m fused silica capillary column (film 0.32 μm) Supelcowax 10 (Supelco, Bellafonte, Palo Alto, California, USA) with helium as carrier gas at a flow rate of 3.5 ml/ml. The column temperature was programmed at an initial temperature of 200°C for 8 min, then increased at 4°C/min to 240°C and held for 16 min. Injector and detector temperatures were 250 and 280°C, respectively, and split ratio was 1:50 for 5 min and 1:10 for the remaining time. The column pressure was 13.5 psi. Peak identification and response factor calculation was carried out using standard GLC-462 (Nu-Chek-Prep, Elysian, Minnesota, USA). Each sample was made in duplicate and injected twice.
Determination of lipid content
Nile Red fluorescence (NRFL) was determined daily to assess lipid content of microalgal cells [19].
Multi-parameter flow cytometry used NR (Riedel de Haën, Buchs SG, Switzerland) using a modified protocol described by de la Jara et al. [9]. A working solution (10 μl) of NR and acetone (0.033 mg/ml) was added to 1.305 ml of a cell suspension ~106 cells/ml (300 events/s). This mixture was gently submitted to vortex and incubated for 2 min at 37°C in darkness. NR fluorescence was determined using a FACScan flow cytometer (Becton-Dickinson Instruments, Erembodegem, Belgium) equipped with a 488-nm argon laser. Upon excitation by a 488-nm argon laser, NR exhibits yellow–gold and red fluorescence when dissolved in neutral and polar lipids, which are detected by the FL2 and FL3 channels, respectively. Non-stained cells were used as an autofluorescence control measured in FL2 and FL3 (AF2 and AF3, respectively), which were always set to the same pre-fixed fluorescence value, in all experiments. The total fluorescence (NRFL) corresponding to total cellular lipids was determined as the sum of the ratios FL2/AF2 and FL3/AF3.
The lipid content in biomass was assumed to be proportional to the total fatty acids obtained directly from transesterification of microalgal powder according to previous references [10, 14].
A correlation between FA% and NRFL was obtained in this work, which was applied to the three different modes of growth. This correlation was then used to estimate the FA content as the total microalgal lipid content.
Glucose determination
Glucose in the medium after separation of the cells was analyzed by 3,5-dinitrosalicylic acid (DNS) assay [21].
Nitrate determination
The ultraviolet spectrophotometric method was used to analyze nitrate in the medium after cells separation [1].
Productivity determination
The biomass productivity in batch was calculated as P = (X i − X 0)/(t i − t 0), at constant culture volume along the time of experiment, and the lipid productivity was calculated as Lp = (X i × %FA i − X 0 × %FA0)/(100 × (t i − t 0)), where X i is the biomass concentration after a period of time t i and X 0 the biomass concentration at the beginning of the experiment (t 0); FA i is the biomass fatty acid content after a period of time t i and FA0 the biomass fatty acid content at the beginning of the experiment (t 0).
Results and discussion
Heterotrophic, mixotrophic, and autotrophic growth
The growth of Chlorella protothecoides under the heterotrophic, mixotrophic and autotrophic conditions was successful, as was in the symbiotic bioreactors. No contamination was detected during the growth.
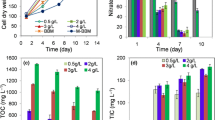
Chlorella protothecoides in the bubble column (Fig. 2) reached its highest biomass concentration (7 g DW/l) in both heterotrophic bioreactors, followed by the mixotrophic bioreactor (5 g DW/l) and, lastly, by the autotrophic bioreactors, that reached only 2 g DW/l.
Different cellular densities from autotrophic to heterotrophic and mixotrophic cultivations were used at the beginning of the trials. Under the photoautotrophic mode of cultivation, growth could be limited by light due to the self-shading effect; therefore operation at low cellular densities in these bioreactors was used (initial cellular density: 0.1 g DW/l). Nevertheless, the heterotrophic mode of cultivation allows the use of higher cellular densities, because it is independent on light intensity (initial cellular density: 0.6 g DW/l). The same cellular density was used for the mixotrophic cultivation.
