Abstract
Animal seed dispersal processes are an important aspect of ecosystem services, as they shape the survival of seed dispersers and the balanced distribution of propagules for many plant communities. Several studies within tropical wild ecosystems have generally shown that seed dispersal processes are highly generalised and robust to extinction. Studies examining seed dispersal networks in highly built-up urban ecosystems and their robustness to species loss or extinction are rare. We examined avian seed dispersal networks across an urban ecosystem characterised by a high human settlement and infrastructure of the built environment in Zambia to determine their network specialisation, interaction evenness and interaction diversity, as these three parameters are critical in driving the resilience of these mutualisms’ interactions against extinction. A total of 405 individuals representing 11 species of birds were observed and recorded feeding on a total of 11 focal fleshy-fruiting plant species. Network specialisation was generally low and remained similar across study areas. Interaction evenness and interaction diversity were not only high but also remained similar across study areas. Low specialisation and high interaction evenness and diversity show that mutualistic interactions in these networks are equally highly generalised, suggesting a stable and robust coexistence of species in plant–frugivore communities within urban ecosystems. Generally, our results seem to broadly suggest that opportunities for conservation still exist in these ecosystems provided urbanisation is accompanied by promoting either the management of remnant fruiting plants or the cultivation of new ones to support the avian communities existing in these areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanisation is a pervasively growing global threat to biodiversity (Elmqvist et al. 2016; Kondratyeva et al. 2020). Research has shown that urbanisation is increasingly contributing towards extensive modification of biological communities at both local and landscape scales by reducing and fragmenting their natural habitats (Mckinney 2002; Emer et al. 2018). Recent forecasts suggest that the amount of natural habitats likely to be transformed by urbanisation is expected to increase, on average, by more than three times between 2000 and 2030 (from 450,000 km2 c. 2000) around the world (Elmqvist et al. 2016). And with 68% of the global human population predicted to live in urban areas by the year 2050 (United Nations 2019), this will most likely result in land use and land cover modifications, and environmental disturbances. Consequently, this will increase the pressure on local diversity of remnant species (Gaston 2010) and will induce the assembly of novel ecological communities (Swan et al. 2011; Kondratyeva et al. 2020). Already, over 80% of most urban areas across the globe are covered by pavement and buildings, of which only less than 20% remain vegetated (Mckinney 2008).
Generally, urbanisation impacts biodiversity and ecosystem services (e.g. soil formation and nutrient cycling, food and fresh water provision, etc.) both directly and indirectly (Elmqvist et al. 2016). Direct impacts consist of degradation, habitat loss, modified soils and other physical transformations caused by urban expansion (Escribano-Avula et al. 2018), whereas indirect impacts include changes in nutrient and water availability as well as an increase in abiotic stressors, like air pollution and changes in herbivory (Pickett and Cadenasso 2009). Arguably, land cover change is the most direct impact of urbanisation on biodiversity globally due to the growth of urban areas and associated activities, such as favouring the growth of non-native and native species that are frequently ecological generalists (Strohbach et al. 2009; Muller et al. 2013). Ultimately, this has been shown to severely affect the structure of ecosystems and can influence species diversity, ecological interactions and key ecological functions, such as animal-driven seed dispersal processes (Cruz et al. 2013; Silva et al. 2015; Valiente-Banuet et al. 2015; Yuan and Lu 2016). In fact, ecological interactions have been shown to be even more vulnerable to human propelled extinction than the species that drive these interactions (Valiente-Banuet et al. 2015). The ecological importance of interactions, such as the animal-mediated mutualistic seed dispersal processes, especially in the context of maintaining biodiversity and ecosystem dynamics are well documented (Howe and Smallwood 1982; Bascompte and Jordano 2007; Shikang et al. 2015; Timoteo et al. 2016). Therefore, altering or causing changes in disperser movement patterns that can affect seed removal and plant recruitment rates across ecosystems could result in several unforeseen consequences on biodiversity as a whole (Ciuti et al. 2012).
In recent years, the study of seed dispersal processes has shifted from examining one-on-one interactions between plants and frugivorous animals (Herrera 2002; Cordeiro and Howe 2003; Farwig et al. 2006), to the use of a community-wide approach involving the mechanistic understanding of seed dispersal network analysis (e.g. Carlo and Yang 2011; Chama et al. 2013; Dugger et al. 2018). This is because seed dispersal is mainly used as a mechanism to predict the consequences of species extinction and environmental perturbation to the entire community, especially in disturbed ecosystems (Guimarães Jr et al. 2006), such as urban environments. In fact, several factors have been shown to affect avian seed dispersers at a community level in disturbed environments, among which include predation pressure, habitat fragmentation size, edge heterogeneity, vegetation structure, human disturbances, food availability and landscape characteristics (e.g. surrounding matrix and patch connectivity; Yuan and Lu 2016; Cote et al. 2017; Gaynor et al. 2018). Many of these ecosystem disturbances affect the biological composition of communities whereby they alter their capacity to sustain key ecological functions, such as seed dispersal (Cruz et al. 2013). Further, most urban areas have also been shown to contain vegetation patches that are poorly connected to a certain degree (Francis and Chadwick 2013), largely driven by an increase in impervious surfaces and promoting the management of landscapes that structurally simplifies vegetation within these areas (Baker and Harris 2007; Mckinney 2008; Warren et al. 2015). Such attributes of habitat disturbance can undeniably impinge dispersal processes. Surprisingly, however, recent research shows that many avian frugivores seem to have developed adaptations in these environments irrespective of the various ecological disturbances they are subjected to (Murgui and Hedblom 2017). Whether these adaptations by avian frugivores translate into stable and thus robust seed dispersal networks in these ecosystems remains unclear.
