Abstract
Birds play a crucial role in plant reproduction, being one of the most important pollinators and seed dispersers among vertebrates. Here, we aim to study plant-bird interactions in the Cerrado biome of Brazil, highlighting existing mutualistic relations and their role in forest regeneration processes. We sampled plants and recorded feeding events and other interactions between frugivorous birds and tree and shrub species in forest and non-forest environments between May 2015 and July 2016. We registered 74 plant species of 36 genera in 23 families, along with 44 bird species, 63.7% of which were frugivores. The rainy season (September-October) offered the highest resource availability for birds, therefore most feeding events and other interactions also occurred during this period. Approximately 64% of the plants observed at the study site had zoochoric dispersal and more than half of them relied on birds. We found a variety of bird species interacting with plants that supplied their food in urban fragments and highlight the relevance of plant-bird interactions to maintaining urban ecosystems. This result demonstrates the importance of maintaining forested environments, as habitat loss reduces ecological interactions, leaving only a few healthy ecological systems as scattered forest fragments within the urban matrix.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecological communities are comprised of diverse populations that interact with each other in a dynamic way (Skarpe 1992). These populations vary spatially and temporally and are affected by interactions with other species (Rosenzweig et al. 2008). These interspecies dynamics depend profoundly on the way species interact (Jordano et al. 2003; Bascompte and Jordano 2006), their development, life cycle and behavior (Trøjelsgaard and Olesen 2016). Ecological interactions between individuals of different species are a crucial component of biodiversity (Guimarães 2010). By understanding these interactions, it is possible to recognize patterns related to ecosystem stability and therefore apply specific conservation actions to maintain biodiversity. Seed dispersal by frugivores is one of the most common plant-animal interactions (Purificação et al. 2014). This interaction is characterized by key bird species that interact with specific plant species, i.e. frugivorous birds that act as seed dispersers of several plants. Since most plants depend on animals for their seed dispersal, the temporal dynamics between plants and animals at different scales (e.g., seasons and life forms) indicate important changes in the structure of the community, since animal populations track the fruiting periods of many plant species (Schupp and Fuentes 1995). This interdependence between plants and birds is essential to maintain phenology and other ecological processes within the community and is also a crucial component of community restoration (Githiru et al. 2002).
In tropical forests, 50 to 90% of plant species produce zoochorous fruits (Howe and Smallwood 1982), which are involved in the vast majority of plant-bird interactions (Marjakangas et al. 2019). Despite the high avian and plant diversity of tropical savannas, few studies have been conducted on avian frugivory and seed dispersal in this biome in the context of interaction networks (Maruyama et al. 2019). One such tropical savanna is the Brazilian Cerrado, which is home to a high number or endemic and threatened species (Maruyama et al. 2019). Plant-bird interaction networks in the Cerrado are weakened by habitat degradation, as well as fragmentation near large urban centers (Souza et al. 2019). Urban fragments form a complex system that act as a refuge for biodiversity, consisting of isolated patches of vegetation that give home to a variety of animal species (Primack and Rodrigues 2001). In seasonal tropical environments, reproduction and seed dispersal occur during the most favorable season, when the diversity of the associated seed dispersers is the highest. Most studies report species richness and abundance, phenotypic traits and general observations without considering more delicate details (Aronson et al. 2017).
These other attributes, such as number of interactions per plant species, number of bird species per interaction, connectivity, specialization, nesting, modularity, completeness and degree distribution can shape the interactions within a community and can be expressed by metrics to inform us about the resilience and diversity process of the community (Aronson et al. 2017). We quantified the number of interactions as binary data and estimated the existing interactions in the community as a whole. Connectivity index is a proportion of observed interactions in relation to total interactions, and its application is based on the observation that more connected networks have higher ecological redundancy and consequently higher stability in the interaction network (Jordano 1987; Jordano et al. 2006). Along with connectivity, specialization and nesting go together and rely on the understanding that mutualistic interactions at the community level are influenced by recurrent nesting detection (Bascompte et al. 2003). Nesting is higher when specialist species interact with generalists, generalists interact with each other and there is no interaction between specialists (Freitas et al. 2014). Specialization makes it possible to identify key species in nesting (e.g., networks with the highest nestedness are the largest connectivity structures in a tropical forest). This nested structure can mitigate secondary extinctions or temporal fluctuations in the abundance and richness of the community. Similarly, modularity can shape and select modules (i.e., subsets of species that interact more with each other than with the other species in the network) of interactions between populations over time. Modularity, completeness and degree distribution are important and often-used metrics that are represented by a node and are connected by links that represent observed interspecific interactions (Bascompte and Jordano 2006).