The mixotrophic cultivation depicted the fastest specific growth rate (0.0402 h−1) among the five cultivations; autotrophic cultivation (μa = 0.0371 h−1, μA = 0.0392 h−1) showed a closed second specific growth rate and the heterotrophic cultivations had the slowest growth rate (μh = 0.0258 h−1, μH = 0.0278 h−1). This order is in accordance with Yuan-Kun Lee [16] who stated that the maximum specific growth rate of algae, cultured heterotrophically on simple sugars, is lower than that of photosynthetic cultures. The mixotrophic specific growth rate was the highest of all, which happens to be also in agreement with the same author that wrote up specific growth rate of the mixotrophic culture is approximately the sum of those under photoautotrophic and heterotrophic conditions, because respiratory and photosynthetic metabolisms operate concurrently.
The symbiotic bioreactor had a specific growth rate increase in comparison to the control bioreactor, due to the positive effect of the aeration becoming richer in O2 for the heterotrophic culture and in CO2 for the autotrophic culture. The positive effect of an extra supply of CO2 from the heterotrophic bioreactor to the autotrophic is much higher than that observed in the heterotrophic symbiotic bioreactor; this may be explained by the relatively small CO2 partial pressure (0.04%) in the air in the incoming flow and a significant increase when leaving the heterotrophic bioreactor. The partial pressure of O2 is high in the air and therefore the variation may not be as significant as depicted in the CO2 variation. For future work, the volume of the autotrophic reactor must be higher compared to the heterotrophic reactor in order to reflect the differences in cellular densities, or the photobioreactor optical path should be narrower in order to induce higher cellular densities, for the same volume.
Glucose consumption
The heterotrophic bioreactor intake air enriched O2 from the autotrophic bioreactor, depleted glucose, which occurred after 6 days of growth (Fig. 3). The heterotrophic bioreactor intake air (control) attained nearly the same result 1 day later. The mixotrophic bioreactor consumed only one-third (31.6%) of the initial glucose, but attained the highest yield coefficient on biomass to glucose (Table 1). This yield does not take into account the carbon consumption from CO2 that also takes place in the mixotrophic cultivation and so, the yield value is lower than that obtained from heterotrophic cultivation.
Despite the apparent highest specific growth rate, the mixotrophic cultivation attained the stationary phase after 48-h growth, compared to the others and, therefore the biomass concentration after 7 days was relatively low. This was the most efficient process to produce biomass with low glucose consumption, although it would take longer to produce the same amount of biomass as compared to any of the heterotrophic cultivation, where a higher biomass concentration was attained after the 7-day growth.
Nitrate consumption
The autotrophic cultivation intaking gases from the heterotrophic bioreactor, showed higher nitrate consumption than the bioreactor intaking air (Table 2). The same trend was observed in the produced biomass and in the yield coefficient for biomass to nitrate consumption; the higher the nitrate consumed, the higher the biomass produced and therefore the higher the observed yield.
Nile Red fluorescence and total lipid content
A correlation between the microalgal total lipid content assayed by the established method (FA) and the NR fluorescence was obtained, analyzing cells collected after 7 days of microalgal batch growth. Data is shown on Table 3, (FA%(w/w) = 0.132 × NRFL + 3.77, R = 0.973).
After a 7-day growth period, a comparison of heterotrophic, autotrophic and mixotrophic Chlorella protothecoides growth for lipid production revealed that heterotrophic production achieved the highest fatty acid content at 23 ± 1% (w/w) (fatty acid/biomass DW), followed by the mixotrophic production (18.1 ± 0.4%) and, lastly, the autotrophic production (4.9 ± 0.2%) (Table 3). The low autotrophic FA production could be due to an excess of nitrate in the medium.
The measured Nile Red fluorescence was proportional to that of the total lipid content, which was nearly constant or showed a slight variation during the autotrophic cultivations; however, a different trend was observed during the glucose cultivation. In the latter case, the fluorescence attained a maximum peak and then decreased, which was followed by a decrease in the glucose concentration (Fig. 3). Indeed glucose concentration was very low (2.7 g/l) in the bioreactor, intaking enriched air after a 5-day period. Simultaneously, the fluorescence started decreasing, which shows evidence that glucose was likely used for lipid accumulation. The heterotrophic bioreactor intaking air, showed that glucose was exhausted only after a 7-day period, and that the fluorescence was lower in the last day of experiment.
