Abstract
Cryptosporidium and Giardia are important intestinal zoonotic pathogens that can infect various hosts and cause diarrhoeal diseases. There are few reports of the epidemiological prevalence and molecular characterization of Cryptosporidium and Giardia in wild birds around Qinghai Lake and in the surrounding areas on the Qinghai-Tibetan Plateau, Northwest China. Therefore, the aim of this study was to determine the Cryptosporidium spp. and Giardia duodenalis genotypes and their epidemiological prevalence in wild birds by PCR amplification. To our knowledge, this is the first report of a variety of Cryptosporidium spp. and G. duodenalis infections in wild birds from that area, with overall prevalence rates of 8.98% (61/679) and 3.39% (23/679), respectively. Furthermore, PCR sequencing confirmed the presence of Cryptosporidium baileyi (n = 3), Cryptosporidium parvum (n = 58), and G. duodenalis assemblage B (n = 19) and E (n = 4) in wild birds from the areas around Qinghai Lake. The results of the present study demonstrated the wide distribution of Cryptosporidium and Giardia among wild birds, which has potential public health significance. Moreover, the study findings also provided useful molecular epidemiological data for monitoring and investigating the two parasitic protozoa in wild animals and surrounding environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryptosporidium spp. and Giardia spp. are common zoonotic enteric protozoan parasites that can infect a wide range of vertebrate hosts, including humans, mammals, and domestic and wild animals worldwide (Feng et al. 2018; Heyworth 2016; Plutzer et al. 2010; Ryan et al. 2014). Currently, birds are mainly infected by four avian Cryptosporidium species based on biological characteristics and genetic differences: Cryptosporidium meleagridis, Cryptosporidium baileyi, Cryptosporidium avium, and Cryptosporidium galli (Plutzer and Karanis 2009; Wang et al. 2019). Meanwhile, other Cryptosporidium species (Cryptosporidium andersoni, Cryptosporidium parvum, Cryptosporidium hominis, Cryptosporidium muris) and genotypes (Cryptosporidium goose genotypes (I–IV), a Cryptosporidium duck genotype, and Cryptosporidium avian genotypes (I–IV)) have also been reported in birds in previous studies (Cui et al. 2018; Nakamura and Meireles 2015; Ryan 2010). C. meleagridis is considered the third most prevalent species known to infect humans after C. hominis and C. parvum (Braima et al. 2019; Una et al. 2001). Based on multiple gene loci analysis, Xiao et al. (2002) have suggested that mammals were the original hosts of C. meleagridis. In general, many of the Cryptosporidium species and genotypes have a host specificity and are not usually considered a public health concern. However, some hosts carry zoonotic species, which contribute to cross-infection between host species (Braima et al. 2019). Furthermore, some genetically distinct avian Cryptosporidium genotypes/species, such as Cryptosporidium avian genotype I, C. avium and C. proventriculi in Psittaciform birds (Holubova et al. 2019), C. parvum in falcons (Azmanis et al. 2018), C. parvum and Cryptosporidium genotype BrPR1 in free-range chickens (Ewald et al. 2017), and Cryptosporidium duck genotype in a mandarin duck (Aix galericulata) (da Cunha et al. 2017), have recently been reported. Likewise, two species of Giardia have been recognized in avian hosts based on the morphology of trophozoites and cysts: G. ardeae and G. psittaci (Ryan and Caccio 2013). Other species/assemblages have been described from bird hosts; for instance, G. duodenalis assemblage A has been detected in a toco toucan (Ramphastos toco) (da Cunha et al. 2017) and G. duodenalis assemblage B, assemblage D, and assemblage F in wild birds from northwest Spain (Reboredo-Fernandez et al. 2015). Assemblages A and B are considered to be zoonotic and pathogenic to humans (Ryan and Caccio 2013).