While natural remnants have received a lot of attention, little is known about plant-bird interactions and metrics in the urban savanna. As these remnants are under continuous anthropogenic pressure, we need further studies on urban fragments to inform the conservation of the vegetation (Piratelli et al. 2017). To understand fruit-frugivore interactions, we need tools that describe ecological processes well, including those in urban environments, where interactions may be more sensitive to change compared to natural habitats (Bender et al. 2018). Few studies have considered temporal interaction networks in highly diverse tropical environments (Vizentin-Bugoni et al. 2018; Souza et al. 2018). Studies in such urban environments may offer new perspectives on the temporal structure of interactions between plants and their mutualistic partners (Weinstein and Graham 2017). Here we focus on mutualistic plant-bird interactions in naturally regenerating forest and non-forest urban fragments. We study plants (including the phenology of tree and shrub species) and evaluate temporal patterns of occurrence of birds in forest and non-forest (Savanna) fragments. We characterize the seasonal dynamics of plant-bird interactions using interaction networks and analyze network metrics to characterize the overall structure (number of interactions per plant species, number of bird species per interaction, connectivity, specialization, nesting, modularity, completeness and degree distribution). Finally, we investigate how these structure networks change in different Cerrado formations and with different plant growth forms at the species and community levels.
Specifically, we test the following three hypotheses: (i) during the rainy season more plant species are in a reproductive phase providing a higher availability of zoochoric fruits, which corresponds to greater bird species richness; (ii) since the plant phenology diversity differs between forest and non-forest vegetation types, their interaction networks vary in their diversity depending on the life form of zoochoric species; and (iii) regenerating vegetation causes generalist bird species to dictate the structure of interactions. Higher resource availability allows for finer niche division, while low specialization is accompanied by high overlap in interspecific interactions, manifested in high nestedness, emphasizing the presence of generalist species in the interaction networks.
Methods
Study site
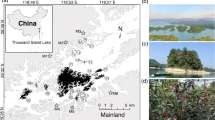
Our two study sites were located in the state of Mato Grosso do Sul in Brazil (20°23’00” S and 54°36’00” W) (Fig. 1), on a property that belongs to the São Vicente Institute, Dom Bosco Catholic University. At the time of this study, 114 hectares of the property were under extensive livestock grazing (Silva and Cheung 2012). One study site has been designated as Permanently Protected Area (Área de Preservação Permanente), with 10 ha of gallery forests and riparian forests, while the other one is a 20 ha Cerrado Legal Reserve (which is the minimum size (20% of the total area)) of native vegetation cover required by law for properties (MMA 2018) composed of forest and non-forest savanna. The region has two well-defined seasons, a dry winter and a rainy summer, with an average annual temperature of 25.6 °C and the average annual precipitation of 254.7 mm (Dom Bosco weather station).
The original vegetation of the study sites was savanna formation (Brazilian Cerrado biome), with regional variations from herbaceous vegetation (commonly phytophysiognomy called “cerrado sensu stricto” in Brazil) to forested savanna (Guimarães et al. 2006). All sites were deforested to accommodate livestock grazing and now only small remnants and regenerating fragments of non-forest vegetation remain (between 5 and 20 years of regeneration). The cerradão area (Brazilian phytophysiognomy) is currently regenerating, with 73% of the plant community composed of pioneer species (Cheung et al. 2016). Therefore, we distinguished two fragment types: forest (fragments composed of riparian and tall forests) and non-forest (fragments composed of dry and closed forests, i.e., “savanna”).
Data collection
We sampled plants and birds on the same five days monthly from May 2015 to July 2016. We conducted both the plant and the bird surveys on four 6 km long transects (two in non-forest (Savanna) and two in forest vegetation). The transects were separated by two km. We surveyed birds during the first three hours of the day, taking advantage of the high activity of diurnal species and also three hours before sunset, corresponding to the end of diurnal bird activities and the beginning of that of nocturnal species. Two observers slowly walked the transects and recorded all species seen or heard, including those flying over the transects. We identified birds at the species level using binoculars and a field guide (Gwynne et al. 2010). Plant surveys were conducted monthly. Shrubs and trees that were in the fruiting phase were identified using specialized literature (Lorenzi 20022009), using nomenclature recommended by Herbarium Virtual Reflora and Flora do Brasil databases (http://floradobrasil.jbrj.gov.br/).