The at-line flow cytometric method used to assess the microalga lipid showed strong advantages related to the established methods for lipid analysis (fatty acids gas chromatography analysis [19], Bligh and Dyer method [5], or soxhlet extraction [20]). The cytometric method is quicker and accurate (each run analyzed 10,000 cells), does not use organic solvents and uses small sample volumes in comparison to the established methods.
Productivity
The variation of biomass productivity with time for each bioreactor individually is represented in Fig. 4. It shows that at the end of the assay the productivity was much lower as compared to the maximum obtained at the beginning of the assay for all the cultivations except for the autotrophic control bioreactor. Both heterotrophic cultivations reached their maximum biomass productivity on the fourth day, but the productivity in the bioreactor intaking air enriched O2 was higher (1.27 g DW l−1 day−1) than the one intaking only air (control bioreactor) (1.17 g DW l−1 day−1).
The mixotrophic cultivation attained the highest biomass productivity (1.33 g DW l−1 day−1) in only 2 days, and afterwards decreased significantly, contrarily to the heterotrophic cultivations where the productivity decreased slowly after reaching its maximum.
The daily fatty acid content (Fig. 5) was estimated from the correlation between FA% versus NRFL, as explained above, obtained from data in Table 3: FA%(w/w) = 0.132 × NRFL + 3.77, with an R = 0.973.
The maximum lipid productivity for heterotrophic cultivations was attained on the fifth day (Fig. 5), 1 day later than the maximum biomass productivity for the same cultivations. The symbiotic bioreactor achieved double lipid productivity compared to the control bioreactor. After that day, lipid productivity decreased indicating that the fifth day is the time when harvesting should be done. At this point the biomass concentration is also close to the maximum 7 g DW/l, attained with these experimental conditions.
The productivity of the symbiotic associations (a → H, h → A) was calculated as the sum of the two individual productivities divided by two, as the two bioreactors comprised in the association have the same volume; results are shown in Table 4.
The biomass productivity of the two bioreactors associations (a → H, h → A) was higher (10 and 20%), than the sum of the productivity of the two bioreactors operating separately (controls), after 4 days of growth, when the maximum productivity was attained.
The lipid productivity of the association ‘a → H’ was 90% higher than the sum of the productivities of the two bioreactors operating separately (controls), however, the association ‘h → A’ had the same lipid productivity as the control (Table 4), on the fifth day, when the maximum was attained.
If the two bioreactors had been inter-connected in a closed loop (A ⇔ H), the biomass productivity gain would be 30% and the lipid productivity gain would be 100% as can be seen by comparing the productivities of the symbiotic association with the sum of the two bioreactors operating separately (controls) (Table 4).
The effect is more remarkable on the fourth day because all cultivations are still growing and have not achieved the stationary phase yet. At this phase, microalgae are actively growing and so, producing CO2 during respiration (heterotrophic bioreactor) or O2 during photosynthesis (autotrophic bioreactor).
These results show an advantage of the symbiotic association of the two bioreactors over the separate operation.
In further scale-up studies with the symbiotic bioreactor a photobioreactor is to be connected to a fermenter with mechanical agitation and improved mass transfer between the gas and liquid phase in order to obtain higher cellular densities and productivities.
Fatty acid composition of microalgae oil
The total cellular fatty acid content was higher when Chlorella was cultivated in glucose, and the heterotrophic cultivation demonstrated (yielding) the highest values, at 22–23% (w/w) (Table 3).
With regards to the microalgae fatty acid composition (Table 5) under different nutritional modes, the heterotrophic oil (h and H) was mainly composed of oleic acid (18:1ω9 54–57%), linoleic acid (18:2ω6 22%) and palmitic acid (16:0 ~ 14%); the mixotrophic oil was quite similar, however, the proportion of the oleic acid to linoleic acid changed and showed a decrease in oleic acid to 39% and an increase in linoleic acid to 35%. The autotrophic oil had quite a different composition, which showed a decrease in oleic acid (26–17%) and in linoleic acid (15%), and an increase in palmitic acid (28%) and in linolenic acid (18:3ω3 15–31%).
