Abstract
Arbuscular mycorrhizal (AM) fungi have traditionally been considered generalist symbionts. However, an increasing number of studies are pointing out the selectivity potential of plant hosts. Plant life form, determined by plant life history traits, seems to drive the AM fungal community composition. The AM fungi also exhibit a wide diversity of functional traits known to be responsible for their distribution in natural ecosystems. However, little is known about the role of plant and fungal traits driving the resultant symbiotic assemblages. With the aim of testing the feedback relationship between plant and fungal traits on the resulting AM fungal community, we inoculated three different plant life forms, i.e. annual herbs, perennial herbs and perennial semi-woody plants, with AM fungal communities sampled in different seasons. We hypothesized that the annual climate variation will induce changes in the mean traits of the AM fungal communities present in the soil throughout the year. Furthermore, the association of plants with different life forms with AM fungi with contrasting life history traits will show certain preferences according to reciprocal traits of the plants and fungi. We found changes in the AM fungal community throughout the year, which were differentially disrupted by disturbance and altered by plant growth form and plant biomass. Both plant and fungal traits clearly contributed to the resultant AM fungal communities. The revealed process can have implications for the functioning of ecosystems since changes in dominant plant life forms or climatic variables could influence the traits of AM fungal communities in soil and hence ecosystem processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizas are the most ancient and widespread symbiotic association in nature (Brachmann and Parniske 2006; Schüßler and Walker 2011). Eighty percent of land plants are able to establish a mutualistic association with arbuscular mycorrhizal (AM) fungi (Brundrett 2009). In the AM symbiosis, the microscopic fungus colonizes plant roots and develops an extraradical mycelium which contributes to an increase in water and nutrient availability to the plant, which, in turn, supplies a part of its C compounds from photosynthesis to the fungus (Koide and Mosse 2004). Additionally fungi provide other benefits to plants such as protection against biotic and abiotic stress factors, and improved soil structure (Barea et al. 2013). There is some support for AM fungi promoting plant diversity and, by extension, affecting ecosystem properties, but this is not a universal phenomenon (Grime et al. 1987; van der Heijden et al. 1998, 2006; Vogelsang et al. 2006; Bever et al. 2010). Such an ecosystem role of AM fungi depends on the degree of mycorrhizal dependency of the plants present in each ecosystem (Urcelay and Díaz 2003).
Both abiotic and biotic factors have been found to drive the distribution of AM fungi in nature (Öpik et al. 2010). Abiotic factors, such as soil properties (Jansa et al. 2014) or the degree of disturbance (López-García et al. 2013), have been found to influence AM fungal communities. The variety of life history strategies exhibited by AM fungi has been proposed to explain these patterns (Chagnon et al. 2013). Since some traits are phylogenetically conserved in AM fungi (Powell et al. 2009) it has been possible to trace ecological patterns as a function of the presence of taxonomic families in different environments. For example, Jansa et al. (2003) found a dominance of Glomeraceae in disturbed environments, Acaulosporaceae have been commonly found in low-pH soils (Oehl et al. 2010), while Gigasporaceae increase their presence in P-limited soils (Johnson et al. 2003). These patterns have been attributed to differences in the production of resistant structures, i.e. sporulating vs. non-sporulating AM fungi (Denison and Kiers 2011), or differences in the investment in extra- vs. intraradical mycelium (Hart and Reader 2002).
Several studies have demonstrated that the host plant is a biotic driver of AM fungal communities, either as a function of plant species identity (Vandenkoornhuyse et al. 2003; Martínez-García and Pugnaire 2011; Sánchez-Castro et al. 2012a, b) or as a function of the functional or ecological group to which the plant belongs (Scheublin et al. 2004; Öpik et al. 2009; Davison et al. 2011). Some studies have focused on the influence of plant traits—mostly life form—on AM fungal communities. Plant life form is the most common and simplest criterion for classifying plants and is based on morphological features that are easy to determine (Schulze 1982) and which can be used to separate plants into trees, shrubs or forbs with different life cycles (Raunkiær 1934). In this manner, Cakan and Karatas (2006) established the relationship between the presence of plants having different life forms in dune succession and the extent of AM fungal colonization. Conversely, Urcelay et al. (2009) did not find differences between AM fungal-spore communities in a removal experiment of plant functional groups. A more recent global meta-analysis by Yang et al. (2012) found a high level of host specificity for AM fungi at different scales, including plant functional type, selected on the basis of their life forms, i.e. grasses, forbs and woody plants. In a more applied study, Lekberg et al. (2013) proposed that invasion-mediated shifts in AM fungal abundance can be estimated based on changes in forb/grass ratios. However, despite such clues, little is known about AM fungal traits interacting with plant traits to drive AM fungal communities, which is an emerging field in soil microbial ecology research (Powell et al. 2013).
The main difficulty of this kind of research arises from a cultivation bias of AM fungal isolates, which results in a dominance of pure cultures by r-strategist fungi (Sýkorová et al. 2011), e.g. heavily sporulating AM fungi. To overcome this limitation, natural soil inoculum can be used to increase the functional diversity of the AM fungi. As different AM fungal taxa exhibit a wide range of rates and timing of sporulation (Oehl et al. 2009), turnover in the composition and traits of AM fungal community can occur throughout the year. Thus, temporal samplings covering a whole year have to be designed to include a wide range of functionally different AM fungal communities. The use of contrasting AM fungal communities to inoculate different plant life forms is expected to reveal different associative patterns between AM fungal and plant traits.