Previous studies have confirmed that Cryptosporidium and Giardia are prevalent in livestock and wild animals (Itagaki et al. 2005; Jian et al. 2018; Oates et al. 2012; Wang et al. 2017, 2018a; Zhang et al. 2018a; Ziegler et al. 2007). Moreover, these two parasitic pathogens have attracted increasing attention, resulting in a series of epidemiological investigations focusing on public and veterinary health. Recently, Cryptosporidium and Giardia have been considered emerging pathogens in poultry and wild bird groups and are becoming prevalent parasites affecting domestic, caged, ornamental, companion, and wild birds. Infection of economic poultry (laying and meat chickens, ducks, and geese) with these two parasites may lead to extensive economic losses (Batz et al. 2012; Holubova et al. 2018; Majewska et al. 2009). Wang et al. (2012) found a 13.1% prevalence of Cryptosporidium from 47 quail farms in Henan, China, where C. baileyi was found in the majority of the positive samples. C. baileyi is generally associated with the respiratory form of cryptosporidiosis in birds and capable of infecting a variety of avian hosts. Most studies have focused on domestic animals (cattle, sheep, goat, yak, horse, chicken, and pig) of commercial interest (Hu et al. 2017; Li et al. 2016a; Majewska et al. 2009; Petersen et al. 2015; Qi et al. 2015, 2019; Squire et al. 2017; Wang et al. 2018a, 2018b; Zhong et al. 2018). McEvoy and Giddings (2009) have reported that while C. parvum was detected on a large turkey farm and post slaughter, C. parvum was not a significant reservoir for Cryptosporidium species. In comparison, relatively fewer studies involved wild birds infected with Cryptosporidium and Giardia (Cano et al. 2016; da Cunha et al. 2017; Majewska et al. 2009; Plutzer and Tomor 2009; Reboredo-Fernandez et al. 2015). Notably, various studies have identified and demonstrated the occurrence of the zoonotic species C. parvum in wild birds, suggesting that infected birds may play an important role in harbouring and disseminating this parasitic pathogen (Plutzer and Tomor 2009; Reboredo-Fernandez et al. 2015). For the zoonotic G. duodenalis assemblages, A and B have also been reported in birds (Cano et al. 2016; da Cunha et al. 2017; Plutzer and Tomor 2009).
Qinghai Lake is located in the north eastern part of the Qinghai-Tibetan Plateau (QTP), with an altitude of approximately 3200 m, covering an area of approximately 4500 km2, and with a circumference of more than 360 km. The sources of water for the lake are from rivers, precipitation, and a spring at the bottom of the lake. The most important water sources are rivers, with more than 40 rivers that, including the Buha River, Shaliu River, Wuha Alam River, and Haage River, deposit into the lake. There are many more rivers on the southwest, northwest, and north coast, with large drainage areas and many tributaries. The environmental conditions and geographic location of Qinghai Lake make it a suitable habitat for wild birds; there are 220 species and more than 160,000 birds, including the bar-headed goose (Anser indicus), brown-headed gull (Chroicocephalus brunnicephalus), great cormorant (Phalacrocorax carbo), Crested duck (Anas platyrhynchos domesticus), ruddy shelduck (Tadorna ferruginea), common merganser (Mergus merganser), Chinese spot-billed duck (Anas zonorhyncha), Northern pintail (Anas acuta), whooper swan (Cygnus cygnus), and black-necked crane (Grus nigricollis), reported from the area. Further, a small study has identified 5/148 (3.38% prevalence) positive samples for C. baileyi genotypes in ruddy shelducks from the Qinghai Lake (Amer et al. 2010). Qinghai Lake has become a major breeding site for migratory birds flying to Australia, India, Siberia, and Southeast Asia via the Central Asian-Indian flyway and the East Asian-Australian flyway (Dong et al. 2017).
However, very few studies on the presence of Cryptosporidium and Giardia in wild birds have been performed in this area. The aim of this study was to determine the prevalence and molecular characterization of Cryptosporidium and Giardia species/genotypes in faecal samples from wild birds around Qinghai Lake on the QTP of China.
Materials and methods
Study sites
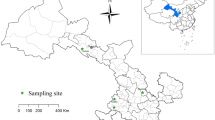
The faecal samples analysed in the present study were collected from wild birds at different locations around Qinghai Lake on the QTP of China (see Fig. 1). The sampling sites were located in the northern (Quanji River estuary, Fairy Bay, Shaliu River estuary, Hadatan wetlands, Naren wetlands and Sheng River estuary) and western (bird rescue centre, Egg Island, Bird Island Park and Cormorant Island) parts of Qinghai Lake, including the river estuaries, wetlands, and islands. These areas are all breeding sites and suitable habitats for wild birds.