The heterotrophic and mixotrophic oils could be used directly as a feedstock for biodiesel, as they comply with the European Standard EN 14214 [11] that limits linolenic acid methyl ester to 12% (w/w) for biodiesel vehicle use. This is not the case for the autotrophic oil, and so an intermediate process like blending the latter with other oils or hydrogenation of the oil is necessary in order to overcome this particular limitation.
Predictions for biodiesel properties from the raw oil FA composition can be made according to Ramos et al. [23] when undergoing a transesterfication process. These researchers found that the critical properties of biodiesel corresponded to the cetane number, oxidation stability, iodine value and cold filter plugging, which happens to depend on the nature of oil.
These researchers concluded that oils having more than 50% of monounsaturated FA, 20% or less of saturated FA and 30% or less of polyunsaturated (dienoic, trienoic) FA, produced quality biodiesel which in turn complied with the limits imposed by the European Standard EN 14214 [11] for critical parameters. The heterotrophic oil obtained in this research had this exact composition (Table 6) and therefore, it had a suitable FA composition for biodiesel production.
When comparing the heterotrophic oil with oils normally used in producing biodiesel (Table 6), we can conclude that it is quite similar to corn and rapeseed oil, and these are also held within the area limited by the saturated, monounsaturated and polyunsaturated (dienoic, trienoic) FA composition, achieving quality biodiesel according to the European Standard EN 14214 [11].
Conclusions
Chlorella protothecoides cultivated under heterotrophic conditions achieved a higher biomass concentration, higher fatty acid content, and higher lipid productivity when compared to mixo- and auto-trophic cultivation conditions. Furthermore, the fatty acid composition from Chlorella protothecoides that was cultivated heterotrophically, was in fact the most suitable for biodiesel production.
The symbiotic bioreactor, which is a photobioreactor or a fermenter that takes air enriched from another bioreactor, increased the biomass and oil productivity of the microalga Chlorella protothecoides. This was due to an extra supply of O2 in the fermenter and extra supply of CO2 in the photobioreactor to the microalgal cells. Therefore, the limitation of these substrates was avoided when cultures were dense. The symbiotic bioreactor association lipid productivity was greater than the sum of the productivities of the two bioreactors operating separately. The production costs of microalgal lipids produced by each of the bioreactors making up the symbiotic system will be lower in comparison to the production costs of the bioreactor operating under the same mode individually.
Moreover, the biomass produced does not have the flue gas contamination coming from the CO2 supply, and therefore the use of biomass or use of its by-products for food, feed, cosmetic and/or pharmaceuticals is also possible with little to no restrictions.
The symbiotic association offers the possibility of decreasing, or even eliminating, CO2 production from the microalgae heterotrophic metabolism, by supplying the photoautotrophic bioreactor with CO2.
It is theoretically possible to raise the dissolved CO2 content in the culture medium to the required level for optimal autotrophic growth simply by modifying the cell density at the heterotrophic stage. The high cell density at the heterotrophic stage requires a high rate of O2 supply. Such requirements will be met by establishing high cell mass density at the autotrophic stage. In future studies, the autotrophic/heterotrophic reactor volume ratio should be carefully designed in order to optimize the overall system.
The symbiosis concept applied to bioreactors associated with two different metabolic modes for cultivation was demonstrated here as a feasible way of producing microalgae for biodiesel, which had a number of advantages over the one metabolic mode of cultivation.
The concept could be easily extended to any heterotrophic culture connected to an autotrophic culture including other promising Single Cell Oil producers such as yeasts.