Taking into account these considerations, the aim of this study was to assess the feedback relationship between plant and fungal traits in the resulting community of symbiotic fungi. As an initial hypothesis we assume that: (1) annual Mediterranean climate variation will induce changes in the mean trait values of the AM fungal community present in the soil throughout the year. A novel approach was used to measure the AM fungal resistance to disturbance, as a function of the production of spore and other resistant propagules, to characterize AM fungal communities. Variation in the survival capacity of the AM fungal community will be reflective of differences in fungal life history strategies and will be related to a certain turnover of AM fungal species composition throughout the year, as has been previously observed (Merryweather and Fitter 1998; Dumbrell et al. 2011; Sánchez-Castro et al. 2012b; López-García et al. (2014). (2) Finally, and never previously observed, it is also hypothesized that the association of plants of different life forms with AM fungi with contrasting life history strategies will show certain preferences according to their reciprocal traits.
Materials and methods
Study site and sampling
The study site was located in the Sierra de Baza Natural Park, at 1,500 m a.s.l. An area of 15,000 m2 was selected using ArcGIS 9.3 (ESRI, Redlands, CA) keeping a constant slope (15–20 %) and orientation (east). The soil is a Calcaric Cambisol. The vegetation is an open autochthonous Mediterranean forest combined with naturalized pines from restoration plans. Components of both Supramediterranean and Mesomediterranean bioclimatic zones are well represented in the area, and include Quercus ilex subsp. ballota (Desf.) Samp., Pinus sp., Juniperus oxycedrus L., Rosmarinus officinalis L., Berberis vulgaris subsp. australis (Boiss.) Heywood or Rosa sp. associated with herbaceous, annual, plant species. The climatic conditions are characterized by low and irregular precipitation with an annual mean of 385 mm, and a temperature of 25 °C in summer and 6 °C in winter, on average.
A representative AM fungal soil inoculum was obtained by sampling 20 randomly selected points in the target area. After removing the first soil layer, approximately 7 L of soil was collected between 2- and 20-cm depth. All soil samples were pooled and mixed in a concrete mixer. The sampling was repeated at the end of each climatic season, i.e. mid-December, mid-March, mid-June and mid-September.
Characterization of the AM fungal soil inoculum
Our aim was to characterize the AM fungal community composition and the survival/resistance capacity against disturbance of the AM fungal inoculum pool throughout the year.
As previously demonstrated, soil sieving has a high impact on AM fungal inoculum potential (Jasper et al. 1989), mainly due to a disruption of hyphal networks. Although the soil could have suffered a major impact during mixing in the concrete mixer, soil aggregates bigger than 5 cm could still be maintained. These aggregates usually harbour intact hyphal networks which are able to colonize new plants. It was thought that a major reduction of the AM fungal colonization potential (hereafter ‘survival capacity’) would result from the sieving treatment of the soil, as previously described by López-García et al. (2014). The relative change in survival capacity of the soil inoculum was measured by comparing the colonization potential in natural and sieved soil (through a 5-mm sieve) for each sampling date. The colonization potential was quantified by following the protocol of the infection unit method described by Franson and Bethlenfalvay (1989). This technique is based on the quantification of the infection units established by AM fungi in the roots of a bait plant (Sorghum vulgare Pers.) during 2 weeks of growth. The schematic diagram of the experimental design is shown in Fig. 1. To get a community mean value of survival capacity of the AM fungal inoculum for each sampling date, the difference between the mean natural and the mean sieved inoculum potential was calculated as a percentage.
Additionally, a comparison between the AM fungal community composition of natural and sieved soil was carried out. For that purpose, a bioassay based on a bait-plant system, modified from that by Johnson et al. (2004), was used to ‘capture’ the different AM fungal taxa. To ensure that community composition data were fully related to mycorrhizal potential assessment, the same plant species (S. vulgare) and timing (2 weeks) as in the infection unit method were used in a first stage. However, a further growth period was needed to allow AM fungal colonization to progress and expand in sorghum roots. Accordingly the experiments included two distinct steps (Fig. 1): plants were grown for 2 weeks in the target soils to allow their AM fungi to colonize the sorghum roots, plants were transferred to sterile soil to allow AM fungi to extend colonization of the root system to levels detectable by the molecular analysis (8 weeks). Thus, 150-ml pots were filled with natural or sieved soil, from samples taken at each sampling date. One sorghum plant per pot was grown under controlled greenhouse conditions (16/8-h day-night light, 24–18 °C). After 2 weeks, plants were removed from the pots, their root system thoroughly cleaned by hand with tap water and then transferred to steam-sterilized soil. This was designed to avoid root colonization by other AM fungal taxa not considered in the colonization-potential assessments. After a further 8-week-period, plants were harvested and their roots cut into 1-cm pieces, mixed and kept in 200-mg aliquots at −80 °C for the molecular characterization of their AM fungal symbiotic assemblages.
Feedback between plant life form and AM fungal community
The aim of this experiment was to evaluate the effect of plants with different life forms on the AM fungal community composition when they were inoculated with functionally contrasting AM fungal communities.
Six different species belonging to two different botanical families with distinct life forms were selected in order to consider both taxonomic distance and life form characteristics (Table 1). The chosen plant species were present in the study area or very close to it, thus share habitat with the AM fungal community living in the target area and can be naturally colonized by these fungi.
The previously characterized soil was used as AM fungal inoculum to set up these experiments at each sampling date. A pre-germinated seedling belonging to each of the selected plant species was sown per pot (1 L), filled with the natural soil. Eight replicates per target plant species were established. Seeds were provided by Real Jardín Botánico (CSIC, Madrid) and Banco de Germoplasma Vegetal Andaluz (Junta de Andalucía, Córdoba). One week before each sampling date, the seeds were germinated in a growth chamber on sterile and wet filter paper, following the protocol described by Kew Royal Botanical Gardens (www.kew.org). Plants were exposed to similar cultivation conditions in the greenhouse (16/8-h day-night light, 24–18 °C) at different sampling dates to try to eliminate factors other than the inoculum type in the experiment. After a growth period of 6 months under controlled conditions plants were harvested. The entire soil–plant system was extracted from the pot and the root system was separated by hand in an attempt to minimize the disruption of soil aggregates. The soil was mixed by hand to be analysed using the same bioassay described in the previous section (“Characterization of the AM fungal soil inoculum”). Plant shoot biomass was measured after drying for 48 h at 65 °C.