Distribution of the sample collection locations (●) in this study. Qinghai Lake is located on the Qinghai-Tibetan Plateau in China. The five-pointed star (★) represents Qinghai Lake, and the number represents the sampling site (sampling site names: 1: Haixinshan Island, 2: Bird Rescue Center, 3: Egg Island, 4: Bird Island Park, 5: Cormorant Island, 6: Quanji River Estuary, 7: Fairy Bay, 8: Shaliu River Estuary, 9: Hadatan Wetlands, 10: Naren Wetlands, 11: Sheng River Estuary)
Specimen collection
A total of 679 individual wild bird faecal samples were collected from the ground around Qinghai Lake in 2016 and 2018. Fresh faecal samples were preferentially chosen when available. The samples were collected on site in cooperation with the staff members of the Qinghai Lake National Nature Reserve Administration, and they were fresh at the time of collection. Upon observing groups of birds, the observers walked towards them and collected the faeces. The main bird species were brown-headed gull, bar-headed goose, great cormorant, and great black-headed gull (Larus ichthyaetus). Each individual fresh faecal sample was placed in a sterile polystyrene tube (50-ml centrifuge tube) with records of the date, location, and identification number. The samples were kept in 2.5% potassium dichromate and transferred in isothermal boxes to the laboratory in Xining where they were stored at − 20 °C until DNA extraction. The total genomic DNA was extracted from each faecal sample with a QIAamp Fast DNA Stool Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions, with the addition of 10 freeze-thaw cycles.
Molecular characterization of Cryptosporidium and Giardia spp.
A two-step nested-PCR technique was performed to amplify a fragment of the 18S rRNA gene to detect Cryptosporidium oocysts. The expected length fragments were obtained after primary amplification with the primers 18SiCF2: 5′-GACATATCA TTCAAGTTTCTGACC-3′ and 18SiCR2: 5′-CTGAAGGAGTAAGGAACAACC-3′; the product was approximately 763 bp. The secondary amplification was conducted with the primers 18SiCF1: 5′-CCTATCAGCTTTAGACGGTAGG-3′ and 18SiCR1: 5′-TCTAAGAATTTCACCTCTGACTG-3′, generating a corresponding 587-bp product (Ryan et al. 2003). Both PCRs were performed with standard mixtures of 50 μl containing 4 μl primer mixtures (10 μM of each primer), 2 μl dNTP mix (10 mM of each dNTP), 5 μl 10 × PCR buffer containing 1.5 mM MgCl2 (Qiagen), 3 μl 3 mM MgCl2 (Qiagen), 0.5 μl 5 U HotStart Taq DNA Polymerase (Qiagen), 3 μl bovine serum albumin (BSA; acetylated, 10 mg/mL) (Promega), 2.5 μl DNA, and 30 μl PCR-grade water. For the primary PCRs, the amplification reactions were run according to the following PCR programme: an initial heat-activation step at 95 °C for 15 min; 35 cycles of 94 °C for 35 s, 58 °C for 35 s, and 72 °C for 50 s; then 72 °C for 10 min and a final hold at 4 °C. For the secondary PCRs, each reaction was prepared as for the primary PCR, but 18SiCF1/R1 primers were used, and the following PCR programme was run: 95 °C for 15 min; 35 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s; then 72 °C for 10 min and a final hold at 4 °C. For the molecular detection of Giardia, a nested PCR was also performed to amplify a 292-bp fragment of the Giardia 18S rRNA gene locus according to Appelbee et al. (2003) to detect Giardia cysts. The protocol used to detect Cryptosporidium, except the primers and the PCR programme, was different as follows: the primary primers used were Gia2029F: 5′-AAG TGT GGT GCA GAC GGA CTC-3′ and Gia2150R: 5′-CTG CTG CCG TCC TTG GAT GT-3′; the secondary primers used were RH11 5′-CAT CCG GTC GAT CCT GCC-3′ and RH4 5′-AGT CGA ACC CTG ATT CTC CGC CAG G-3′; and the annealing temperatures were 55 °C and 59 °C, respectively. A positive control and negative control were included in each amplification. The amplified PCR products were analysed using 1.5% agarose gel containing ethidium bromide (0.6 mg/mL) and were observed under UV light.