Fruit-eating birds in wild tropical ecosystems have been shown to have access to a variety of fruits, largely attributed to the presence of a variety of fleshy-fruiting plants, almost all year round, in those regions (Howe and Smallwood 1982; Chama et al. 2013). Several studies seem to generally suggest that such plant–frugivore interactions are robust to extinction in tropical wild ecosystems (Gonzalez-Castro et al. 2012; Dugger et al. 2018). However, studies examining how the community interactions between frugivores and their plant mutualists respond to ecological disturbances, such as urbanisation, are still rare (but see Cruz et al. 2013). Yet, seed dispersal processes play an important role in shaping plant recruitment and forest regeneration (D'Avila et al. 2010), albeit the benefits generated from the seed dispersal relationship between frugivorous animals and fleshy-fruiting plants are reciprocal or mutual. Thus, any declines in plant population may affect the population of seed dispersers since fleshy fruits are important food resources for many frugivorous animals (Carlo and Yang 2011). Similarly, if seed dispersers decrease in numbers, it will affect the regeneration and restoration process of plant communities (Guimarães Jr et al. 2006). Unless we increase our understanding of the response of these ecologically critical mutualistic interactions to environmental disturbance, we are at risk of facing a total collapse in biological communities in the face of increased threats resulting from urbanisation. Although research has shown that dispersal networks in the tropical and subtropical ecosystems are generally resilient and thus robust to species extinction (e.g. Schleuning et al. 2011; Dugger et al. 2018), it remains unclear whether this also applies to those (seed dispersal networks) in highly built-up tropical urban areas, especially given the ecological disturbances that they are regularly subjected to.
Using community ecological parameters, such as network specialisation, interaction evenness and diversity, this study examined the effects of urbanisation on avian seed dispersal networks especially in the context of their robustness to species extinction. We predicted that avian seed dispersal networks in the urban ecosystem are highly specialised and therefore weakly structured. Effectively, this means that avian seed dispersal networks in urban ecosystems are not robust to species extinction as compared to those reported in the wild tropical and subtropical ecosystems (Albrecht et al. 2012; Dugger et al. 2018).
Materials and methods
Study area
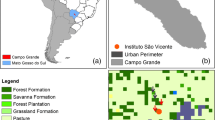
This study was conducted in Kitwe district (− 12° 48′ 8.75″ S/28° 12′ 47.63″ E; elevation of 1213 m asl, Fig. 1), within the Copperbelt Province of Zambia, from 30th Oct. 2018 to 31st Mar. 2019. Located in the central part of the province and covering approx. 777 km2, Kitwe is the second largest city in terms of population size (517,543) in Zambia (after the capital, Lusaka). The city was founded in 1936 and obtained its status as a city in 1966 (Mwitwa et al. 2016). The city whose mean annual rainfall and temperature are roughly 1288 mm and 20 °C, respectively, is one of the fairly developed commercial and industrial areas in the nation, hosting a complex of mining activities on its North-western and Western edges (Mwakikagile 2010). Further, the city is composed of several townships and suburbs, some of which were selected in the context of undertaking this research (Fig. 1).
Research design
Within Kitwe’s urban environment, we selected four townships, namely, Riverside, Nkana west, Chimwemwe and Chipata Compound, based on their socio-economic designations (henceforth referred to as high-cost, medium-cost, mixed-cost and low-cost residential areas, respectively; Fig. 1). Here, high cost represents areas of high-household income, medium cost represents areas of moderate or medium income; low cost represents areas of low income, while mixed cost denotes residential areas that have both medium- and low-cost attributes. We used these criteria because it has the potential to influence the richness of fleshy-fruiting plants found across designations. For example, a pattern of higher biodiversity has been observed in affluent residential areas, than in low-income and predominantly minority populated neighbourhoods (Hope et al. 2003; Leong et al. 2018). Therefore, our assumption was that the richness of fruiting plant species could increase with increasing socio-economic status of a residential area. This is because highly affluent communities are likely to have the capacity to not only plant but also pay for the costs (e.g. water bills, outsourcing labour, etc.) of managing fruiting plant species in their backyard gardens. Consequently, such areas are likely to attract more avian frugivores than those of lower socio-economic status.
In each of the study areas, we first conducted extensive random reconnaissance walks to identify the fruiting plants with a view to establishing sampling plots and observation points. Thus, three replicates of 50 m by 100 m plots were established in each study area. A distance of at least 700 m apart from each plot was employed, in order to reduce the potential for counting the same birds more than once (Chama et al. 2013).
Assessment of plant–frugivore interactions
Field observations were undertaken to assess the interactions between frugivorous birds and all fleshy-fruiting plant species in each study area. All fleshy-fruiting plants in each plot were identified to species level (Forest Dept. 1979; Palgrave 2002) and observed for feeding activities by visiting avian frugivores. Where several individuals of a single plant species occurred, only one individual was randomly selected as a focal plant for undertaking the observations. Observations were conducted during the main fruiting season from November, 2018 to March, 2019 when most plant species were at their peak of ripening (Forest Dept. 1979; Palgrave 2002). We split the observation surveys in two sessions, i.e. early morning (07:30–10:30 a.m.) and late afternoon (3:00–5:00 p.m.), as these are times when most birds are active (Menke et al. 2012; Chama et al. 2013). On each focal plant in a study plot, observations were conducted on three different days per week with the aid of a pair of binoculars (Bushnell Legend E-Series—10 × 42), from a hidden position and at a distance of 10–15 m.
All fruit-eating birds on each focal fruiting plant were observed for a period of 30 min, before moving to the next plant within the 5 h’ time per day (Albrecht et al. 2012). Thus, each plant was observed for a total of 1.5 h (i.e. 30 min/day × 3 observation days). We identified (i.e. using Sinclair and Ryan 2010) and recorded all visiting birds that were seen consuming fruit on each focal fleshy-fruiting plant under observation. The number of visitations that the bird made within the time (30 min) of observation per day were also recorded. The number of individual birds and the time they spent feeding was equally recorded. In cases where a group of conspecific birds visited a plant and individual observations could not be done simultaneously, only one individual that was best visible was randomly chosen and observed with regard to its fruit consumption (Menke et al. 2012). The total number of observation hours for all the focal plants was 67.5.