References
American Public Health Association, America Water Works Association, and Water Environment Federation (1998) Standard methods for the examination of water and wastewater. NO3 − ultra-violet spectrophotometric screening method. 20th edn. APHA, AWWA, WEF, Washington, DC, pp 4–115
Becker EW (1994) Measurement of algal growth. In: Baddiley Sir J, Carey NH, Higgings IJ, Potter WG (eds) Microalgae, biotechnology, & microbiology. Cambridge University Press, Cambridge, pp 56–62
Behzadi S, Farid MM (2007) Review: examining the use of different feedstock for the production of biodiesel. Asia-Pac J Chem Eng 2:480–486. doi:10.1002/apj.085
Benemann JR (1994) Systems and economic analysis of microalgae ponds for conversion of CO2 to biomass. 4th Quarterly Technical Prog. Report, DE-FG22-93PC93204, pp 44–51
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (1992) Algal biotechnology products and processes—matching science and economics. J Appl Phycol 4:267–279
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. doi:10.1016/j.biotechadv.2007.02
Chisti Y (2008) Response to Reijnders: do biofuels from microalgae beat biofuels from terrestrial plants? Trends Biotechnol 26(7):351–352
de la Jara A, Mendoza H, Martel A, Molina C, Nordström L, de la Rosa V, Diaz R (2003) Flow cytometric determination of lipid content in a marine dinoflagellate, Crypthecodinium cohnii. J Appl Phycol 15:433–438. doi:10.1023/A:1026007902078
de Swaaf ME, De Rijk TC, Eggink G, Sijtsma L (1999) Optimisation of docosahexaenoic acid production in batch cultivations by Crypthecodinium cohnii. J Biotechnol 70:185–192
European Standard EN 14214 (2004) Automotive fuels—fatty acid methyl esters (FAME) for diesel engines—requirements and test methods. AFNOR, Saint-Denis. (http://ec.europa.eu/energy/res/sectors/bioenergy_en.htm; http://ec.europa.eu/energy/green-paper-energy/doc/2006_03_08_gp_document_en.pdf
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36(2):269–274. doi:10.1007/s10295-008-0495-6
Gudin C, Chaumont D (1991) Cell fragility—the key problem of microalgae mass production in closed photobioreactors. Biores Tech 38(2–3):145–151
Kimura K, Yamaoka M, Kamisaka Y (2004) Rapid estimation of lipids in oleaginous fungi and yeasts using Nile Red fluorescence. J Microbiol Methods 56:331–338
Lee J-S, Kim D-K, Lee J-P, Park S-C, Koh J-H, Cho H-S, Kim SW (2002) Effects of SO2 and NO on growth of Chlorella sp. KR-1. Biores Tech 82:1–4
Lee YK (2004) Algal nutrition: heterotrophic carbon nutrition. In: Richmond Amos (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell, London, pp 116–124
Lee YK (2001) Microalgal mass culture systems and methods: their limitation and potential. J Appl Phycol 13:159–168
Lepage G, Roy C (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–119
Lopes da Silva T, Santos CA, Reis A (2009) Multi-parameter flow cytometry as a tool to monitor heterotrophic microalgal batch fermentations for oil production towards biodiesel. Biotechnol Bioproc Eng 14:330–337
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Biores Tech 97:841–846
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Pack MY (1991) Symbiotic production method for microalgae and fishes. US Patent 5,040,486
Ramos MJ, Fernandez CM, Casas A, Rodriguez L, Perez A (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Biores Tech 100:261–268
Soeder C (1978) Economic considerations concerning the autotrophic production of microalgae at the technical scale. Arch Hydrob Beih 11:259–273
Tredici MR (2004) Mass cultivation of microalgae. Mass production of microalgae: photobioreactors. In: Richmond Amos (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell, London, pp 178–214
Vonshak A (1986) Laboratory techniques for the cultivation of microalgae. In: Richmond A (ed) Handbook of microalgal mass culture. CRC Press, Boca Raton, pp 117–143
Acknowledgments
Santos CA was financially supported by a PhD grant of Fundação para a Ciência e a Tecnologia (SFRH/BD/38516/2007), Portugal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santos, C.A., Ferreira, M.E., Lopes da Silva, T. et al. A symbiotic gas exchange between bioreactors enhances microalgal biomass and lipid productivities: taking advantage of complementary nutritional modes. J Ind Microbiol Biotechnol 38, 909–917 (2011). https://doi.org/10.1007/s10295-010-0860-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0860-0