Sequencing and phylogenetic analysis
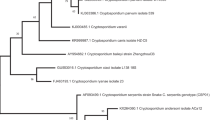
The positive PCR products were sequenced by SUZHOU GENEWIZ Company (Suzhou, China). To confirm their genotypes, the sequences were analysed by Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) and BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) alignment with reference sequences in GenBank. The phylogenetic analyses of Cryptosporidium and Giardia were performed with the neighbour-joining (NJ) method, which was calculated by the Jukes-Cantor model with 2000 bootstrap replicates.
Results
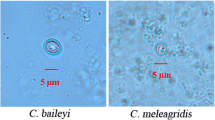
In this study, a total of 679 fresh wild bird faecal samples were collected from different locations around Qinghai Lake on the QTP of China (Fig. 1) from 2016 to 2018 to study the prevalence of Cryptosporidium and Giardia by PCR and sequencing analysis. Among the samples, 61 specimens were Cryptosporidium-positive, and 23 were Giardia-positive, as confirmed by PCR amplification of the rRNA genes, with infection rates of 8.98% (61/679) and 3.39% (23/679), respectively. In detail, the results showed that Cryptosporidium spp. infection in wild birds was prevalent at the bird rescue centre, Egg Island, Quanji River estuary, and Fairy Bay. Notably, Giardia infection in wild birds was found in more places: the bird rescue centre, Egg Island, Fairy Bay, Shaliu River estuary, the Hadatan wetlands, and the Naren wetlands. The numbers of co-infections of Cryptosporidium and Giardia were three for Egg Island (C. parvum, n = 3, G. duodenalis assemblage B, n = 3) and one for Fairy Bay (C. parvum, n = 1, G. duodenalis assemblage B, n = 1). For Cryptosporidium spp., sequencing and phylogenetic analyses identified the following: fifteen Cryptosporidium-positive faecal samples were detected from the bird rescue centre (15/153, 9.80%), 41 from Egg Island (41/311, 13.18%), one from the Quanji River estuary (1/17, 5.88%), and four from Fairy Bay (4/95, 4.21%); the species were identified as C. parvum (n = 58) and C. baileyi (n = 3). The sequencing and phylogenetic analyses of Giardia were as follows: five Giardia-positive faecal samples were detected from the bird rescue centre (5/153, 3.27%, assemblage B), 12 from Egg Island (12/311, 3.86%, assemblages E (n = 1) and B (n = 11), three from Fairy Bay (3/95, 3.16%, assemblage B), and one each from the Shaliu River estuary (1/21, 4.76%), Hadatan wetlands (1/10, 10.00%), and Naren wetlands (1/14, 7.14%); all identified as assemblage E.
For Cryptosporidium spp., the partial sequences of the 18S rRNA locus identified the species C. baileyi, with 97% similarity with C. baileyi (MH062741/2, MF498750, KY448455/6/8, KY352487/8/9) with a query coverage of 99%. For C. parvum, there was 100% similarity with C. parvum (MF589923, MH477699, MH074867, KY514066, KT948751, KP994663, KP730314, KP004203, KJ808693, KC886318, EU553550, EF175936, DQ833278, DQ656354, AJ853993/4, AJ849463, AF308600) with a query coverage of 100%. With respect to Giardia spp., the partial sequences of the 18S rRNA determined the presence of G. duodenalis assemblage E, which showed 100% similarity to assemblage E (MK573336/28, MG958618, KF843921, JF957620, KR048478-91) with a query coverage of 100%. Moreover, G. duodenalis assemblage B presented 100% similarity with assemblage B (MG018739, KY658186/7, JX972180, HQ616612) with a query coverage of 100%. The nucleotide sequences identified in our study were deposited in the GenBank database under the accession numbers MK992409-MK992469 for Cryptosporidium and MK993304-MK993326 for Giardia. The phylogenetic analyses employing the NJ method indicated that all 18S rRNA representative gene sequences of the Cryptosporidium and Giardia species identified in the present study formed well-defined clusters with their respective reference sequences (Figs. 2 and 3).