Molecular analysis
Terminal restriction fragment length polymorphism (T-RFLP) was used to fingerprint AM fungal communities by analysing gene polymorphism in a ca. 800-base pairs (bp) section of the small subunit (SSU) rDNA. For this purpose, DNA from 200 mg of roots per sample was extracted using a DNeasy plant mini-kit (Qiagen, Crawley, UK) and eluted in 75 μl double-distilled H2O. A partial ribosomal SSU DNA fragment was amplified using a nested polymerase chain reaction (PCR). First, an initial reaction with the universal eukaryotic primers NS1 and NS4 amplified a ca. 1,000-bp region. PCR were carried out using the illustra Pure-Taq Ready-To-Go PCR beads (GE Healthcare UK, Buckinghamshire, UK) and 5 μM of each primer. As a template, 1 μl of extracted DNA was used in all reactions in a final volume of 25 μl. The program was: an initial denaturation at 94 °C for 3 min, followed by 30 cycles at 94 °C for 30 s, 40 °C for 1 min, 72 °C for 1 min, followed by a final extension period at 72 °C for 10 min.
The resulting amplicons were used to carry out the nested PCR. The primer set used was AML1-AML2 (Lee et al. 2008), a specific pair of primers for AM fungal DNA amplification. This primer set has been found to be very specific in previous studies of our research group, thus avoids amplifications of other fungal groups or even plant sequences (López-García et al. 2014). For these amplifications the conditions defined by Lee et al. (2008) were modified as follows: 1 min at 94 °C, 30 cycles at 94 °C for 1 min, 62 °C for 1 min, and 72 °C for 1 min, followed by a final extension period at 72 °C for 5 min. Since T-RFLP profiles were analysed in multiplex, two independent and analogous reactions were done for each sample, one with 6-carboxy-fluorescein (6-FAM)-labelled AML1 primer, and the other with hexachloro-6-carboxy-fluorescein (HEX)-labelled AML1 primer. One microlitre of the product of the universal PCR previously diluted 1/10 was used as template. The PCR was carried out using the illustra Pure-Taq Ready-To-Go PCR beads (GE Healthcare UK) and 5 μM of primer in the 6-FAM reactions and 20 μM of primer in the HEX reactions. This difference in the protocol was based on a differential amplification capacity of both reactions. Using these conditions both reactions produced a similar final concentration of DNA. Afterwards, PCR products were cleaned using illustra GFX PCR DNA and Gel Band Purification Kit (GE Healthcare Life Sciences, Freiburg, Germany).
PCR products were digested using 1.5 units of MboI (for 6-FAM labelled products) and HinfI (for HEX) restriction enzymes. Both have been widely used in T-RFLP analysis of AM fungi (Dickie and FitzJohn 2007). After a in silico analysis, the combination of the products of the digestion with MboI and HinfI were found to have similar TRF size variability in the 800-bp rDNA fragment (AML1-AML2) as found for a typically used 550-bp fragment (NS31-AM1) (data not shown). Both digestions used 40 ng of PCR product and were mixed and cleaned together (illustra GFX PCR DNA and Gel Band Purification Kit; GE Healthcare Life Sciences). TRF size determination was carried out in the Unidad de Genómica y Síntesis de DNA, Instituto de Biomedicina y Parasitología López Neyra (Granada, Spain). Profiles were processed using GeneMapper software version 3.7 (Applied Biosystems).
Peaks were filtered by setting the minimal cut-off height under 100 fluorescence units. TRFs smaller than 50 bp and bigger than 800 bp were not considered. TRFs occurring in only one sample were considered artifacts of the T-RFLP procedure and were also excluded. Although a similar fluorescence level was found in each sample, peak areas were normalized as the percentage of the total fluorescence in each sample and those which contributed less than 1 % to the total peak area were excluded. The diversity and composition of the AM fungal community of each sample were estimated from the TRF abundance.
The use of peak area as a measure of relative abundance is controversial due to the bias caused by the PCR (Dickie and FitzJohn 2007). However, a quantitatively based T-RFLP approach is appropriate in some circumstances (e.g. Mummey et al. 2005; Mummey and Rillig 2008; Martínez-García and Pugnaire 2011). To the extent of our knowledge, no studies have addressed the correspondence between the abundance of AM fungi in roots and their TRF profiles; however, empirical data have shown a correlation between the TRF peak area and the number of root tips colonized by ectomycorrhizal fungi (Burke et al. 2006).
Data analysis
Differences in colonization potential of the AM fungi for each treatment and dry shoot biomass of different life forms at different sampling dates were tested by two-way ANOVA. The data met ANOVA assumptions of normality and equality of variance. A Tukey post hoc test was used to analyse the detected differences.
The effects of the experimental variables on the AM fungal community composition were investigated by permutational multivariate ANOVA (PERMANOVA) (McArdle and Anderson 2001), using Bray-Curtis distance as a measure of community dissimilarity. The effect of plant species in such an analysis was taken into account by nesting within the plant family level. To ensure that the detected effects were not a consequence of differences in multivariate dispersion rather than compositional change, a β-dispersion analysis were carried out within and between groups (Anderson et al. 2006). The Bray-Curtis similarity matrix was used to perform a non-metric multidimensional scaling (NMDS) ordination for both data sets to graphically visualize community composition patterns. The same matrix was used to perform two-way analysis of similarity (ANOSIM) pairwise comparisons to test for significant differences in AM fungal community composition associated with the different treatments (Clarke 1993). P-values were subsequently adjusted using Bonferroni correction. A regression analysis was performed to look at the relation of AM fungal community composition (NMDS axes) and plant dry shoot biomass.
Dufrêne-Legendre indicator species analysis (Dufrêne and Legendre 1997) was carried out to identify AM fungal operational taxonomic units (OTUs) tied to specific levels of the tested variables that could serve as indicator species.