Statistical analysis
To test the effects of urbanisation on the robustness of seed dispersal networks, we analysed the response of key indices at network level, namely, specialisation (H2'), interaction evenness and interaction diversity. Network specialisation (H2') defines the link complementarity across all species in a community (Kaiser-Bunbury and Blüthgen 2015). It is quantified with the values ranging from 0 to 1, where 0 denotes complete generalization and vice versa. Thus, a higher specialisation denotes a high dependency of each species on few limited partners. Effectively, this implies a narrow niche breadth and a highly specialised seed dispersal process (Blüthgen et al. 2006). On the other hand, low specialisation indicates a higher functional redundancy among partners (Blüthgen 2010), which implies that there is high niche breadth of both plants and frugivores, and reveals that plants benefit from seed dispersal of generalised frugivores. Unlike network specialisation, interaction evenness defines the uniformity in interaction frequencies across all links in the networks, with high values (closer to 1) reflecting more uniform spread of interaction among species (Blüthgen et al. 2008; Kaiser-Bunbury and Blüthgen 2015), while interaction diversity is the quantitative similarity to the total number of links across species in the network (Kaiser-Bunbury and Blüthgen 2015; Landi et al. 2018). Higher interaction diversity (greater than 1) implies a higher community stability and robustness.
Thus, at the onset of our analysis, we computed the plant–frugivore interaction data to plot seed dispersal networks by means of the bipartite package. The bipartite package uses the R environment to provide statistical functions for visualising food webs and calculating a series of indices commonly used to describe two-trophic-level ecological webs, e.g. seed–disperser, plant–pollinator and predator–prey systems (Dormann et al. 2008). Thus, we compiled the interaction frequencies of each fleshy-fruiting plant species (p) with each frugivore species (f) for each study site in the quantitative interaction matrix. Here, the interaction frequencies (between plants and frugivores) were calculated using the number of feeding visits that each identified bird made on each fruiting plant species (Vazquez et al. 2012). From these data, we calculated, analysed and compared the specialisation (H2'), interaction evenness and interaction diversity across networks from each study area using mixed-effects models with maximum likelihood. Further, we undertook a post hoc test with Tukey Honest Significant Difference (HSD) to compare the variance in the mean network specialisation, interaction evenness and interaction diversity across study areas. We then used the mean network indices in our seed dispersal networks to compare with what has already been documented in the wild ecosystems (e.g. Schleuning et al. 2011; Menke et al. 2012). Finally, we used repeated-measures analysis to test if the richness of both fleshy-fruiting plants and avian frugivores differed across study areas. We then performed linear regression models to test if our network parameters (specialisation (H2'), interaction evenness and interaction diversity) correlated with the richness of both the fleshy-fruiting plants and avian frugivores following Chama et al. (2013). All statistical analyses were performed in R statistical software version 4.1.3 (The R Development Core Team 2022).
Results
Plant species composition
We observed a total of 11 species of focal fleshy-fruiting plants (including both native and exotic plants, i.e. representing a total of 45 individuals across all four study areas; Table 1). Of these, nine were in the high-cost, seven in the mixed-cost and four each in both the medium- and low-cost residential areas. Plant species richness remained similar across study areas (F3:8 = 3.66; p > 0.05). Plant species that recorded the most interactions or fruit-eating visits by frugivores included the Pawpaw (Carica papaya; n = 108), followed by Woodland water berry (Syzygium guineense; n = 78), Guava (Psidium guajava; n = 74) and the Sycamore fig (Ficus sycomorus; n = 53). In contrast, the Avocado (Persea americana) and Mango (Mangifera indica) had the least number of visitors with a total count of 1 and 2 birds, respectively (Fig. 4; Table 2).
Bird species composition
A total of 11 avian frugivore species, representing a total of 405 individuals, were recorded feeding on focal fleshy-fruiting plant species (Table 3). Of these, 11 were in the high-cost, 8 in the medium-cost and 6 each in the mixed-and low-cost residential areas. Frugivores species richness remained similar across study areas (F3:8 = 3.68; p > 0.05). Avian frugivores species with the most fruit-eating interactions across study areas were the Common Bulbul (Pycnonotus barbatus; n = 106), followed by the Speckled Mousebird (Colius striatus; n = 92), African Yellow White-eye (Zosterops senegalensis; n = 88), and the Red-faced Mousebird (Urocolius indicus; n = 59; Table 3; Fig. 4).
Network specialisation across study sites
The mean network specialisation (H2') ranged from 0.177 ± 0.039 (SD, if not otherwise stated; high cost) to 0.179 ± 0.075 (mixed cost), 0.421 ± 0.164 (medium cost) and 0.257 ± 0.214 (low cost). Network specialisation remained similarly low across study areas (F3:8 = 0.258; p = 0.854; Fig. 2a). Moreover, it was unaffected by the increase in the richness of both fleshy-fruiting plants (F1:10 = 1.65; p > 0.05) and avian frugivores (F1:10 = 1.44; p > 0.05).
Network interaction evenness across study sites
The mean network interaction evenness ranged from 0.817 ± 0.07 (low cost) to 0.822 ± 0.035 (medium cost), 0.822 ± 0.029 (mixed cost) and 0.823 ± 0.027 (high cost). It remained similarly high across study areas (F3:8 = 0.01; p > 0.05; Figs. 2b and 4). Like network specialisation, interaction evenness was unaffected by the richness of both the fleshy-fruiting plants (F1:10 = 1.65; p > 0.05) and avian frugivores (F1:10 = 0.329; p > 0.05).