Discussion
This is the first large parasitological study involving the molecular characterization and epidemiological prevalence detection of Cryptosporidium and Giardia species in wild birds around Qinghai Lake on the QTP in China. For this location and the wild birds in this area, the main focus so far has been on avian influenza. Little is known about the occurrence of Cryptosporidium and/or Giardia in wild birds here, with only one study to date (Amer et al. 2010). Many researchers suggest to monitor migratory waterfowl as a model for potential source contamination for water supplies that extend to humans, farms, and wildlife (Rao et al. 2009). Most previous studies from other locations around the world have also focused on the transmission of these two parasitic pathogens in aquatic and migratory birds (Cano et al. 2016; Elmore et al. 2013; Plutzer and Tomor 2009).
The overall prevalence of Cryptosporidium in the samples was 8.98%, as determined by PCR analysis. Prevalence data from other studies on waterbirds (see Table 1) indicate a wide scope of infection rates, ranging from 0.5% in Canada geese (Branta canadensis) (Zhou et al. 2004) to 100% in ducks (da Cunha et al. 2017; Kuhn et al. 2002), the black-headed gull (Chroicocephalus ridibundus) (Medema 1999), great cormorant (Plutzer and Tomor 2009), and hooded merganser (Lophodytes cucullatus) (Kuhn et al. 2002), although in all cases with 100% infection rates, sample size was small, with only one to three faecal samples analysed. Similar results to ours have been reported in various species, with infection rates of 2% and 3.4% in the Greylag goose (Anser anser) (Plutzer and Tomor 2009), 2.8% in the common merganser (Majewska et al. 2009), 3.38% in the ruddy shelduck (Amer et al. 2010), 0.5 to 6.8% in the Canada goose (Jellison et al. 2004; Zhou et al. 2004), 4.95% in the black-headed gull and European herring gull (Larus argentatus) (Smith et al. 1993), 7.7% in the White stork (Ciconia ciconia) (Reboredo-Fernandez et al. 2015), and 8% in the great cormorant (Rzymski et al. 2017). Moreover, infection with Cryptosporidium is found in a wide geographic range (Table 1). Most studies are from Europe, including the Czech Republic (Pavlasek 1993), Germany (Richter et al. 1994), Hungary (Plutzer and Tomor 2009), the Netherlands (Medema 1999), Poland (Majewska et al. 2009; Rzymski et al. 2017), Scotland (Smith et al. 1993), and Spain (Cano et al. 2016; Reboredo-Fernandez et al. 2015), and the Americas, with the USA (Jellison et al. 2004; Kassa et al. 2004; Kuhn et al. 2002; Zhou et al. 2004) and Brazil (Bomfim 2013; da Cunha et al. 2017; Nakamura et al. 2009). There are fewer studies from Asia, including China (Amer et al. 2010; Wang et al. 2010), Iran (Larki et al. 2018; Shemshadi et al. 2014, 2016), and Thailand (Koompapong et al. 2014) with one study from Australia (Ng et al. 2006). It is obvious that Cryptosporidium is a widespread parasite, widely dispersed among waterbirds and continents.
Additionally, Cryptosporidium has been found in domestic, captive and wild terrestrial avian hosts worldwide (Nakamura and Meireles 2015). Studies on domestic birds, for example, have reported prevalence rates of 0.82% in domestic pigeons in Guangdong Province, southern China (Li et al. 2015); 3.8% in free-ranging, captive, and domestic birds in western Poland (Majewska et al. 2009); 7% in carrier pigeons in Brazil (Oliveira et al. 2017a); 7.03% in turkeys and chickens in Germany (Helmy et al. 2017); 10.20% in farmed chickens in Hubei Province, China (Liao et al. 2018); 12.6% in three chicken production systems in Brazil (Santana et al. 2018); 13.1% in farmed quail in Henan, China (Wang et al. 2012); 14.8% in poultry in Brazil (da Cunha et al. 2018); 15.8% in 3 large farm turkeys flocks in America (McEvoy and Giddings 2009); 25.6% in free-range chickens in Brazil (Ewald et al. 2017); and 50% in domestic ducks in Ahvaz, southwest Iran (Larki et al. 2018). Studies on captive birds have reported prevalence rates of 2.3% in captive birds in Brazil (da Cunha et al. 2017); 3.22% in pet parrots in North China (Zhang et al. 2015); 5% in caged exotic psittacines in Brazil (Ferrari et al. 2018); 9.1% in companion birds in Japan (Iijima et al. 2018); 10.64% in wild captive psittacines in Brazil (Oliveira et al. 2017b); and 19.1% in introduced monk parakeets (Myiopsitta monachus) in Santiago, Chile (Briceno et al. 2017). Studies on wild terrestrial avian hosts have reported prevalence rates of 11.7% in wild quail in the rolling plains ecoregion of Texas and Oklahoma, USA (Xiang et al. 2017); 13.42% in Java sparrows (Lonchura oryzivora) of northern China (Yao et al. 2017); and 17.1% in North American red-winged blackbirds (Agelaius phoeniceus) in the USA (Chelladurai et al. 2016). Cryptosporidium seems to be widely dispersed among all avian taxa with implications for transmission of infections to humans via environmental media, such as contamination of water sources by waterbirds, and direct transmission via food and companion birds.