All functions used to develop these analyses are found in the vegan 2.0-5 and labdsv 1.6-1 packages (R project 2.15.2, Oksanen et al. 2011, http://CRAN.R-project.org/package=vegan; and Roberts 2010, http://cran.r-project.org/web/packages/labdsv). Data concerning each set of experiments (see sections “Characterization of the AM fungal soil inoculum” and “Feedback between plant life form and AM fungal community”), were analysed separately.
Results
Characterization of the AM fungal soil inoculum
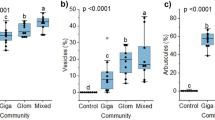
As expected for a Mediterranean area, the contrasting conditions of the different climatic seasons caused significant differences in the AM fungal colonization potential of the soil (date of sampling, F = 7.391, P < 0.001) (Fig. 2). Sieving had also an effect on the colonization potential (F = 22.862, P < 0.001), as well as the interaction between sieving treatment and date of sampling (F = 5.041, P < 0.01) (Fig. 2). The colonization potential tended to increase from September onwards to reach a maximum in June (Fig. 2). It seems that summer conditions, with scarce rainfall and high temperatures, could have been responsible for the highest reduction in the AM fungal colonization potential of the natural soil in September. In the sieved treatment, however, the AM fungal colonization potential did not change significantly throughout the year (Fig. 2).
The mycorrhizal potential was similar for both natural and sieved soils in September. This suggests that most of the AM fungal propagules present in the soil at the end of summer survived after soil sieving (85.7 % of survival; Fig. 2). From December, however, the colonization potential of the natural soil was progressively more affected by the sieving treatment. Thus, it seems that at the end of the Mediterranean summer (September) the more resistant AM fungi dominated, while the more sensitive AM fungi dominated at the end of the spring (June; only 46.4 % survival), with intermediate levels in December and March.
PERMANOVA showed a significant influence of the sampling date and an interaction of date with the soil treatment on AM fungal community composition (R 2 = 0.454, P < 0.001 and R 2 = 0.125, P < 0.001, respectively). As revealed by ANOSIM pairwise comparison, the AM fungal community composition in September was not affected by the sieving treatment (Fig. 3). However, the AM fungal communities of December (R = 0.5884, P < 0.05) and March (R = 0.5884, P < 0.05) differed between the sieved and natural treatments. Since β-dispersion analysis showed significant differences among treatments (F = 8.1271, P < 0.001), it could have biased the result of the PERMANOVA. However, the post hoc comparisons revealed differences in β-diversity among soil treatments exclusively for June (P < 0.001), although no differences in composition were shown by the ANOSIM for that date.
Feedback between plant life form and AM fungal community
PERMANOVA evidenced a clear influence of the survival capacity of the initial AM fungal community on the AM fungal community at the end of the experiment (Table 2). Part of this variation was explained by differences in the β-dispersion (F = 8.8096, P < 0.001), which showed significantly less dispersion of the community composition in September than for the rest of the sampling dates (data not shown). The variables related to host traits (taxonomy and plant life form traits) only showed significant differences in the comparison of plant growth form, i.e. semi-woody vs. herbaceous plants (Table 2). These differences could not be attributed to the dispersion patterns since no differences in β-dispersion were detected among life form treatments. At the plant species level only partial significant differences were found (Table 2). Finally, plant biomass showed a clear influence on the final AM fungal community composition (Table 2). The regression of plant biomass against NMDS axes showed low R 2-values (Online Resource 1); however, in March the relation was greater between variables (R 2 = 0.1578).
ANOSIM pairwise comparison showed no significant differences between AM fungal communities associated with plants with different life forms except for the March sampling (R = 0.2878, P < 0.001). At this sampling time, the resulting AM fungal community appears to be rather different in perennial semi-woody plants and the two herbaceous plant life forms (P < 0.026 for both comparisons), in agreement with results of PERMANOVA. ANOSIM also showed that AM fungal communities promoted by each plant life form had different trends throughout the year. For example, the AM fungal community associated with semi-woody plants showed wider differences among seasons, having a particular composition at every sampling date except in June (Fig. 4). By contrast, the AM fungal communities associated with herbaceous annual and herbaceous perennial plants were more constant between sampling dates, showing only two different community compositions (Fig. 4).
Non-metric multidimensional scaling ordination (k = 2, stress = 0.196) of the AMF communities promoted in different seasons by different plant species, grouped according to plant life form. a Herbaceous annuals [Trifolium angustifolium (Ta), Xeranthemum inapertum ( Xi)]; b herbaceous perennials [Medicago sativa (Ms), Inula montana (Im)]; c semi-woody perennials [Ononis natrix (On), Santolina canescens ( Sc)]. Different letters indicate significant differences in the community composition as found by analysis of similarity pairwise comparisons. DEC December, MAR March, JUN June, SEP September
ANOVA of plant shoot biomass data revealed a clear influence of the sampling date (F = 34.3700, P < 0.001). Plant life form was also found to be significant (F = 14.8748, P < 0.001) as well as the interaction of both variables (F = 5.7828, P < 0.001). The three analysed plant life forms showed a similar trend throughout the year, with some exceptions (Fig. 5), for example in December.
Dry shoot biomass of plant life forms at the end of the feedback experiment in soil from different sampling dates. Different letters indicate significant differences (P < 0.05) according to the Tukey’s post hoc test. Bars denote mean ± SE. For abbreviations, see Fig. 4
Indicator species analysis showed particular OTUs associated with specific traits and sampling dates (see Online Resource 2 for more complete information). However, the most interesting patterns can be seen in Table 3 where results from both experiments are crossed. Most of the semi-woody and perennial indicator OTUs were also indicative of March and June sampling dates and of natural, unsieved, soil. Conversely, those OTUs more frequent in September and December were primarily associated with annual and herbaceous plants.