Interaction diversity across study sites
The mean interaction diversity across networks ranged from 2.186 ± 0.498 (medium cost) to 2.220 ± 0.258 (low cost), 2.366 ± 0.339 (mixed cost) and 3.068 ± 0.424 (high cost). Species interaction diversity remained similar across study areas (F3:8 = 3.361; p = 0.076; Fig. 2c and Figs. 4). However, it increased significantly with increasing richness in both the fruiting plants (F1:10 = 38.34; p < 0.001; Fig. 3a) and avian frugivores (F1:10 = 86.04; p < 0.001; Fig. 3b).
Discussion
Our aim was to test the fitness of avian seed dispersal networks in the urban ecosystems and establish if they are as robust as those reported in tropical wild ecosystems. Overall, our results indicated a similarly low specialisation across all seed dispersal networks, whereas interaction evenness remained similarly high. In contrast, species interaction diversity varied marginally across networks. It was slightly higher in the high-cost than in the rest of the residential areas studied, all of which remained similar. Generally, the results suggest that the avian seed dispersal networks in the studied urban ecosystems are stable and well organised. This is contrary to our earlier prediction that avian seed dispersal networks in highly built-up tropical urban ecosystems are not robust in the context of facing extinction. Although species interaction diversity differed slightly across networks, the difference was marginal, suggesting that these seed dispersal networks have either adapted or are broadly highly resilient to disturbance, and they also do not seem to be affected by the socio-economic status of human communities in which they exist.
Network specialisation across study areas
The mean network specialisation remained similarly low across study areas, suggesting a high redundancy threshold in the associations between plants and frugivores. A high redundancy in associations depicts a stable synchronicity of species in these plant–frugivore communities. Effectively, this could mean that these networks are highly generalised, which potentially increases their robustness to extinction. High functional redundancy has been shown to contribute to the persistence of networks such that even if some interactions disappear, the plant–frugivore community will still remain robust towards land use and land cover changes (Bascompte and Jordano 2007).
The low specialisation depicted in these networks from a highly built-up human-dominated urban ecosystem is similar to those found in the wild or areas with less ecosystem disturbance (e.g. Schleuning et al. 2011; Chama et al. 2013). Generally, seed dispersal networks in the wild ecosystems of the tropics have been shown to be highly robust, as they exhibit very low mean community specialisation (H2' < 0.5; Dugger et al. 2018). Thus, the fact that the specialisation in the studied networks was comparably very low suggests that seed dispersal networks in the tropics will remain robust even in highly built-up environment, provided the opportunities that drives these interactions, such as the presence of fleshy-fruiting plant species and avian frugivores, continue to exist. In this case, the preservation or growth of fleshy-fruiting plant species should especially be strongly promoted, as this is crucial towards making urban ecosystems attractive for avian frugivores to thrive.
Network interaction evenness across study areas
Overall, the results from this study shows that the mean network interaction evenness remained similarly high across study areas. Effectively, this suggests that the frequency of the interaction assembly among species of both plants and their avian frugivore mutualists was uniformly spread in the community, albeit in a highly heterogeneous pattern. The assemblages of mutualistic communities have been shown to be centred on highly asymmetric and coevolutionary interactions that have shaped their persistence to environmental change over time (Jordano et al. 2003). This means that while a bulk of species may have a few interactions, a few other species have many more interactions than expected by chance (e.g. if a plant species depends strongly on an animal species, the animal depends weakly on the plant) and this pattern is uniformly spread across the network (Bascompte et al. 2006; Fig. 4). Thus, even if some species may only depend on a few partners, the higher and uniform interaction evenness suggests that higher functional robustness occurs within these networks, as the risks of losing a link or entire species or even fluctuations of frequencies are spread evenly (Kaiser-Bunbury and Blüthgen 2015).
Seed dispersal networks for high-, medium-, mixed- and low-cost residential study areas. In each of the networks, the grey bars (on the left) represent a node of different fleshy-fruiting plant species (letter codes T1 to T11), while the black bars (on the right) represent a node of different bird species (B1 to B11). The light-grey lines between the two nodes highlights the partners that each species (of plants and birds) interacts with in the network. The size of the line indicates the strength of the interaction, i.e. the thicker the line, the stronger the mutualistic interaction and vice versa. Full names of plants and birds represented by each letter code in the networks are shown in Tables 1 and 3, respectively
Asymmetric coevolutionary interactions have been shown to be often stable and less sensitive to habitat disturbance, and their capabilities to remain resilient are strengthened at community rather than one-on-one interaction basis (Pauw et al. 2009; Fontúrbel and Murúa 2014). This is because interacting parties in these relationships are more likely to persist in spite of changes in environmental conditions and the demographic changes of their interacting parties (Fontúrbel and Murúa 2014). Our results highlight the importance of asymmetrically even interactions in mutualistic networks, as this is critical in driving both the diversity and coexistence of species in these communities (Paine 1980; Schoener 1983; Bascompte et al. 2006), especially in the face of threats, such as those coming from urbanisation. Even in an extreme scenario, plant–animal interactions assembled on a higher interaction evenness (albeit asymmetrically) could coevolve to develop strategies that are suitable for them to survive in such conditions (Fontúrbel and Murúa 2014). In this case, it seems highly unlikely that the networks in our study area could easily collapse even if one or two individuals are eliminated from the community due to extinction (Yachi and Loreau 1999; Wang and Loreau 2014).
Network interaction diversity across study areas
As observed from our results, the mean interaction diversity was significantly high and broadly similar across seed dispersal networks. Ecologically, a higher interaction diversity signals a higher heterogeneity in the magnitude of interacting partners and their complementary response to environmental disturbance (Almqvist et al. 2003; Hooper et al. 2005). Generally, however, interaction diversity can sometimes suffer from interference between consumer species, which could impact negatively on potential complementarity effects, and thus reduce ecosystem function even in the face of increasing consumer diversity (Montoya et al. 2003; Finke and Denno 2004; Tylianakis et al. 2010). Nonetheless, a higher interaction diversity has also been shown to not only increase network stability but also the functional performance in the context of enhancing the fitness of the community (Hector et al. 1999) to environmental threats.
