Abstract
Mix cultivation of microalgae (Spirulina platensis) and yeast (Rhodotorula glutinis) for lipid production was studied. Mixing cultivation of the two microorganisms significantly increased the accumulation of total biomass and total lipid yield. Dissolved oxygen and medium components in the mixed fermentation medium were analyzed. Mix cultivation in monosodium glutamate wastewater was further studied. Result indicated 1,600 mg/L of biomass was obtained and 73% of COD were removed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Researches concerning lipid production by microorganisms were stimulated decades ago with the increasing shortage of crude oil [1, 2]. Lipid produced by microorganisms was not only an alternative of crude oil, but also could be used as a raw material of biodiesel, which was a promising alternative fuel in the future [3, 4]. Compared with traditional techniques of lipid production (using vegetables or beans), using microorganisms to produce lipid has many merits such as less affected by the territory and climates than plants, more abundant and cheaper substrate needed, and so on [5].
Numerous studies on lipid production by microorganisms have been documented ever since World War I. Several kinds of microorganisms (fungi, bacteria, microalgae, etc.) were reported to have the ability to produce lipid. According to Seraphim Papanikolaou [6], single-cell oil was obtained when fungal mycelia was cultivated in high-sugar and nitrogen-limited media. Yen-Hui Lin [7] used marine microalgal strain Isochrysis galbana to produce docosahexaenoic acid. Stredansky [8] studied the production of polyunsaturated fatty acids by oleaginous fungus in solid-state cultivation. Wenmin Peng [9] investigated the pyrolytic characteristics of Spirulina platensis and Chlorella protothecoides as renewable energy source. Lipid production by S. platensis [9–11] or Rhodotorula glutinis [3, 12] has been elaborately studied; however, few have focused on mix cultivation of different strains to produce lipid. In addition, Jose [13] reported that mix cultivation of S. platensis and lactic acid bacteria could boost biomass accumulation. Thus, herein for the first time, we tried the mix cultivation of S. platensis and R. glutinis for lipid production. The aim of this study is to discuss a new method of producing lipid to increase lipid content and to decrease the cost of raw materials by utilizing the reciprocity between the strains in the mixed culturing system or even in monosodium glutamate wastewater.
Materials and Methods
Strains
The S. platensis (UTEX 1926) and R. glutinis (2.541) used were stored in our laboratory after being provided by Institute of Process Engineering, Chinese Academy of Sciences and China National Research Institute of Food and Fermentation Industry.
Culture Media
Medium I
Yeast medium (in grams per liter) [12]: industrial glucose, 40; yeast extract, 1.5; (NH4)2SO4, 2; KH2PO4, 7; Na2SO4, 2; MgSO4·7H2O, 1.5; initial pH = 5.5.
Medium II
Zarrouk medium (in grams per liter) [14]: NaHCO3, 16.8; K2HPO4, 0.5; NaNO3, 2.5; NaCl, 1.0; MgSO4·7H2O, 0.2; K2SO4, 1.0; FeSO4·7H2O, 0.01; ethylenediaminetetraacetic acid (EDTA), 0.08; CaCl2, 0.004; H3BO3, 0.00286; (NH4)6MO7O24, 0.00002; MnCl2·4H2O, 0.0018; CuSO4·5H2O, 0.000125; ZnSO4·7H2O, 0.00022.
Mixed Medium (in Grams per Liter)
MgSO4·7H2O, 1.0; (NH4)2SO4, 1.0; NaNO3, 2.5; K2SO4, 1.5; industrial glucose, 40; NaCl, 1.0; KH2PO4, 5.0; NaHCO3, 10.0; FeSO4·7H2O, 0.01; EDTA, 0.08; CaCl2, 0.004; H3BO3, 0.00286; (NH4)6MO7O24, 0.00002; MnCl2·4H2O, 0.0018; CuSO4·5H2O, 0.000125; ZnSO4·7H2O, 0.00022.
Wastewater Medium
The monosodium glutamate wastewater with initial COD of 43,210 mg/L and initial pH 2.0–2.5, provided by Hongmei, was used after pH was adjusted to 5.5.