We determined avian trophic guilds using definitions by Levey et al. (1984) sensu lato, i.e., a species is classified as a frugivore if it includes fruit in its diet for at least one season or life phase, and not just those that feed exclusively on fruit. We considered plant-bird interactions as defined in the literature, i.e., any visit for food consumption by a frugivorous animal (Van der Pijl 1982; Oliveira et al. 2015). We recorded as “interaction” when a bird consumes the fruits of a plant as described in the literature (1) or did not consume fruits (0). Using this definition, we identified which plant species the birds were feeding on.
Data analysis
First, we quantified seasonal variations in the observed interactions, evaluating the monthly average number of frugivorous bird species and the plant species they fed on. For our first hypothesis (the availability of fruiting plants influences bird species richness), we used a linear regression of plant species richness of forest and non-forest fragments on bird species richness. To test our second hypothesis (the relation between feeding events in forest and non-forest fragments and the tree and shrub species present), we performed a chi-square test (χ²) using contingency tables (Zar 1999) to ascertain if there were more records of feeding events and interactions associated with the different life forms (trees and shrub) and to determine how these values differed between forest formations and non-forest formations.
Subsequently, we transformed the raw data (weighted matrix) into six binary (presence/absence) matrices, considering only presence (1) or absence (0) of the interaction: forest shrubs, non-forest shrubs, forest trees, non-forest trees, forest and non-forest trees and shrubs. Using the resulting matrix, we calculated the metrics for ecological networks, such as number of interactions per plant species, number of bird species per interaction, completeness, connectivity, specialization, nesting, modularity, sampling intensity and degree distribution, using the methods described by Melo et al. (2016). To calculate the number of interactions from the matrix formed, we used the function “sum” and “rowSums” for the number of interactions per plant. For the number of bird species per number of interactions, we use the “sapply” function in R version 3.3.4 (Core Development Team 2018). If after evaluating the number of interactions we found no difference between shrubs and trees, we used the Chao2 species richness estimator (Chao 1987) to analyze sample completeness using the “iNEXT”, “estimateR”, and “ChaoSpecies” functions of the bipartite (Dormann et al. 2008) and iNEXT packages (Hsieh et al. 2016) in R.
Sample completeness was calculated from the occurrence of each plant species (only forest and non-forest) with repeated sampling of interactions (thereby constructing the typical species-species interaction matrix). We calculated the connectivity and specialization rates using the “networklevel” function of the bipartite R package. To calculate per species specialization (both plant and bird), we used the “specieslevel” function with index “d”. For this index, the values range from 0 (no specialization) to 1 (full specialist). We calculated nesting using the “NODF2” method in the “nested” function, where the closer the value is to 100, the more nested the network. For modularity we applied the “computeModules” function using steps randomized matrix (10,000,000) from the real matrix, using the null model 2 of Bascompte et al. (2003) in the vegan package (Oksanen et al. 2013), where the closer the value is to 1, the higher the modularity.
We calculated sampling intensity (forest and non-forest) dividing the square root of the number of interactions by the square root of the product of the number of species in each guild per network. For the degree distribution we applied the “degreedistr” function of the bipartite package, selected by the Akaike Information Criterion. To represent interactions, we drew six networks of the six gross (weighted) matrices elaborated using the network package (Oliveira et al. 2015). The significance of nesting was estimated by applying a t-test for each sample using the null model, in which the probability of an interaction between an animal and a plant is proportional to all of their interactions (Almeida-Neto and Ulrich 2011). From the randomized matrices that were created to calculate modularity, we ran the modularity for each matrix and compared the significance using the Z test (t-test for a sample) considering a significance of 5%. To calculate the correlation between the matrices, we used the Mantel test, using the “Spearman” method with 5000 permutations. All analyses were conducted in the program R.
Results
Occurrence
Among all 67 plant species recorded, 48 had zoochoric dispersal. Among the 44 bird species, 34 were considered frugivorous (Table S1). Plant species with the highest resource potential for birds were Xylopia aromatica (n = 26), Cecropia pachystachya (n = 24) and Inga vera (n = 22) (Table S1). The richest plant families were Annonaceae and Fabaceae, with six species each. For birds, Psittacidae and Tyrannidae were the richest families, with nine and eight species, respectively (Table S1). Considering plant phenology data, resource availability was highest in September and October, with a peak of fruiting species (Fig. 2a).