Discussion
With the aim of gaining insight into the interaction of plant and AM fungal traits, an experimental approach was designed to check for the resultant AM fungal community when different plant life forms are inoculated with AM fungal communities differing in fundamental traits. We documented changes throughout the year in the AM fungal community, which was differentially disrupted by disturbance and altered by plant growth form and plant biomass. Both plant and fungal traits clearly contributed to the AM fungal communities we observed in the field at any given time.
We measured the AM fungal survival capacity of the initial inoculum, as an indicator of the ability to produce resistant structures, and found that AM fungal communities exhibiting extreme values of this trait, i.e. AM fungal communities very sensitive (June) or very resistant to disturbance (September), did not respond differentially to the presence of different plant life forms. By contrast, inoculating with AM fungal communities with intermediate values (March sampling date) resulted in the promotion of different OTU composition by plant life forms. This result clearly suggests that plants with different life forms have the ability to promote distinct AM fungal communities when the latter are functionally heterogeneous.
We found that annual herbaceous plants promoted more similar AM fungal communities over time than semi-woody plants, suggesting a higher capacity of their preferred symbionts to persist in the soil throughout the year. In addition, annual herbaceous plants were associated with AM fungal OTUs present in soil in September and December, i.e. the sampling dates with AM fungal communities showing the highest survival ability. A higher ability to persist has been suggested for sporulating AM fungi (Schnoor et al. 2011) and is considered a ruderal trait (Chagnon et al. 2013). Since annual herbs are classified usually as ruderal strategists (Grime 1977), this finding links both plant and fungal life history traits, as previously hypothesized by Chagnon et al. (2013). By contrast, perennial vegetation has been suggested to be associated with AM fungi not primarily dispersed by spores but by hyphal networks (Rosendahl and Stukenbrock 2004). The lack of resistant structures would increase the sensitivity of AM fungi to environmental changes and consequently would imply a wider turnover in terms of the presence and composition of the AM fungal community in the soil throughout the year. This trend was found for the AM fungal community associated with semi-woody perennial plants in the current study. Indeed, the indicator OTUs promoted by semi-woody perennials corresponded with those present mainly in March and June, which can be considered the most favourable period in the Mediterranean climate (López-Bermúdez et al. 1990) and showed the lowest survival values for the AM fungal community. In other words, semi-woody plants seem to be associated with the most sensitive fungal components.
Previous findings have documented changes in AM fungal traits between seasons, for example differential sporulation dynamics (Oehl et al. 2009). Furthermore, our group has also recently highlighted the seasonal replacement of AM fungal phylogenetic groups in individual roots (López-García et al. 2014), which, due to the phylogenetic trait conservatism of AM fungi (Powell et al. 2009), can be understood as a change in their fungal traits. However, the controversy in the literature regarding the existence of seasonal changes in AM fungal community composition (Dumbrell et al. 2011; Sánchez-Castro et al. 2012a, b; Bennet et al. 2013) or its lack (Santos-González et al. 2007; Davison et al. 2011, 2012) must be addressed in order to examine to what extent functional seasonal changes in the AM fungal community can play a role in ecosystem functioning.
Plant shoot biomass, as an intra-species plant trait variable, was also found to drive AM fungal communities. Although temperature and light period were constant in the greenhouse during the whole experiment, the variability of external sunlight could cause differences in photosynthetic rates and consequently differences in the plant growth and C supplied to the fungus, inducing a change in the AM fungal community, as suggested by Heinemeyer et al. (2003) and Dumbrell et al. (2011). We did not find that plant taxonomy was a driver of the AM fungal community assemblage. This contrasts with some studies that found a differential AM fungal community for legumes with respect to other plant species/families (Scheublin et al. 2004; König et al. 2010). However, the AM fungal community composition by plant life form found in the present study is in agreement with previous findings (see the meta-analysis by Yang et al. 2012). Another interesting line of research has pointed out the relation of generalist and specialist AM fungal species with similar ecological plant groups (Öpik et al. 2009; Davison et al. 2011; Torrecillas et al. 2014). Unfortunately, quantifying the role of plant traits in these studies is difficult since they focus on single plant life forms. In the context of invasion biology this duality, i.e. generalist/specialist, is closely related to the exotic-native classification of AM fungi (Kivlin and Hawkes 2011; Moora et al. 2011). In this regard, it is noteworthy that some studies which examine invasive processes have appealed for the use of trait-based approaches for an understanding of the ecology of AM fungi in these processes (Lekberg et al. 2013).
It is important to note that the only moment in which plants with different life forms could promote distinct AM fungal communities was in the March sampling, which is the beginning of the growing season in a Mediterranean climate and when plant seeds are expected to germinate. Despite small differences in phenology of the plant species used in the study, all of them have a typical Mediterranean life cycle, i.e. they flower in spring (March–June) (Blanca et al. 2009) and, although we have no data, they supposedly germinate a little bit earlier, also in the spring, when there is water availability and temperatures are moderate (López-Bermúdez et al. 1990). This would allow the maintenance of plant and AM fungal assemblages since new seedlings could be associated with their better-adapted symbionts. Nevertheless, although we found clear trends in AM symbiosis ecology in the present study, these should be carefully interpreted due to the experimental method. For example, the short time span in which trap plants were colonized, which could be shown for only the fastest AM fungal colonizers; and also the tracking for only 1 year, or the focus on only two plant families. Nevertheless, the agreement of our results with the preliminary hypotheses and the existing literature is noteworthy.
In summary, our results add new insights to the understanding of processes in plant and AM fungal assemblages. Also of importance is the capacity of plant functional traits to affect ecosystem properties by changing the soil dynamics, e.g. by rhizodeposition (Hirsch et al. 2013), by controlling litter decomposition (Cornwell et al. 2008) or by changing the soil C content (Gill and Burke 1999); and, as demonstrated, by impacting on the AM fungal community composition and, even more importantly, on relationships with the life history traits of the AM fungi. These feedback interactions will determine the assemblage of the symbiotic fungal community and consequently can alter ecosystem properties with a role in important processes such as biological invasions.