Culturing Conditions
A plate culture of cells (S. platensis or R. glutinis) was incubated at 30 °C for 24 h, the cells transferred into 250 mL Erlenmeyer flasks containing 50 mL of corresponding culture medium. The flasks were incubated at 30 °C and 140 rpm. S. platensis was incubated for 7 days with continuous light illumination (4,000 lx), while R. glutinis was incubated for 24 h without special light illumination.
Flasks containing 50 mL culture medium was sterilized at 121 °C for 20 min, inoculated with 10% (v/v) seed cultures of R. glutinis or 20% (v/v) seed cultures of S. platensis or the mixture of both depending on different culture purpose. The culture system with S. platensis (i.e., mix culture, culture with waste water, or culture of S. platensis) was cultivated for 5 days under continuous light illumination (4,000 lx).
Determination of Biomass Concentration and Total Lipid
Biomass concentration was determined gravimetrically. Samples containing 40 mL fermentation broth withdrawn from the flasks were centrifuged at 4,000 rpm for 10 min, the cell pellet was collected and washed by distilled water twice, and then dried at 60 °C to constant weight.
Total lipid yield was measured according to Bligh’s means [15]. Dry cells were ground into a fine powder; 0.3 g powder was blended with 5 mL chloroform/methanol (2:1) and the mixture was agitated for 30 min at room temperature. The liquid phase was recovered by centrifugation and transferred into weighed test tubes. The process was repeated three times. The combined solvent was evaporated at 70 °C and the test tubes were thoroughly dried before weighed again.
Analysis of Lipid Composition and Metabolites in Fermentation Broth
Lipid composition was analyzed by the gas chromatography (GC-2010, made in Shimadzu, Japan). The condition of GC analysis was depicted as follows: flame ionization detector, 350 °C; DB-1ht, 30 m (length) × 0.25 mm (inner diameter) × 0.1 μm (thickness); PTV sample entrance, 33 cm/s; diffluent ratio, 1:5; carrier gas, N2.
The analysis method was established by Fei Shang [16]. Organic acids in the broth were quantified by high-performance liquid chromatography (HPLC) (LC-10Atvp, Shimadzu, Kyoto, Japan; Aminex HPX-87H ion exclusion column, 300 × 7.8 mm; mobile phase, 5 mmol/L H2SO4; flow rate, 0.6 mL/min; column temperature, 65 °C; organic acids were detected using an ultraviolet photometric detector at 210 nm).
Results and Discussion
Comparison of Mix Cultivation and Single Cultivation
The result of mix cultivation in the mixed medium was compared with R. glutinis cultivated in medium I or S. platensis cultivated in medium II. During mix cultivation, it was observed that the color of S. platensis was yellow rather than green, which meant that the synthesis of chlorophyll a was restrained and mixotrophic process was conducted instead because of the presence of glucose. According to Marquez [17], mixotrophic culture is much better than phototrophic culture, for the growth rate of mixotrophic culture is the sum of the phototrophic culture and the heterotrophic culture.
As shown in Table 1, the highest total biomass concentration in the R. glutinis culture was 4,784 mg/L and the highest lipid content was 16.02% in the S. platensis culture; the highest total lipid yield was 467 mg/L obtained from the mix cultivation, which was 3.18 times of R. glutinis culture and 3.92 times of S. platensis culture.
Interaction of R. glutinis and S. platensis in Mixed Medium
Mixed medium was used to cultivate R. glutinis and S. platensis separately. As shown in Table 1, the R. glutinis biomass concentration was 1,702 mg/L and the S. platensis biomass concentration was 203 mg/L. The sum of biomass concentration of the two microorganisms was 1,905 mg/L, which was much smaller than that of the mix cultivation 3,673 mg/L. The result of the total lipid yield of mix cultivation (467 mg/L) was also much more than the sum of the result of R. glutinis (135 mg/L) and S. platensis (13 mg/L). This suggested that, in the cell growth and lipid synthesis of the microorganisms, the interaction between the two microorganisms played a more important role than mixed medium itself. Several parameters of the culture medium were detected to explain this conclusion.