Plant and bird species richness in fragments from May 2015 to July 2016. (a) monthly patterns in fruiting plant species richness in non-forest fragments (dark green), forest remnants (green), and bird species richness (grey). (b) bird species richness (grey), and fruiting plant species richness (dark green) in forest and non-forest fragments. Different letters indicate statistical differences between samples
Peak bird species richness coincided with the fruiting peak (p < 0.05; R² = 0.86, se = 3.751 (df = 14). Most plant species (81.8%) had a single fruiting event, while about a fifth of the species (18.2%) had two, showing a bimodal pattern (Fig. 2a). The rest of the species, such as Tococa guianensis and Miconia albicans fructified year round. Shrubs and trees represented 45.4% and 54.6% of all species, respectively. While forest remnants had similar plant species richness, their bird species richness differed (Fig. 2b).
Interaction Network
In general, tree species and forest fragments had the most interactions (124, Table S2), followed by non-forest fragments (79). Non-forest fragments on average had half of the interactions than forest fragments (maximum of 113 interactions, Fig. 3). The network of avian frugivore interactions in urban environments showed a significantly nested pattern for both tree and shrub species in forest (NODF = 55.77 and 50.16, respectively, p < 0.01, Fig. 4). The species with the highest number of interactions was Pera glabrata, followed by Inga vera. The number of bird species per interaction reached 14, for 11 interactions (on average 1.2 bird species per interaction). The matrices showed a high correlation between the forms of life (Mantel statistic r = 0.39, p < 0.01, Fig. 4), but not to types of fragments (Mantel statistic r = 0.19, p > 0.05).
Left: Examples of habitats and representative interactions: (a) Tersina viridis feeding on Cecropia pachystachya fruit in a forest, and (b) Tangara sayaca with the fruit of Jacaranda spp. in non-forest habitat. Right: Networks of interactions (plant species indicated in green and birds in grey on Fruchterman-Reingold graphs) for trees, shrubs and both for forest formations (top three) and non-forest formations (bottom three) in May 2015 – July 2016. Lines correspond to interactions and bold lines correspond to stronger interspecific interactions. Species that showed no interactions are drawn without links
The plant-bird connectivity was higher for trees compared to shrubs (c = 0.30) (Table S2, Fig. 4). When comparing the six matrices, network modularity was not significant (p = 0.16, see Table S2). Considering fragments, non-forests had higher modularity (M = 0.36) and nesting (NODF = 23.25) compared to forests (M = 0.26; NODF = 45.39). (Table S2). The degree of distribution and intensity of the networks was higher for trees in both forests and non-forests (AIC= -15; p < 0.05, see Table S2).
Considering diet, the most specialized bird species was Columbina squammata (h2 = 0.37), feeding only on the shrub Bauhinia spp., and on two tree species, Bauhinia forficata and Mouriri elliptica (h2 = 0.40, Fig. 5). However, we found no difference between forest and non-forest formations. The Index of Relative Importance was highest for species in forest fragments (Fig. 5). Most plant-bird interactions (65%) were recorded in forest formations with shrubs (χ² = 35.59; p < 0.001), with a difference between forests and non-forests (χ² = 31.48; p < 0.001).
Discussion
As we hypothesized, the time scale for birds and plants was similar and higher during the rainy season, when the species richness was high. Similar to the results of previous studies on Neotropical birds, which found that most species were frugivores (Jordano 1994; Francisco and Galetti 2002; Fadini and Marco 2004), our results showed that 63.7% of birds were frugivorous in urban remnants. Following the categorization of Francisco and Galetti (2002), approximately 64% of plants had zoochoric dispersal and among them 38.2% were dispersed by birds. These high values highlight the relevance of the role of birds in urban fragments and their interactions with plants that provide their food source. Similar to published studies (Francisco and Galetti 2002), we found the families Thraupidae, Turdidae, Psittacidae and Tyrannidae particularly important in seed dispersal and plant pollination. However, members of Psittacidae can be considered “seed destroyers”. While feeding on the fruits, parrots break the structure and therefore damage the embryo, contributing little to the dispersal processes (Sick 1997), thereby decreasing their contribution to the natural restoration of the habitat.
Tyrannidae is a heterogeneous group, with mostly insectivores (Gwynne et al. 2010). Nevertheless, a previous study found that insectivorous Tyrannidae species also feed on fruits and thereby contribute to seed dispersal (Lasky and Keitt 2012), as observed in our study. The most abundant plant families were Annonaceae and Fabaceae, both of high species richness and wide distribution in the Cerrado biome, including fragments (Nunes 2012). As we hypothesized, we confirmed a difference in interactions between forest and non-forest fragments. This difference was particularly strong for frugivorous birds in forests. The high level of interaction between bird and plant species in these forest fragments, mainly involving tree life forms, suggests that this is caused by the high number of tree species at the study site or by the higher visibility of fruits due to the height of the plant or the presence of certain tree species.