Our findings agree with Cruz et al. (2013) suggesting that seed dispersal networks can be complex and variable even in a highly managed urban area and that a network approach remains an important monitoring tool to detect the status of crucial ecosystem functions in such rapidly changing and human-dominated environments. Therefore, any potential effects of interaction interference are likely to be temporal rather than long-term where interaction diversity is high, like in the case of the studied seed dispersal networks. Besides, a higher interaction diversity generally also suggests higher richness and evenness of species as well as generalization of interactions. This is further supported by the fact that interaction diversity in the studied networks was positively correlated with the richness of both fleshy-fruiting plants (Fig. 3a) and avian frugivores (Fig. 3b). In fact, several species of fleshy-fruiting plants (namely, Carica papaya, Syzygium guineense, Psidium guajava and Ficus sycomorus) and avian frugivores (namely, Pycnonotus barbatus, Colius striatus, Zosterops senegalensis and Urocolius indicus) had more interactions than others in our study area. Such species have a disproportionately greater contribution to the overall organization and cohesion of networks (Mello et al. 2011, 2015; Sebastián-González 2017; Bomfim et al. 2018). Obviously, by occupying central network positions, these plants benefit from the dispersal services provided by many species of frugivores, while the birds are able to consume fruits on multiple plant species (Jordano 2000; Chama et al. 2013). Nonetheless, the removal of such highly connected species could diminish crucial ecological interactions and consequently compromise the stability or integrity of these networks (Kaiser-Bunbury et al. 2010; Galetti et al. 2013; Correa et al. 2016). Therefore, such species of both plants and birds should especially be at the centre of conservation efforts if these interactions and their ecosystem functions are to be preserved.
Our findings are similar to those found in the study of pollination networks where species interaction diversity was shown to increase with increased abundance of beneficial resources, such as plant species diversity and pollinators that provide pollination services to crop plants (Albrecht et al. 2007). Although Albrecht et al. (2007) further showed that interaction diversity tends to somewhat decline more rapidly in disturbed environments, our results show that human-settled urban ecosystems have the potential to support stable and robust ecological processes if they can promote the hosting of a diversity of plant and animal species that are especially critical in driving these ecological systems.
Conclusion
Our findings broadly suggest that avian seed dispersal networks in urban ecosystems are highly generalised. This means that there is a high redundancy in plant—frugivore associations (links), signifying stability of coexistence among species in these communities. Thus, even if some species may only have one species that they interact with, the chances that such species would go extinct are rare because the few partners that they interact with are potentially linked to several others, as highlighted by the high interaction evenness across networks. And collectively, such a combination of interactions shapes the fitness of both fleshy-fruiting plant species and avian frugivores against the threats of extinction. For plants, this provides an assurance that their seeds or propagules will be dispersed, while for frugivores this ensures that there is adequate availability of fruits for them to feed on, and this is critical for their fitness. Our findings bring out some lessons of conservation importance about urban ecosystems, i.e. they have the potential to support key ecological processes, such as seed dispersal networks even in the face of heightened human disturbance. Therefore, there is a need for the creation of awareness about the conservation opportunities that exist in these areas. Activities, such as the planting of a diversity of fleshy-fruiting plants and educating the human communities living in these areas about the ecological importance of birds, should especially be promoted. The planting of native rather than exotic fruiting plant species should especially be encouraged. For key conservation agencies, these results should propel them to widen their conservation efforts beyond the boundaries of protected areas, which are often wild and remote, by also considering resource preservation opportunities in urban ecosystems. Species of both plants and avian frugivores with higher interactions are especially critical not only in the maintenance of network stability but also ecosystem function and should be the focus of conservation efforts (Correa et al. 2016).
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable to this study.
References
Albrecht M, Duelli P, Schmid B, Muller CB (2007) Interaction diversity within quantified insect food webs in restored and adjacent intensively managed meadows. J Anim Ecol 76(5):1015–1025. https://doi.org/10.1111/j.1365-2656.2007.01264
Albrecht J, Neuschuz EL, Farwig N (2012) Impact of habitat structure and fruit abundance on avian seed dispersal and fruit predation. Basic Appl Ecol 13:347–354. https://doi.org/10.1016/j.baae.2012.06.005
Almqvist T, Folke C, Nystron M, Peterson G, Bengtsson J, Walker B, Norberg J (2003) Response diversity, ecosystem change, and resilience. Front Ecol Environ 1(9):488–494. https://doi.org/10.1890/1540-9295(2003)001[0488:RDECAR]2.0.CO;2