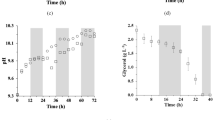
Dissolved oxygen (DO) in the R. glutinis culture was monitored using an autoclavable O2 sensor (Mettler Toledo, Greifensee, Switzerland). In the early phase of yeast cultivation, the DO decreased rapidly because of continuous consumption of oxygen. When S. platensis was added into the culture, DO increased rapidly from 7.45% to 120.5% in 5 h (Fig. 1), which meant that the addition of S. platensis could provide extra oxygen for yeast to enhance aerobic metabolism. Thus, the yeast could grow better. During yeast metabolism, organic acids were synthesized and the pH of the culture decreased then carbon dioxide was released in acidified environment containing NaHCO3. On one hand, the culture was adjusted to a less acidic environment so that the inhibition of yeast growth was alleviated. On the other hand, in the photosynthesis process of S. platensis, carbon dioxide, from yeast metabolism of mixed medium, was utilized as carbon source by S. platensis, and simultaneously, oxygen was released leading to DO increasing.
Components of the medium after cultivation were analyzed by HPLC. Several organic acids in the R. glutinis culture were detected, such as pyruvic acid, formic acid, and acetic acid, none of which ever appeared in the S. platensis culture. As to mix cultivation, less pyruvic acid was detected, which was presumed to be utilized by S. platensis, and the acetate in the culture could be utilized by S. platensis as carbon source during mixotrophic process [18]. Ragaerta [19] detected a wide range of organic compounds produced by yeast, such as ethanol, ethyl butyrate, 1-propanol, and so on. Among all the metabolites of yeast, many compounds might be utilized by algae.
In the photosynthesis process of S. platensis, carbon dioxide was transformed into carbohydrates. Jose [13] analyzed carbon, hydrogen, and nitrogen percentage in the S. platensis culture: for the Zarrouk medium alone, the corresponding values were 7.3%, 1.7%, and 2.0% before the growth of S. platensis and 11.5%, 8.9%, and 1.3% after cultivating it to late log phase, respectively. The changes recorded above showed that S. platensis consumed nitrogen and produced exopolysaccharide and other compounds [20], which should be favorable for the growth of yeast.
Result of Mix Cultivation of S. platensis and R. glutinis in Monosodium Glutamate Wastewater
The result above showed that mix cultivation of S. platensis and R. glutinis could greatly increase the accumulation of total biomass and total lipid yield. However, the mixed medium was so entangled that it would not be suitable to scale up culturing. Thus, monosodium glutamate wastewater instead of mixed medium was used to culture the two microorganisms. Fortunately, monosodium glutamate wastewater was found to be favorable for mix cultivation of S. platensis and R. glutinis. As the result indicated (Table 2), 1,600 mg/L of biomass and 220 mg/L of total lipid yield were obtained. At the same time, 73% of COD degradation, 100% of glutamic acid, 94% of reducing sugar, and 35% of NH4 +–N were removed, respectively. However, the lipid content was much lower than that in the basic medium (mixed medium) as show in Table 1. The reason should be that the wastewater is not rich in carbon source (as Table 3 shows) for lipid synthesis. So, further studies might be focused on finding a cheap carbon source for lipid production in the mix culture system.
Conclusions
Since most of studies were performed to enhance the lipid content by increasing the nutrimental components, especially glucose content, in the medium [5, 21], a new culture strategy was investigated in this paper. The mechanism of mix cultivation of R. glutinis and S. platensis was proposed. Mix cultivation in monosodium glutamate wastewater indicated that mix cultivation of R. glutinis and S. platensis would be a promising way to synthesize lipid using wastewater as medium if a carbon source was added.
References
Keith, E. C., James, B. G., Scott, A. W., & Patrik, R. J. (1987). Journal of Microbiological Methods, 6, 333–345. doi:10.1016/0167-7012(87)90019-4.
Edward, C., Cirilo, N. H., Genta, K., Kenji, S., & Ayaaki, I. (2001). Process Biochemistry, 37, 65–71. doi:10.1016/S0032-9592(01)00178-9.