This relationship is possibly linked to seasonal differences in the environment, given the strong climatic seasonality of the Cerrado. Annual precipitation patterns affect bird community composition and also the amount of food supplied by different shrub and tree species, which differ in their flowering and fruiting patterns (Macedo 2002; Malhães 2003; Vieira et al. 2013). We found strong plant-bird interactions for both shrubs and trees (Fig. 4), indicating that most bird species were generalists (i.e., they can feed on various plant species and possibly at different times of the year). This type of behavior suggests a potentially crucial ecological interaction in the urban community: if one species is no longer present, there are others that can replace it and still maintain seed dispersal and pollination activities (Jordano et al. 2010). While specificity is a very important factor in plant-bird interactions, ensuring the dynamics in the environment, it is generalists, such as members of the Tyrannidae and Columbidae families (Melo et al. 2003). Likewise, from a functionality point of view, members of Columbidae are usually seeded predators (granivores), decreasing their performance as effective seed dispersers (Dennis and Westcott 2006; Vidal et al. 2014). Nevertheless, contrary to the published literature (Dennis and Westcott 2006), in our study Columbidae behaved more as specialist than generalist. A reason could be that unlike previous studies, our observations occurred in urban fragments.
Previous studies on bird communities in urban forest fragments showed that 13.5% of the species were unique to the study area (Corral and Valério 2019). Among these restricted species, we had the first record of Phibalura flavirostris for the municipality of Campo Grande, showing an expansion from its previously known geographical distribution (BirdLife International 2020). This shows the importance of species that favor regenerating forest fragments and, thus contribute to the creation of habitats that can provide shelter to other bird species in the urban area (Corral et al. 2018). Other studies have found that compared to trees, shrubs have higher species richness and more interactions with birds. The reason for this is that the vast majority (about 90%) of tropical shrub species have zoochoric dispersal (Howe and Smallwood 1982). In our study, interactions between shrubs and birds were fewer compared to those between trees and birds, but still showed a significantly similar pattern for tree and shrub species. Overall, increasing forest connectivity and maintaining or restoring plant-bird interactions are important strategies to maintain urban biodiversity (Aronson et al. 2017). Most bird species in the Cerrado are highly mobile, capable of moving from open areas to forests, even crossing through a hostile matrix (Plein et al. 2013; Souza et al. 2019). However, in our study, the connectivity index revealed a greater interaction between birds and tree species compared to interactions between birds and shrubs, possibly due to the characteristics of the local landscape, as the study was conducted in a fragmented habitat (Plein et al. 2013). This may affect urban fragments, as well as large cities that have mostly tree species and lack shrubs (e.g., as a result of landscaping). In studies conducted in urban environments, bird species richness was related to the number of trees, which may further affect bird diversity, as birds move into urban centers (Souza et al. 2019).
A recent study conducted in the city of Campo Grande indicates that a large proportion of the bird community does not respond to urbanization, as many forest species are also found in the city, possibly because of the large number of trees in the city center and the high connectedness among smaller urban forest fragments (Souza et al. 2019) In addition, these results may indicate changes in bird movements, as individuals from adjacent fragments that are threatened by the constant loss of habitat seek forested areas in the urban matrix, altering the established species composition (Corral and Valério 2019). The low modularity index and the increased nesting indicate the generalization and asymmetry of the networks and the points with few interactions indicate plant species that are linked to generalist bird species, and therefore suggest that interactions are not restricted (Bascompte et al. 2003; Olesen et al. 2007). Therefore, community interactions are determined by generalist species and thus become more resistant to species loss, in which case this process can be an alternative way for the system to respond to disturbance (Bascompte et al. 2003).
In accordance with our hypotheses, when comparing urban and non-urban forest fragments, we found higher fruit production during the rainy season, and observed an effect of seasonality-dependent phenology on bird species richness (Camargo et al. 2018). What differentiates urban and non-urban fragments are the biogeographic characteristics, such as the size of the fragments and their distance from each other (Corral and Valério 2019). With regard to phenology, we need to consider the premise that interactions are more specialized when resources are scarce and in the rainy season interactions tend to be more comprehensive, i.e., plants are most commonly visited by generalist bird species (Souza et al. 2018). Similar studies can inform conservation strategies for forest fragments and thereby contribute to the conservation of ecological interaction networks and existing richness patterns (Carlo and Yang 2011; Schleuning et al. 2011).