Baker PJ, Harris S (2007) Urban mammals: What does the future hold? An analysis of the factors affecting patterns of use of residential gardens in Great Britain. Mamm Rev 37(4):297–314. https://doi.org/10.1111/j.1365-2907.2007.00102
Bascompte J, Jordano P (2007) Plant–animal mutualistic networks: the architecture of biodiversity. Annu Rev Ecol Evol Syst 38:567–593. https://doi.org/10.1146/annurev.ecolsys.38.091206.095818
Bascompte J, Jordano P, Olesen JM (2006) Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312(5772):431–433. https://doi.org/10.1126/science.1123412 (PMID: 16627742)
Blüthgen N (2010) Why network analysis is often disconnected from community ecology: a crique and an ecologists guide. Basic Appl Ecol 11(3):185–195. https://doi.org/10.1016/j.baae.2010.01.001
Blüthgen N, Menzel F, Blüthgen N (2006) Measuring Specialization in species interaction networks. BMC Ecol 6(9):1–12. https://doi.org/10.1186/1472-6785-6-9
Blüthgen N, Frund J, Vazquez DP, Menzel F (2008) What do interaction network metric tell us about specialization and biological traits? Ecol 89(12):3387–3399. https://doi.org/10.1890/07-2121.1
Bomfim JDS, Guimarães PR Jr, Peres CA, Carvalho G, Cazetta E (2018) Local extinctions of obligate frugivores and patch size reduction disrupt the structure of seed dispersal networks. Ecography 41(11):1899–1909. https://doi.org/10.1111/ecog.03592
Carlo TA, Yang AS (2011) Network models of frugivory and seed dispersal: challenges and opportunities. Acta Oecol 37(6):619–624. https://doi.org/10.1016/j.actao.2011.08.001
Chama L, Berens DG, Downs CT, Farwig N (2013) Habitat characteristics of forest fragments determine specialization of plant-frugivore networks in a mosaic forest landscape. PLoS One 8(1):e54956. https://doi.org/10.1371/journal.pone.0054956
Ciuti S, Muhly TB, Paton DG, McDevitt AD, Musiani M, Boyce MS (2012) Human selection of elk behavioural traits in a landscape of fear. Proc R Soc B 279:4407–4416. https://doi.org/10.1098/rspb.2012.1483
Cordeiro NJ, Howe HF (2003) Forest fragmentation severs mutualism between seed dispersers and an endemic African tree. PNAS 100(24):14052–14056. https://doi.org/10.1073/pnas.2331023100
Correa SB, Araujo JKM, Penha JMF, Nunes da Cunha C, Stevenson PR, Anderson JT (2015) Overfishing disrupts an ancient mutualism between frugivorous fishes and plants in Neotropical wetlands. Biol Conserv 191:159–167. https://doi.org/10.1016/j.biocon.2015.06.019
Correa SB, Arujo JK, Penha J, Nunes da Cunha C, Bobier KE, Anderson JT (2016) Stability and generalization in seed dispersal networks: a case study of frugivorous fish in Neotropical wetlands. Proc R Soc B 283(1836):20161267. https://doi.org/10.1098/rspb.2016.1267
Cote J, Bestion E, Jacob S, Travis J, Legrand D, Baguette M (2017) Evolution of dispersal strategies and dispersal syndromes in fragmented landscape. Ecography 40(1):56–73. https://doi.org/10.1111/ecog.02538
Cruz JC, Ramos JA, Silva LP, Tenreiro PJ, Ruben H (2013) Seed dispersal networks in an urban novel ecosystem. Eur J for Res 132(5–6):887–897. https://doi.org/10.1007/s10342-013-0722-1
D’Avila G, Gomes-Jr A, Canary AC, Bugoni AL (2010) The role of avian frugivores on germination and pontential Seed dispersal of the Brazilian Pepper Schninus terebinthifolius. Biota Neotrop 10(3):47–51. https://doi.org/10.1590/S1676-06032010000300004
Dormann CF, Gruber B, Fründ J (2008) The bipartite package. Version 0.73. R Project for Statistical Computing, Vienna
Dugger PJ, Blendinger PG, Böhning-Gaese K, Chama L, Correia M, Dehling DM, Emer C, Farwig N, Fricke EC, Galetti M, García D, Grass I, Heleno R, Jacomassa FAF, Moraes S, Moran C, Muñoz MC, Neuschulz EL, Nowak L, Piratelli A, Pizo MA, Quitián M, Rogers HS, Ruggera RA, Saavedra F, Sánchez MS, Sánchez R, Santillán V, Schabo DG, da Silva FR, Timóteo S, Traveset A, Vollstädt MGR, Schleuning M (2018) Seed-dispersal networks are more specialized in the Neotropics than in the Afrotropics. Glob Ecol Biogeogr 28(2):1–14. https://doi.org/10.1111/geb.12833
Elmqvist T, Zipperer W, Güneralp B (2016) Urbanization, habitat loss, biodiversity decline: solution pathways to break the cycle. In: Seto KC, Solecki WD, Griffith CA (eds) The Routledge handbook of urbanization and global environmental change. Routledge, Abingdon, pp 139–151
Emer C, Galetti M, Pizo MA, Guimarães PR Jr, Moraes S, Piratelli A, Jordano P (2018) Seed-dispersal interactions in fragmented landscapes—a metanetwork approach. Ecol Lett 21(4):1–10. https://doi.org/10.1111/ele.12909
Escribano-Avula G, Lara-Romero C, Heleno R, Traveset A (2018) Tropical seed dispersal networks: emerging patterns, biases and keystone species traits. In: Dattilo W, Rico-Gray V (eds) Tropical Seed dispersal networks. Springer Internatiional Publishing AG, Mallorca, pp 93–110
Farwig N, Böhning-Gaese K, Bleher B (2006) Enhanced seed dispersal of Prunus africana in fragmented and disturbed forests? Oecologia 147(2):238–252. https://doi.org/10.1007/s00442-005-0288-9
Finke DL, Denno RF (2004) Predator diversity dampens trophic cascades. Nature 429:407–410. https://doi.org/10.1038/nature02554
Fontúrbel FE, Murúa MM (2014) Microevolutionary effects of habitat fragmentation on plant–animal interactions. Adv Ecol Res. https://doi.org/10.1155/2014/379267
Forest Department (1979) Know your trees: some of the common trees found in Zambia. Forest Department, Lusaka
Francis RA, Chadwick MA (2013) Urban ecosystem: understanding the human environment, 1st edn. Routledge, London