The prevalence of Cryptosporidium was between Cryptosporidium infection rates found in environmental media, e.g. sewage and river water (2.2%) (Ma et al. 2019) and water samples (27.3%) (Ma et al. 2014b). Compared to studies on livestock, the pattern is varied, with infection rates in livestock ranging from lower values than this study, e.g. yaks (2.53%) (Ren et al. 2019), to mostly similar and some higher rates, e.g. 1–2-month-old highland yaks (11.3%) (Wang et al. 2018a), young domestic farm animals (cattle (14.4%) and sheep (6.2%)) (Zhang et al. 2018b), Tibetan sheep (12.3%) and yaks (28.5%) (Li et al. 2016b), yaks (30.0%) (Ma et al. 2014a), yaks (24.2%) (Mi et al. 2013), farm yaks (12.5%), and farm goats (35.7%) (Karanis et al. 2007) on the QTP in China. Interestingly, similar rates to this study have been found in wild animals, e.g. Qinghai voles (8.9%), and wild plateau pikas (6.25%) (Zhang et al. 2018a), but much higher ones have been found in zoo animals (80%) (Karanis et al. 2007). In comparison, the prevalence of Giardia in this study (3.39%) was less than Giardia infection rates in environmental media, e.g. water samples (15.4%) (Ma et al. 2014b) and sewage and river waters (21.3%) (Ma et al. 2019) and similar to studies in livestock, e.g. 1–2-month-old highland yaks (5.2%) (Wang et al. 2018a), Zangxiang pigs (6.2%) (Zhang et al. 2019), cattle (10%) (Jian et al. 2018), Tibetan sheep (13.1%) and yaks (10.4%) (Jin et al. 2017), and 4–7-month-old yaks (5.4%) (Wang et al. 2017) on the QTP in China. The large differences in the prevalence of Cryptosporidium spp. and Giardia spp. can be attributed to factors including project design and sample collection methods, bird population movement and density, and the intervention of livestock (yaks, cattle, and sheep) and humans.
The prevalence of Giardia in this study (3.39%) was generally lower than that reported in previous studies carried out in other locations. In studies on waterbirds (see Table 2), only prevalence rates of 1.4% in common merganser and 4.2% in White stork in Poland (Majewska et al. 2009) and 2% in Greylag geese at Lake Balaton in Hungary (Plutzer and Tomor 2009) were similar to the results of this study. All other studies on waterbirds reported higher prevalence rates (Table 2), ranging from 8.3% in ‘waterbirds’ (Cano et al. 2016) to 100% in Northern pintail (Kuhn et al. 2002), White stork (Franssen et al. 2000), and great cormorant (Plutzer and Tomor 2009). However, in all cases with 100% infection rates, only one faecal sample was analysed per study. Moreover, infection of wild waterbirds with Giardia is widespread but found in less regions than Cryptosporidium (Table 2). Studies from Europe include Hungary (Plutzer and Tomor 2009), the Netherlands (Franssen et al. 2000), Poland (Majewska et al. 2009), and Spain (Cano et al. 2016; Reboredo-Fernandez et al. 2015). Studies from other regions are fewer, with two from the USA (Erlandsen et al. 1990; Kuhn et al. 2002) and Australia (Forshaw et al. 1992; McRoberts et al. 1996) and one from Asia (Iran) (Shemshadi et al. 2014).
In other avian taxa, prevalence rates ranged from 1.2% in captive birds in Brazil (da Cunha et al. 2017) and 5.2% in free-ranging, captive, and domestic birds in western Poland (Majewska et al. 2009) to 25.9% in captive Psittaciformes in Brazil (Ichikawa et al. 2019).