Conclusions
In the surveyed urban fragment, plants and birds showed great diversity and interactions. The most specialized plant and bird species were Mouriri elliptica and Columbina squammata, respectively. Species with the highest number of interactions were Inga vera and Pera glabrata. Having dispersed resources, the urban environment can influence plant and animal species richness and the interactions among these species. We found a large number of plant species in the fruiting stage at the beginning of the rainy season, increasing food availability for birds. In addition, forest fragments presented more interactions and trees provided the main food source for birds. Nevertheless, when fruit availability was high, the number of granivores (seed-predators) also increased. Even with low specialization of the networks, this result demonstrates the importance of maintaining forest environments, as they support higher bird species diversity, given increased resource availability in forests, connecting complex ecological systems. With habitat loss, ecological interactions are reduced, leaving only a few healthy ecological systems scattered in forest fragments within the urban matrix.
References
Almeida-Neto M, Ulrich W (2011) A straight forward computational approach for measuring nestedness using quantitative matrices. Environ Model Softw 26:173–178. https://doi.org/10.1016/j.envsoft.2010.08.003
Aronson MJF, Lepczyk C, Evans KL et al (2017) Biodiversity in the city: Key challenges for urban green space management. Front Ecol Environ 15:189–196. https://doi.org/10.1002/fee.1480
Bascompte J, Jordano P (2006) The structure of plant animal mutualistic networks. In: Pascual M, Dunne JA (eds) Ecological networks: linking structure to dynamics in food webs. Oxford University Press, Oxford, pp 143–159
Bascompte J, Jordano P, Melían CJ et al (2003) The nested assembly of plant-animal mutualistic networks. Proc Natl Acad Sci 100(16):9383–9387
Bender IMA, Kissling WD, Blendinger G et al (2018) Morphological trait matching shapes plant–frugivore networks across the Andes. Ecography 41:1910–1919. https://doi.org/10.1111/ecog.03396
BirdLife International (2020) Species factsheet: Phibalura flavirostris. Downloaded from http://www.birdlife.org on 04/02/2020
Camargo R, Boucher-Lalonde V, Currie D (2018) At the landscape level, birds respond strongly to habitat amount but weakly to fragmentation. Divers Distrib. https://doi.org/10.1111/ddi.12706
Carlo TA, Yang S (2011) Modelos de rede de frugivoria e dispersão de sementes: desafios e oportunidades. Acta Oecol 37:619–624 (10.1016/j.actao.2011.08.001)
Chao A (1987) Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43:783–791
Cheung KC, dos Reis LK, Jesus CCC (2016) Análise fitossociológica de um fragmento de Cerrado em Campo Grande, MS. Multitemas, 21(49)
Corral A, Valério LM (2019) Efeito do tamanho e distância de fragmentos florestais urbanos na composição de aves no perímetro urbano de Campo Grande – MS. Atualidades Ornitológicas. Campo Grande 210:33–46
Corral A, Silva CLR, Carvalho CME et al (2018) First record of Swallow-tailed Cotinga, Phibalura flavirostris Vieillot, 1816 (Aves, Cotingidae) in Campo Grande, Mato Grosso do Sul, Brazil . CheckList 14:495–497. https://doi.org/10.15560/14.2.495
Dennis AJ, Westcott DA (2006) Reducing complexity when studying seed dispersal at community scales: a functional classification of vertebrate seed dispersers in tropical forests. Oecologia 149(4):620–634. https://doi.org/10.1007/s00442-
Dormann CF, Gruber B, Frund J (2008) Introducing the bipartite package analysing ecological networks . RNews 8:8–11
Fadini RF, Marco P (2004) Interações entre aves frugívoras e plantas em um fragmento de mata atlântica de Minas Gerais. Ararajuba, São Paulo 12:97–103
Franciso MR, Galetti M (2002) Aves como potenciais dispersores de sementes Ocoteapulchella numa área de vegetação de Cerrado do sudeste brasileiro. Rev Bras Bot 25:11–17
Freitas L, Vizentin-Bugoni J, Wolowski M, Souza JMT, de Varassin IG (2014) Interações planta-polinizador ea estruturação das comunidades. In: Biologia da Polinização. Rech (Org) et al. Editora projeto cultural. 1st edn. Rio de Janeiro