Galetti M, Guevara R, Côrtes MC, Fadini R, Matter SV, Leite AB, Labecca F, Ribeiro T, Carvalho CS, Collevatti RG, Pires MM, Guimarães PR, Brancalion PH, Ribeiro MC, Jordano P (2013) Functional extinction of birds drives rapid evolutionary changes in seed size. Science 340(6136):1086–1090. https://doi.org/10.1126/science.1233774
Gaston K (2010) Urban ecology. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511778483
Gaynor J, Hojnowski C, Carter N, Brashares J (2018) The influence of human distance on wildlife nocturnality. Science 360(6394):1232–1235. https://doi.org/10.1126/science.aar7121
Gonzalez-Castro A, Traveset A, Nogales M (2012) Seed dispersal interactions in the Mediteranean Region: contrasting patterns between Islands and Mainland. J Biogeogr 39(11):1938–1947. https://doi.org/10.1111/j.1365-2699.2012.02693
Guimarães PR Jr, Gray VR, Dos Reis SF, Thompson JN (2006) Asymmetries in specialization in ant-plant mutualistic networks. Proc R Soc B 273(1597):2041–2047. https://doi.org/10.1098/rspb.2006.3548
Hector A, Schmid B, Beierkuhnlein C, Caldeira MC, Diemer M, Dimitrakopoulos PG, Finn JA, Freitas H, Giller PS, Good J, Harris R, HöGberg P, Huss-Danell K, Joshi J, Jumpponen A, KöRner C, Leadley PW, Loreau M, Minns A, Mulder CPH, O’donovan G, Otway SJ, Pereira JS, Prinz A, Read DJ, Scherer-Lorenzen M, Schulze ED, Siamantziouras ASD, Spehna EM, Terry C, Troumbis AY, Woodward FI, Yachi S, Lawton JH (1999) Plant diversity and productivity experiments in European grassland. Science 286(5442):1123–1127. https://doi.org/10.1126/science.286.5442.1123
Herrera CM (2002) Seed dispersal by vertebrates. In: Herrera CM, Pellmyr O (eds) Plant–animal interactions: an evolutionary approach. Blackwell Science, Oxford, pp 85–210
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75(1):3–35. https://doi.org/10.1890/04-0922
Hope D, Gries C, Zhu W, Fagan WF, Redman CL, Grimm NB, Nelson AL, Martin C, Kinzig A (2003) Socioeconomics drive urban plant diversity. PNAS 100(15):8788–8792. https://doi.org/10.1073/pnas.1537557100
Howe HF, Smallwood J (1982) Ecology of seed dispersal. Annu Rev Ecol Evol Syst 13(1):201–228. https://doi.org/10.1146/annurev.es.13.110182.001221
Jordano P (2000) Fruits and frugivory. In: Fenner M (ed) Seeds: the ecology of regeneration in natural plant communities, 2nd edn. Commonwealth Agricultural Bureau International, Wallingford, pp 125–166. https://doi.org/10.1079/9780851994321.0125
Jordano P, Bascompte J, Olesen JM (2003) Invariant properties in coevolutionary networks of plant–animal interactions. Ecol Lett 6(1):69–81. https://doi.org/10.1046/j.1461-0248.2003.00403.x
Kaiser-Bunbury CN, Blüthgen N (2015) Integrating network ecology with applied conservation: a synthesis and guide to implementation. Ann Bot 7:plv076. https://doi.org/10.1093/aobpla/plv076
Kaiser-Bunbury CN, Muff S, Memmott J, Müller CB, Caflisch A (2010) The robustness of pollination networks to the loss of species and interactions: a quantitative approach incorporating pollinator behaviour. Ecol Lett 13(4):442–452. https://doi.org/10.1111/j.1461-0248.2009.01437.x
Kondratyeva A, Knapp S, Durka W, Kühn I, Vallet J, Machon N, Martin G, Motard E, Grandcolas P, Pavoine S (2020) Urbanization effects on biodiversity revealed by a two-scale analysis of species functional uniqueness vs. redundancy. Front Ecol Evol. https://doi.org/10.3389/fevo.2020.00073
Landi P, Minoarivelo HO, Brännström A, Hui C, Dieckmann U (2018) Complexity and stability of ecological networks: a review of the theory. Popul Ecol 60(4):1–28. https://doi.org/10.1007/s10144-018-0628-3
Leong M, Dunn RR, Trautwein MD (2018) Biodiversity and socioeconomics in the city: a review of the luxury effect. Biol Lett 14:20180082. https://doi.org/10.1098/rsbl.2018.0082
Mckinney ML (2002) Urbanization, biodiversity, and conservation: the impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. Bioscience 52(10):883–890. https://doi.org/10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2
Mckinney ML (2008) Effects of urbanization on urban ecosystem: a review of plants and animals. Urban Ecosyst 11(2):161–176. https://doi.org/10.1007/s11252-007-0045-4
Mello MAR, Marquitti FMD, Guimarães PR, Kalko EKV, Jordano P, de Aguiar MAM (2011) The missing part of seed dispersal networks: structure and robustness of bat-fruit interactions. PLoS One 6:e0017395. https://doi.org/10.1371/journal.pone.0017395
Mello MAR, Rodrigues FA, Costa LDF, Kissling WD, Şekercioğlu ÇH, Marquitti FMD, Kalko EKV (2015) Keystone species in seed dispersal networks are mainly determined by dietary specialization. Oikos 124(8):1031–1039. https://doi.org/10.1111/oik.01613
Menke S, Bohning-Gaese K, Schleuning M (2012) Plant-frugivore networks are less specialized and more robust at forest-farmland edges than in the interior of a tropical forest. Oikos 121(10):1553–1566. https://doi.org/10.1111/j.1600-0706.2011.20210
Montoya JM, Rodriguez MA, Hawkins BA (2003) Food web complexity and higher-level ecosystem services. Ecol Lett 6(7):587–593. https://doi.org/10.1046/j.1461-0248.2003.00469.x
Muller N, Ignatieva M, Nilon CH, Werner P, Zipper WC (2013) Patterns and trends in urban biodiversity and landscape design. Urbanization, biodiversity and ecosystem services: challenges and opportunities. Springer, Dordrecht, pp 123–174. https://doi.org/10.1007/978-94-007-7088-1_10
Murgui E, Hedblom M (2017) Ecology and conservation of birds in urban environments. Springer, Cham. https://doi.org/10.1007/978-3-319-43314-1
Mwakikagile G (2010) The land and its people, 1st edn. Continental Press, Pennsylvania