From the Cryptosporidium species detected in this study, the common species infecting the birds was C. baileyi (3 isolates), which was found at two sites and was also identified in yaks from Qinghai Province on the QTP with a prevalence rate of 3.85% (Ren et al. 2019). The zoonotic C. parvum genotype (58 isolates) was predominant with 8.54% prevalence; this genotype was also identified in water samples (Ma et al. 2019), yaks (Mi et al. 2013; Wang et al. 2018a), Qinghai voles, wild plateau pikas (Zhang et al. 2018a), and domestic farm animals (cattle and sheep) (Zhang et al. 2018b) on the QTP in China. With respect to G. duodenalis detected in this study, 19 isolates were identified as assemblage B, which was also detected in Zangxiang pigs (Zhang et al. 2019) and yaks (Wang et al. 2018a). Importantly, among the eight G. duodenalis assemblages, assemblage B is primarily associated with humans, livestock, and wild animals, which suggests that the presence of assemblage B in wild birds is a cause of public health concern. On the other hand, 4 isolates were identified as assemblage E, which was also found in water samples (Ma et al. 2019), Zangxiang pigs (Zhang et al. 2019), yaks (Wang et al. 2018a), cattle (Jian et al. 2018), and Tibetan sheep and yaks (Jin et al. 2017; Wang et al. 2017) on the QTP in China.
Wild birds around Qinghai Lake are mostly migratory; Qinghai Lake is a major breeding site on several migratory bird flyways. Previous studies on the global transmission of avian influenza viruses showed that the virus was spread to Mongolia, Russia, Europe, and Africa along bird migratory flyways (Dong et al. 2017). Similarly, these two zoonotic parasites, Cryptosporidium and Giardia, may also be transmitted during wild bird migration. Therefore, because of the geographic location of Qinghai Lake and the bird species present in the area, Cryptosporidium and Giardia may be of public health concern. Importantly, the surrounding areas of Qinghai Lake support human travel and sheep, goats, cattle, and yak grazing, and the water sources are shared with wild animals. In addition, if the wild birds are infected with Cryptosporidium and Giardia, these parasitic pathogens can spread into or out of the Qinghai Lake area when the wild birds migrate, resulting in a potential threat of further cross-contamination. Therefore, it is imperative to carry out epidemiological investigations in this area.
Conclusions
The 679 faecal samples collected from wild birds in Qinghai Lake areas were screened for the presence of Cryptosporidium and Giardia. To our knowledge, this is the first study to report a variety of protozoan pathogens (C. baileyi, C. parvum, and G. duodenalis assemblages B and E) in wild birds from the Qinghai Lake area. The results obtained in this study demonstrate the wide prevalence of Cryptosporidium and/or Giardia in wild birds. Further studies are needed to investigate seasonal effects and the effects of yaks, cattle, sheep, and human environmental factors on the transmission dynamics of Cryptosporidium and/or Giardia in wild birds on the QTP in China.