Githiru M, Lens L, Bennur LA, Ogol CPKO (2002) Effects of site and fruit size on the composition of avian frugivore assemblages in a fragmented Afrotropical forest. Oikos 96:320–330
Guimarães PR (2010) A estrutura e dinâmica evolutiva de redes mutualísticas. Ciência e Ambiente 39:137–148
Guimarães LD, Silva MAD, Anacleto TC (2006) Natureza viva: Cerrado. Editora da UCG, Goiânia
Gwynne JA et al (2010) Aves do Brasil: Pantanal e Cerrado. Editora Horizonte, São Paulo
Howe HF, Smallwood J (1982) Ecology of seed dispersal. Annu Rev Ecol Syst 13:201–228
Hsieh TC, Ma KH, Chao A (2016) iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol (in revision)
Jordano P (1987) Patterns of mutualistic interactions in pollination and seed dispersal: connectance, dependence asymmetries, and coevolution. Am Nat 129:657–677
Jordano P (1994) Spatial and temporal variation in the avian-frugivore assemblage of Prunus mahaleb: patterns and consequences. Oikos 71:479–471
Jordano P, Bascompte J, Olesen JM (2003) Invariant properties in coevolutionary networks of plant–animal interactions. Ecol Lett 6:69–81
Jordano P, Bascompte J, Olesen JM (2006) The ecological consequences of complex topology and nested structure in pollination webs. In: Waser NM, Ollerton J (eds) Plant-pollinator interactions: from specialization to generalization. The University of Chicago Press, Chicago, pp 173–199
Jordano P, Lambert JE, Forget PM et al (2010) Frugivores and seed dispersal: mechanisms and consequences for biodiversity of a key ecological interaction. Biol Let 7:321–323. https://doi.org/10.1098/rsbl.2010.0986
Lasky J, Keitt T (2012) The effect of spatial structure of pasture tree cover on avian frugivores in eastern amazonia. Biotropica 44:489–497. https://doi.org/10.1111/j.1744-7429.2012.00857.x
Levey DJ, Moermond TC, Denslow JS (1984) Fruit choice in neotropical birds: The effect of distance between fruits on preference patterns. Ecology 65:844–850. https://doi.org/10.2307/1938058
Lorenzi H (2002) Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil, 2rd edn. Instituto Plantarum, Nova Odessa
Lorenzi H (2009) Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil, 3rd edn. Instituto Plantarum, Nova Odessa
Macedo RHF (2002) Nos Cerrados do Brasil: ecologia e história natural de uma savana neotropical. Columbia University Press, Nova Iorque
Malhães MA (2003) Variação sazonal da dieta e comportamento alimentar de traupídeos (Passeriformes: Emberezidae) em Ibitipoca, Minas Gerais, Brasil. Ararajuba 11:45–55
Marjakangas EL, Abrego N, Grotan V et al (2019) Fragmented tropical forests lose mutualistic plant–animal interactions. Divers Distrib 00:1–15. https://doi.org/10.1111/ddi.13010
Maruyama PK, Bonizario C, Marcon AP et al (2019) Plant-hummingbird interaction networks in urban areas: Generalization and the importance of trees with specialized flowers as a nectar resource for pollinator conservation. Biol Conserv 230:187–194
Melo C, Bento EC, Oliveira PE (2003) Frugivory and dispersal of Faramea cyanea (Rubiaceae) in cerrado woody plant formations. Braz J Biol 63:75–82. https://doi.org/10.1590/S0101-81752008000400013
Melo MAR, Muylaert ML, Pinheiro RBP, Ferreira GMF (2016) Guia para análise de redes ecológicas. 1ed. http://www.marcomello.org. Accessed 01 July 2020
MMA (2018) Ministério do Meio Ambiente. Reserva Legal. IOP Publishing: <https://www.mma.gov.br/>. Accessed 4 Feb 2020
Nunes K (2012) Interações entre aves frugívoras e plantas: um estudo comparativo em formações savânicas e florestais do cerrado. Universidade do Estado de Mato Grosso, Nova Xavantina
Olesen JM, Bascompte J, Yoko L et al (2007) The modularity of pollination networks. Proc Natl Acad Sci 104:19891–19896
Oliveira DSF, Franchin AG, Marçal J et al (2015) Bird-plant interaction networks, a study on frugivory in Brazilian urban areas. Biotemas 28:83–97
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB, Wagner H (2013) Community ecology package. R package version, 2-0. https://cran.r-project.org/web/packages/vegan/index.html. Accessed 1 July 2020
Piratelli AJ, Franchin AG, Marín-Gómez OH (2017) Urban conservation: Toward bird-friendly cities in Latin America. Springer International Publishing, Cham. https://doi.org/10.1007/978-3-319-63475-3_8