Mwitwa J, Sibanjene M, Chipoya GC, Namiluko Y (2016) City region system situational analysis. RUAF Foundation, Kitwe
Paine RT (1980) Food webs: linkage, interaction strength and community infrastructure. J Anim Ecol 49(3):666–685. https://doi.org/10.2307/4220
Palgrave MC (2002) Keith coates palgrave trees of southern Africa. Struik Publishers, Cape Town
Pauw A, Stofberg J, Waterman RJ (2009) Flies and flowers in Darwin’s race. Evolution 63(1):268–279. https://doi.org/10.1111/j.1558-5646.2008.00547
Pickett S, Cadenasso M (2009) Altered resources, disturbances and heterogeniety: a frame work for comparing urban and non-urban soils. Urban Ecosyst 12:23–44. https://doi.org/10.1007/s11252-008-0047
Schleuning M, Bluthgen N, Florchinger M, Braun J, Schaefer HM, Bohning-Gaese K (2011) Specialization and interaction strength in a tropical plant-frugivore network differ among forest strata. Ecology 92(1):26–36. https://doi.org/10.1890/09-1842.1
Schoener TW (1983) Field experiments on interspecific competition. Am Naturalist 122(2): 240–285 http://www.jstor.org/stable/2461233
Sebastián-González E (2017) Drivers of species’ role in avian seed-dispersal mutualistic networks. J Anim Ecol 86(4):878–887. https://doi.org/10.1111/1365-2656.12686
Shikang S, Fuqin W, Yuehua W (2015) Does the passage of seeds through frugivore gut affect their storage: a case study on the endangered plant Euryodendron excelsum. Sci Rep 5(1):11615. https://doi.org/10.1038/srep11615
Silva FR, Montoya D, Furtado R, Memmott J, Pizo MA, Rodrigues RR (2015) The restoration of tropical seed dispersal networks. Restor Ecol 23(6):1–9. https://doi.org/10.1111/rec.12244
Sinclair and Peter Ryan (2010) Birds of Africa: south of the Sahara. Struik Nature, Cape Town
Strohbach MW, Haase D, Kabisch N (2009) Birds and City: Urban biodiversity, landuse and socioeconomic. Ecol Soc 14(2):31. https://doi.org/10.5751/ES-03141-140231
Swan CM, Pickett STA, Szlavecz K, Waren P, Willey T (2011) Biodiversity and community composition in urban ecosystems: coupled human, spatial, and metacommunity processes. In: Niemela J (ed) Urban ecology: patterns, processes, and application. Oxford University Press, Oxford, pp 179–186. https://doi.org/10.1093/acprof:oso/9780199563562.003.0021
The R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Timoteo S, Ramos JA, Vaughan IP, Memmott J (2016) High resilience of seed dispersal webs highlighted by the experimental removal of the dominat disperser. Curr Biol 26(7):910–915. https://doi.org/10.1016/j.cub.2016.01.046
Tylianakis JM, Laliberté E, Nielsen A, Bascompte J (2010) Conservation of species interaction networks. Biol Conserv 143(10):2270–2279. https://doi.org/10.1016/j.biocon.2009.12.004
United Nations, Department of Economic and Social Affairs, Population Division (2019) World urbanization prospects: the 2018 revision (ST/ESA/SER.A/420). United Nations, New York
Valiente-Banuet A, Aizen MA, Alcántara JM, Arroyo J, Cocucci A, Galetti M, García MB, García D, Gómez JM, Jordano P, Medel R, Navarro L, Obeso JR, Oviedo R, Ramírez N, Rey PJ, Traveset A, Verdú M, Zamora R (2015) Beyond species loss: the extinction of ecological interactions in a changing world. Funct Ecol 29(3):299–307. https://doi.org/10.1111/1365-2435.12356
Vazquez DP, Morris WF, Jordano P (2012) Interaction frequency as a surrogate for the total effects of animal mutualist on plants. Ecol Lett 8(10):1088–1094. https://doi.org/10.1111/j.1461-0248.2005.00810
Wang S, Loreau M (2014) Ecosystem stability in space: alpha, beta and gamma variability. Ecol Lett 17(8):891–901. https://doi.org/10.1111/ele.12292
Warren JR, Pearson SM, Henry S, Rossouw K, Love JP, Olejniczak MJ, Elliott K, Bradford MA (2015) Cryptic indirect effects of exurban edges on a woodland community. Ecosphere 6(11):1–13. https://doi.org/10.1890/ES15-00318.1
Yachi S, Loreau M (1999) Biodiversity and ecosystem productivity in a fluactuating environment: the insurance hypothesis. PNAS 96(4):1463–1468. https://doi.org/10.1073/pnas.96.4.1463
Yuan B, Lu C (2016) Effects of urbanization on bird diversity: a case study in Yizhou, Guangix Province, China. Asia Life Sci 25(1):79–96
Acknowledgements
The authors would like to thank the residents of Kitwe particularly in the four study areas for their cooperation during the period that we conducted this study within their premises. Special thanks to Stephen Syampungani for providing help during the data collection exercise.
Funding
This study was funded by the government loans board, administered through the Ministry of Higher Education in Zambia. The funders had no role to play in the study design, data collection, analysis, manuscript development and choice of journal to which this work was submitted.
Author information
Authors and Affiliations
Contributions
SM and LC originally conceived and formulated the research idea. SM, assisted by MC and NN, undertook the fieldwork. DP did the mapping of the study area. LC and SM analysed the data. SM, LC, NN, SS, DP and MC wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable to this study.
Consent for publication
Not applicable to this study.
Additional information
Communicated by Francisco E. Fonturbel.
Rights and permissions
About this article
Cite this article
Mubamba, S., Nduna, N., Siachoono, S. et al. Plant–frugivore networks are robust to species loss even in highly built-up urban ecosystems. Oecologia 199, 637–648 (2022). https://doi.org/10.1007/s00442-022-05213-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-022-05213-9