References
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693. doi:10.1111/j.1461-0248.2006.00926.x
Barea JM, Pozo MJ, López-Ráez JA, Aroca R, Ruíz-Lozano JM, Ferrol N, Azcón R, Azcón-Aguilar C (2013) Mycorrhizas and their significance in promoting soil-plant systems sustainability against environmental stresses. In: Rodelas B, González-López J (eds) Beneficial plant-microbial interactions: ecology and applications. CR, USA, pp 353–387
Bennet AE, Daniell TJ, Öpik M, Davison J, Moora M, Zobel M, Selosse MA, Evans D (2013) Arbuscular mycorrhizal fungal networks vary throughout the growing season and between successional stages. PLoS One 8:e83241. doi:10.1371/journal.pone.0083241
Bever JD, Dickie IA, Facelli E, Facelli JM, Klironomos JN, Moora M, Rillig MC, Stock WD, Tibbett M, Zobel M (2010) Rooting theories of plant community ecology in microbial interactions. Trends Ecol Evol 25:468–478. doi:10.1016/j.tree.2010.05.004
Blanca G, Cabezudo B, Cueto M, Fernández López C, Morales Torres C (2009) Flora Vascular de Andalucía Oriental. Consejería de Medio Ambiente, Junta de Andalucía, Sevilla
Brachmann A, Parniske M (2006) The most widespread symbiosis on Earth. PLoS Biol 4:e239. doi:10.1371/journal.pbio.0040239
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77. doi:10.1007/s11104-008-9877-9
Burke DJ, Martin KJ, Rygiewicz PT, Topa MA (2006) Relative abundance of ectomycorrhizas in a managed loblolly pine (Pinus taeda) genetics plantation as determined through terminal restriction fragment length polymorphism profiles. Can J Bot 84:924–932. doi:10.1139/B06-046
Cakan H, Karatas C (2006) Interactions between mycorrhizal colonization and plant life forms along the successional gradient of coastal sand dunes in the eastern Mediterranean, Turkey. Ecol Res 21:301–310. doi:10.1007/s11284-005-0134-x
Chagnon PL, Bradley RL, Maherali H, Klironomos JN (2013) A trait-based framework to understand the life history of mycorrhizal fungi. Trends Plant Sci 18:484–491. doi:10.1016/j.tplants.2013.05.001
Clarke K (1993) Non parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. doi:10.1111/j.1442-9993.1993.tb00438.x
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071. doi:10.1111/j.1461-0248.2008.01219.x
Davison J, Öpik M, Daniell TJ, Moora M, Zobel M (2011) Arbuscular mycorrhizal fungal communities in plant roots are not random assemblages. FEMS Microbiol Ecol 78:103–115. doi:10.1111/j.1574-6941.2011.01103.x
Davison J, Öpik M, Zobel M, Vasar M, Metsis M, Moora M (2012) Communities of arbuscular mycorrhizal fungi detected in forest soil are spatially heterogeneous but do not vary throughout the growing season. PLoS One 7:e41938. doi:10.1371/journal.pone.0041938
Denison RF, Kiers ET (2011) Life histories of symbiotic rhizobia and mycorrhizal fungi. Curr Biol 21:r775–r785. doi:10.1016/j.cub.2011.06.018
Dickie IA, FitzJohn RG (2007) Using terminal restriction fragment length polymorphism (T-RFLP) to identify mycorrhizal fungi: a methods review. Mycorrhiza 17:259–270. doi:10.1007/s00572-007-0129-2
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. doi:10.2307/2963459
Dumbrell AJ, Ashton PD, Aziz N, Feng G, Nelson M, Dytham C, Fitter AH, Helgason T (2011) Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol 190:794–804. doi:10.1111/j.1469-8137.2010.03636.x
Franson RL, Bethlenfalvay GJ (1989) Infection unit method of vesicular-arbuscular mycorrhizal propagule determination. Soil Sci Sco Am J 53:754–756
Gill RA, Burke IC (1999) Ecosystem consequences of plant life form changes at three sites in the semiarid United States. Oecologia 121:551–563. doi:10.1007/s004420050962
Grime JP (1977) Evidence for existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194. doi:10.1086/283244
Grime JP, Mackey JML, Hillier SH, Read DJ (1987) Floristic diversity in a model system using experimental microcosms. Nature 328:420–422. doi:10.1038/328420a0
Hart MM, Reader JR (2002) Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol 153:335–344. doi:10.1046/j.0028-646X.2001.00312.x
Heinemeyer A, Ridway KP, Edwards EJ, Benham DG, Young JPW, Fitter AH (2003) Impact of soil warming and shading on colonization and community structure of arbuscular mycorrhizal fungi in roots of a native grassland community. Glob Change Biol 10:52–64. doi:10.1046/j.1529-8817.2003.00713.x
Hirsch PR, Miller AJ, Dennis PG (2013) Do root exudates exert more influence on rhizosphere bacterial community structure than other rhizodeposits? In: de Bruijn FJ (ed) Molecular microbial ecology of the rhizosphere. Wiley, Hoboken, pp 229–242
Jansa J, Mozafar A, Kuhn G, Anken T, Ruh R, Sanders IR, Frossard E (2003) Soil tillage affects the community structure of mycorrhizal fungi in maize roots. Ecol Appl 13:1164–1176. doi:10.1890/1051-0761(2003)13[1164:STATCS]2.0.CO;2
Jansa J, Erb A, Oberholzer HR, Smilauer P, Egli S (2014) Soil and geography are more important determinants of indigenous arbuscular mycorrhizal communities than management practices in Swiss agricultural soils. Mol Ecol 23:2118–2135. doi:10.1111/mec.12706
Jasper DA, Abbott LK, Robson AD (1989) Soil disturbance reduces the infectivity of external hyphae of vesicular arbuscular mycorrhizal fungi. New Phytol 112:93–99. doi:10.1111/j.1469-8137.1989.tb00313.x
Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB (2003) Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84:1895–1908. doi:10.1890/0012-9658(2003)084[1895:NEAMAA]2.0.CO;2
Johnson D, Vandenkoornhuyse PJ, Leake JR, Gilbert L, Booth RE, Grime JP, Young JPW, Read DJ (2004) Plant communities affect arbuscular mycorrhizal fungal diversity and community composition in grassland microcosms. New Phytol 161:503–515. doi:10.1046/j.1469-8137.2003.00938.x
Kivlin SN, Hawkes C (2011) Differentiating between effects of invasion and diversity: impacts of aboveground plant communities on belowground fungal communities. New Phytol 189:526–535. doi:10.1111/j.1469-8137.2010.03494.x
Koide RT, Mosse B (2004) A history of research on arbuscular mycorrhiza. Mycorrhiza 14:145–163. doi:10.1007/s00572-004-0307-4
König S, Wubet T, Dormann CF, Hempel S, Renker C, Buscot F (2010) TaqMan Real-Time PCR assays to assess arbuscular mycorrhizal responses to field manipulation of grassland biodiversity: effects of soil characteristics, plant species richness, and functional traits. Appl Environ Microbiol 76:3765–3775. doi:10.1128/AEM.02951-09
Lee J, Lee S, Young JPW (2008) Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 65:339–349. doi:10.1111/j.1574-6941.2008.00531.x
Lekberg Y, Gibbons SM, Rosendahl S, Ramsey PW (2013) Severe plant invasions can increase mycorrhizal fungal abundance and diversity. ISME J 7:1424–1433. doi:10.1038/ismej.2013.41
López-Bermúdez F, Albadalejo J, Stocking MA, Díaz E (1990) Factores ambientales de la degradación del suelo en el área mediterránea. In: Albadalejo J, Stocking MA, Díaz E (eds) Degradation and rehabilitation of soil in Mediterranean environmental conditions. CSIC, Murcia, pp 15–45
López-García A, Hempel S, Miranda JD, Rillig MC, Barea JM, Azcón-Aguilar C (2013) The influence of environmental degradation processes on the arbuscular mycorrhizal fungal community associated with yew (Taxus baccata L.), an endangered tree species from Mediterranean ecosystems of Southeast Spain. Plant Soil 370:355–366. doi:10.1007/s11104-013-1625-0
López-García A, Palenzuela J, Barea JM, Azcón-Aguilar C (2014) life history strategies of arbuscular mycorrhizal fungi determine succession into roots of Rosmarinus officinalis L., a characteristic woody perennial plant species from Mediterranean ecosystems. Plant Soil 379:247–260. doi:10.1007/s11104-014-2060-6
Martínez-García LB, Pugnaire FI (2011) Arbuscular mycorrhizal fungi host preference and site effects in two plant species in a semiarid environment. Appl Soil Ecol 48:313–317. doi:10.1016/j.apsoil.2011.04.003
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82:290–297. doi:10.1111/j.1461-0248.2006.00926.x
Merryweather J, Fitter A (1998) The arbuscular mycorrhizal fungi of Hyacinthoides non-scripta. II.. Seasonal and spatial patterns of fungal populations. New Phytol 138:131–142. doi:10.1046/j.1469-8137.1998.00889.x
Moora M, Berger S, Davison J, Öpik M, Bommarco R, Bruelheide H, Kühn Y, Kunin WE, Metsis M, Rortais A, Vanatoa A, Vanatoa E, Stout JC, Truusa M, Westphal C, Zobel M, Walther GR (2011) Alien plants associate with widespread generalist arbuscular mycorrhizal fungal taxa: evidence from a continental-scale study using massively parallel 454 sequencing. J Biogeogr 38:1305–1317. doi:10.1111/j.1365-2699.2011.02478.x
Mummey DL, Rillig MC (2008) Spatial characterization of arbuscular mycorrhizal fungal molecular diversity at the submetre scale in a temperate grassland. FEMS Microbiol Ecol 64:260–270. doi:10.1111/j.1574-6941.2008.00475.x
Mummey DL, Rillig MC, Holben WE (2005) Neighboring plant influences on arbuscular mycorrhizal fungal community composition as assessed by T-RFLP analysis. Plant Soil 271:83–90. doi:10.1007/s11104-004-2066-6
Oehl F, Sieverding E, Ineichen K, Mäder P, Wiemken A, Boller T (2009) Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agric Ecosyst Environ 134:257–268. doi:10.1016/j.agee.2009.07.008
Oehl F, Laczko E, Bogenrieder A, Stahr K, Boesch R, van der Heijden MGA, Sieverding E (2010) Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol Biochem 42:724–738. doi:10.1016/j.soilbio.2010.01.006
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H. 2011. Vegan: community ecology package. R package version 2.0-1. http://CRAN.R-project.org/package=vegan
Öpik M, Metsis M, Daniell TJ, Zobel M, Moora M (2009) Large-scale parallel 454 sequencing reveals host ecological group specifity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol 184:424–437. doi:10.1111/j.1469-8137.2009.02920.x
Öpik M, Vanatoa A, Vanatoa E, Moora M, Davidson J, Kalwij JM, Reier U, Zobel M (2010) The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol 188:223–241. doi:10.1111/j.1469-8137.2010.03334.x
Powell JR, Parrent JL, Hart MM, Klironomos JN, Rillig MC, Maherali H (2009) Phylogenetic trait conservatism and the evolution of functional trade-offs in arbuscular mycorrhizal fungi. Proc R Soc B 276:4237–4245. doi:10.1098/rspb.2009.1015
Powell JR, Anderson IC, Rillig MC (2013) A new tool of the trade: plant-trait based approaches in microbial ecology. Plant Soil 365:35–40. doi:10.1007/s11104-012-1581-0