Plein M, Längsfeld L, Neuschulz EL et al (2013) Constant properties of plant–frugivore networks despite fluctuations in fruit and bird communities in space and time. Ecology 94:1296–1306
Primack RB, Rodrigues E (2001) Biologia da Conservação. Editora Planta, Londrina Purificação KN (2014) Interactions between frugivorous birds and plants in savanna and forest formations of the Cerrado. Biota Neotropica 14(4)
Purificação KN, Pascotto MC, Pedroni F, Pereira JMN, Lima NA (2014) Interactions between frugivorous birds and plants in savanna and forest formations of the Cerrado. Biota Neotropica 14(4):1–14. https://doi.org/10.1590/1676-06032014006814
Rosenzweig C, Karoly D, Vicarelli M et al (2008) Attributing physical and biological impacts to anthropogenic climate change. Nature 453:353–358. https://doi.org/10.1038/nature06937
Schleuning M, Blüthgen N, Flörchinger M et al (2011) Specialization and interaction strength in a tropical plant–frugivore network differ among forest strata. Ecology 92:26–36. https://doi.org/10.1890/09-1842.1
Schupp EW, Fuentes M (1995) Spatial patterns of seed dispersal and the unification of plant population ecology. Écoscience 3:267–275. https://doi.org/10.1080/11956860.1995.11682293
Sick H (1997) Ornitologia Brasileira. Revisão: José Fernando Pacheco. Editora Nova Fronteira, Rio de Janeiro
Silva IC, Cheung KC (2012) Levantamento da araneofauna (Arachnida, Araneae), presente em três fragmentos distintos no Instituto São Vicente, Lagoa da Cruz, Campo Grande, MS. Brasil. Monography, Universidade Católica Dom Bosco
Skarpe C (1992) Dynamics of savanna ecosystems. J Veg Sci :3293–300. https://doi.org/10.2307/3235754
Souza CS, Maruyama PK, Aoki C et al (2018) Temporal variation in plant-pollinator networks from seasonal tropical environments: Higher specialization when resources are scarce. J Ecol. https://doi.org/10.1111/1365-2745.12978
Souza FL, Valente-Neto F, Severo-Neto F et al (2019) Impervious surface and heterogeneity are opposite drivers to maintain bird richness in a Cerrado city. Landsc Urban Plann 192:1–10
Trøjelsgaard K, Olesen JM (2016) Ecological networks in motion: micro- and macroscopic variability across scales. Funct Ecol :1–10. https://doi.org/10.1111/1365-2435.12710
Van der Pijl L (1982) Principles of dispersal in higher plants. Springer Verlag, New York
Vidal MM, Hasui E, Pizo MA, Tamashiro JY, Silva WR, Guimarães PR (2014) Frugivores at higher risk of extinction are the key elements of a mutualistic network. Ecology 95(12):3440–3447. https://doi.org/10.1890/13-1584.1
Vieira FM, Castilho LS, Purificação KN et al (2013) Estrutura histórica da avifauna de quatro fitofisionomias do Cerrado no Parque Estadual da Serra Azul. Ornithologia 543 – 57
Vizentin-Bugoni J, Maruyama PK, Souza CS et al (2018) Plant-pollinator networks in the tropics: A review. In: Dáttilo W. Rico-Gray V (eds) Ecological networks in the tropics. Cham, pp 73–91
Weinstein BG, Graham CH (2017) Persistent bill and corolla matching despite shifting temporal resources in tropical hummingbird-plant interactions. Ecol Lett 20:326–335. https://doi.org/10.1111/ele.12730
Zar JH (1999) Biostatistical analysis. Upper Saddle River, New Jersey
Acknowledgements
This study was financed in part by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (PIBIC-CNPq). We thank all contributors for field support and logistics (particularly the project coordinator, Cristiano Carvalho) and the members of the EcoFrag group (Nicolle Prado, Klysman Fernandes and Mônica Moreira) for making this article possible. We are also grateful to the editor Dr. Glenn R. Guntenspergen and two anonymous reviewers for their valuable input on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 240 kb)
Rights and permissions
About this article
Cite this article
Corral, A., Valério, L.M., Cheung, K.C. et al. Plant-bird mutualistic interactions can contribute to the regeneration of forest and non-forest urban patches in the Brazilian Cerrado. Urban Ecosyst 24, 205–213 (2021). https://doi.org/10.1007/s11252-020-01029-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-020-01029-8