Xue, F. Y., Zhang, X., Luo, H., & Tan, T. W. (2006). Process Biochemistry, 41, 1699–1702. doi:10.1016/j.procbio.2006.03.002.
Zhao, Z. B. (2005). Biotech-world (Chinese Journal), 12, 62–63.
Yan, Z., & Chen, J. (2003). Journal of Cereal & Oils (Chinese Journal), 7, 13–15.
Seraphim, P., Michael, K., & George, A. (2004). Bioresource Technology, 95, 287–291. doi:10.1016/j.biortech.2004.02.016.
Lin, Y.H., et al. (2007). Biochemical Engineering Journal, 37, 166–176. doi:10.1016/j.bej.2007.04.014.
Stredansky, M., Conti, E., & Salaris, A. (2000). Enzyme and Microbial Technology, 26, 304–307. doi:10.1016/S0141-0229(99)00146-5.
Peng, W. M., Wu, Q. G., Tu, P. G., & Zhao, N. M. (2001). Bioresource Technology, 80, 1–7. doi:10.1016/S0960-8524(01)00072-4.
Aaronson, S., Berner, T., & Dubinsky, Z. (1980). Microalgae. As a source of chemicals and natural products. In G. Shelef, & C. J. Soeder (Eds.), Algae biomass, production and use, (575–601). Amsterdam, Netherlands: Elsevier-North Holland.
Kiet, P. Q., & Jean, P. D. (1997). Biochimica et Biophysica Acta, 1346, 237–246.
Shi, A. H., & Zhou, B. (2003). Chinese Journal of Biotechnology, 24, 48–51.
Jose, L. P., Gloria, Z. C., Maria, C., & Zaccaro, M. (1998). International Journal of Food Microbiology, 45, 225–228. doi:10.1016/S0168-1605(98)00151-2.
Yoshitomo, W., & David, O. H. (1995). Energy Conversion, 36, 721–724. doi:10.1016/0196-8904(95)00106-N.
Bligh, E. G., & Dyer, W. J. (1959). Canadian Journal of Biochemistry and Physiology, 35, 911–917.
Shang, F., Wen, S. H., Wang, X., & Tan, T. W. (2006). Journal of Bioscience and Bioengineering, 101, 38–41. doi:10.1263/jbb.101.38.
Marquez, F. J. J. (1993). Journal of Fermentation and Bioengineering, 76, 408–410. doi:10.1016/0922-338X(93)90034-6.
Chen, T. F., Zheng, W. J., Yang, F., & Bai, Y. (2006). Enzyme and Microbial Technology, 39, 103–107. doi:10.1016/j.enzmictec.2005.10.001.
Ragaerta, P., Devliegherea, F., Loosa, S., Dewulfb, J., Langenhoveb, H. V., & Debeverea, J. (2006). Food Microbiology, 23, 154–161. doi:10.1016/j.fm.2005.02.002.
Jorge, A., Vieira, C., Luciane, M. C., & Paulp, F. D. (2004). Bioresource Technology, 92, 237–241. doi:10.1016/j.biortech.2003.09.013.
Li, Y., Zhao, Z. B., et al. (2007). Enzyme and Microbial Technology, 41, 312–317. doi:10.1016/j.enzmictec.2007.02.008.
Acknowledgements
We wish to express our thanks for the support from the National Natural Science Foundation of China (grant no. 20576013), Nature Fund for Outstanding Youth (grant no. 20325622), Beijing WWF (grant no. 2071002), Beijing Science And Technology Projects (grant no. D0205004040211), the National Basic Research 973 Program of China (grant nos. 2007CB707804 and 2007CB714304), and the National High Technology Research and Development 863 Program of China (grant nos. 2006AA020103, 2006AA020102, and 2006AA020201).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, F., Miao, J., Zhang, X. et al. A New Strategy for Lipid Production by Mix Cultivation of Spirulina platensis and Rhodotorula glutinis . Appl Biochem Biotechnol 160, 498–503 (2010). https://doi.org/10.1007/s12010-008-8376-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8376-z