Raunkiær C (1934) Life forms of plants and statistical plant geography. Claredon, Oxford
Roberts DW. 2010. labdsv: Ordination and multivariate analysis for ecology. R package version 1.4-1. http://CRAN.R-project.org/package=labdsv
Rosendahl S, Stukenbrock EH (2004) Community structure of arbuscular mycorrhizal fungi in undisturbed vegetation revealed by analyses of LSU rDNA sequences. Mol Ecol 13:3179–3186. doi:10.1111/j.1365-294.2004.02295.x
Sánchez-Castro I, Ferrol N, Barea JM (2012a) Analyzing the community composition of arbuscular mycorrhizal fungi colonizing the roots of representative shrubland species in a Mediterranean ecosystem. J Arid Environ 80:1–9. doi:10.1016/j.jaridenv.2011.12.010
Sánchez-Castro I, Ferrol N, Cornejo P, Barea JM (2012b) Temporal dynamics of arbuscular mycorrhizal fungi colonizing roots of representative shrub species in a semi-arid Mediterranean ecosystem. Mycorrhiza 22:449–460. doi:10.1007/s00572-011-0421-z
Santos-González JC, Finlay RD, Tehler A (2007) Seasonal dynamics of arbuscular mycorrhizal fungal communities in roots in a seminatural grassland. Appl Environ Microbiol 73:5613–5623. doi:10.1128/AEM.00262-07
Scheublin TR, Ridgway KP, Young JPW, van der Heijden MGA (2004) Nonlegumes, legumes, and root nodules harbor different arbuscular mycorrhizal fungal communities. Appl Environ Microbiol 70:6240–6246. doi:10.1128/AEM.70.10.6240-6246.2004
Schnoor TK, Lekberg Y, Rosendahl S, Olsson PA (2011) Mechanical soil disturbance as a determinant of arbuscular mycorrhizal fungal communities in semi-natural grassland. Mycorrhiza 21:211–220. doi:10.1007/s00572-010-0325-3
Schulze ED (1982) Plant life forms and their carbon, water and nutrient relations. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology. II. Encyclopedia of plant physiology. Springer, Berlin, pp 615–676
Schüßler A, Walker C (2011) Evolution of the ‘plant-symbiotic’ fungal phylum, Glomeromycota. In: Pöggeler S, Wöstemeyer J (eds) Evolution of fungi and fungal-like organisms. Springer, Berlin, pp 163–185
Sýkorová Z, Ineichen K, Wiemken A, Redecker D (2011) The cultivation bias: different communities of arbuscular mycorrhizal fungi detected in roots from the field, from bait plants transplanted to the field, and from a greenhouse trap experiment. Mycorrhiza 18:1–14. doi:10.1007/s00572-007-0147-0
Torrecillas E, Alguacil MM, Roldán A, Díaz G, Montesinos-Navarro A, Torres MP (2014) Modularity reveals the tendency of arbuscular mycorrhizal fungi to interact differently with generalist and specialist plant species in gypsum soils. Appl Environ Microbiol 80:5457–5466. doi:10.1128/AEM.01358-14
Urcelay C, Díaz S (2003) The mycorrhizal dependence of subordinates determines the effect of arbuscular mycorrhizal fungi on plant diversity. Ecol Lett 6:388–391. doi:10.1046/j.1461-0248.2003.00444.x
Urcelay C, Díaz S, Gurvich DE, Chapin FS, Cuevas E, Domínguez LS (2009) Mycorrhizal community resilience in response to experimental plant functional type removals in a woody ecosystem. J Ecol 97:1291–1301. doi:10.1111/j.1365-2745.2009.01582.x
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72. doi:10.1038/23932
van der Heijden MGA, Streitwolf-Engel R, Riedl R, Siegrist S, Neudecker A, Ineichen K, Boller T, Wiemken A, Sanders IR (2006) The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol 172:739–752. doi:10.1111/j.1469-8137.2006.01862.x
Vandenkoornhuyse P, Ridgway KP, Watson IJ, Fitter AH, Young JPW (2003) Co-existing grass species have distinctive arbuscular mycorrhizal communities. Mol Ecol 12:3085–3095. doi:10.1046/j.1365-294X.2003.01967.x
Vogelsang KM, Reynolds HL, Bever JD (2006) Mycorrhizal fungal identity and richness determine the diversity and productivity of a tallgrass prairie system. New Phytol 172:554–562. doi:10.1111/j.1469-8137.2006.01854.x
Yang H, Zang Y, Yuan Y, Tang J, Chen X (2012) Selectivity by host plants affects the distribution of arbuscular mycorrhizal fungi: evidence from ITS rDNA sequence metadata. BMC Evol Biol 12:50–62. doi:10.1186/1471-2148-12-50
Acknowledgments
Dr Álvaro López-García thanks the Formación de Personal Investigador Programme (Ministerio de Ciencia e Innovación, Spain) for financial support. This research was supported by the Spanish government under the Plan Nacional de I+D+I (project CGL-2009-08825). We sincerely thank Dr John N. Klironomos and Dr Alexander Koch for their suggestions in the early stages of the experimental design and Mr Domingo Álvarez for technical assistance. We also thank the Consejería de Medio Ambiente, Junta de Andalucía (Spain) for permission to work in the Sierra de Baza Natural Park as well as the Real Jardín Botánico (Madrid, CSIC) and the Banco de Germoplasma Vegetal Andaluz (Córdoba, Junta de Andalucía) for providing us with the seeds used in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Joel Sachs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
López-García, Á., Azcón-Aguilar, C. & Barea, J.M. The interactions between plant life form and fungal traits of arbuscular mycorrhizal fungi determine the symbiotic community. Oecologia 176, 1075–1086 (2014). https://doi.org/10.1007/s00442-014-3091-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3091-7