References
Amer S, Wang C, He H (2010) First detection of Cryptosporidium baileyi in Ruddy Shelduck (Tadorna ferruginea) in China. J Vet Med Sci 72:935–938
Appelbee AJ, Frederick LM, Heitman TL, Olson ME (2003) Prevalence and genotyping of Giardia duodenalis from beef calves in Alberta, Canada. Vet Parasitol 112:289–294
Azmanis P, di Somma A, Pappalardo L, Silvanose CD, Bangoura B (2018) First detection of Cryptosporidium parvum in falcons (Falconiformes): diagnosis, molecular sequencing, therapeutic trial and epidemiological assessment of a possible emerging disease in captive falcons. Vet Parasitol 252:167–172
Batz MB, Hoffmann S, Morris JG Jr (2012) Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J Food Prot 75:1278–1291
Bomfim TCB (2013) The importance of poultry in environmental dissemination of Cryptosporidium spp.. Open Vet. Sci J 7: 12–17
Braima K, Zahedi A, Oskam C, Reid S, Pingault N, Xiao L, Ryan U (2019) Retrospective analysis of Cryptosporidium species in Western Australian human populations (2015-2018), and emergence of the C. hominis IfA12G1R5 subtype. Infect Genet Evol 73:306–313
Briceno C, Surot D, Gonzalez-Acuna D, Martinez FJ, Fredes F (2017) Parasitic survey on introduced monk parakeets (Myiopsitta monachus) in Santiago, Chile. Rev Bras Parasitol Vet 26:129–135
Cano L, de Lucio A, Bailo B, Cardona GA, Muadica AS, Lobo L, Carmena D (2016) Identification and genotyping of Giardia spp. and Cryptosporidium spp. isolates in aquatic birds in the Salburua wetlands, Alava, Northern Spain. Vet Parasitol 221:144–148
Chelladurai JJ, Clark ME, Kvac M, Holubova N, Khan E, Stenger BL, Giddings CW, McEvoy J (2016) Cryptosporidium galli and novel Cryptosporidium avian genotype VI in North American red-winged blackbirds (Agelaius phoeniceus). Parasitol Res 115:1901–1906
Cui Z, Song D, Qi M, Zhang S, Wang R, Jian F, Ning C, Zhang L (2018) Revisiting the infectivity and pathogenicity of Cryptosporidium avium provides new information on parasitic sites within the host. Parasit Vectors 11:514
da Cunha MJR, Cury MC, Santin M (2017) Molecular identification of Enterocytozoon bieneusi, Cryptosporidium, and Giardia in Brazilian captive birds. Parasitol Res 116:487–493
da Cunha MJR, Cury MC, Santin M (2018) Molecular characterization of Cryptosporidium spp. in poultry from Brazil. Res Vet Sci 118:331–335
Dong J, Bo H, Zhang Y, Dong L, Zou S, Huang W, Liu J, Wang D, Shu Y (2017) Characteristics of influenza H13N8 subtype virus firstly isolated from Qinghai Lake region, China. Virol J 14:180
Elmore SA, Lalonde LF, Samelius G, Alisauskas RT, Gajadhar AA, Jenkins EJ (2013) Endoparasites in the feces of arctic foxes in a terrestrial ecosystem in Canada. Int J Parasitol Parasites Wildl 2:90–96
Erlandsen SL, Bemrick WJ, Wells CL, Feely DE, Knudson L, Campbell SR, van Keulen H, Jarroll EL (1990) Axenic culture and characterization of Giardia ardeae from the great blue heron (Ardea herodias). J Parasitol 76:717–724
Ewald MPC, Martins FDC, Caldart ET, Vieira FEG, Yamamura MH, Sasse JP, Barros LD, Freire RL, Navarro IT, Garcia JL (2017) The first study of molecular prevalence and species characterization of Cryptosporidium in free-range chicken (Gallus gallus domesticus) from Brazil. Rev Bras Parasitol Vet 26:472–478
Feng Y, Ryan UM, Xiao L (2018) Genetic diversity and population structure of Cryptosporidium. Trends Parasitol 34:997–1011
Ferrari ED, Nakamura AA, Nardi ARM, Santana BN, da Silva CV, Nagata WB, Bresciani KDS, Meireles MV (2018) Cryptosporidium spp. in caged exotic psittacines from Brazil: evaluation of diagnostic methods and molecular characterization. Exp Parasitol 184:109–114
Forshaw D, Palmer DG, Halse SA, Hopkins RM, Thompson RC (1992) Giardia in straw-necked ibis (Threskiornis spinicollis) in Western Australia. Vet Rec 131:267–268
Franssen FF, Hooimeijer J, Blankenstein B, Houwers DJ (2000) Giardiasis in a white stork in The Netherlands. J Wildl Dis 36:764–766
Helmy YA, Krucken J, Abdelwhab EM, von Samson-Himmelstjerna G, Hafez HM (2017) Molecular diagnosis and characterization of Cryptosporidium spp. in turkeys and chickens in Germany reveals evidence for previously undetected parasite species. PLoS One 12: e0177150
Heyworth MF (2016) Giardia duodenalis genetic assemblages and hosts. Paras 23:13
Holubova N, Sak B, Hlaskova L, Kvetonova D, Hanzal V, Rajsky D, Rost M, McEvoy J, Kvac M (2018) Host specificity and age-dependent resistance to Cryptosporidium avium infection in chickens, ducks and pheasants. Exp Parasitol 191:62–65
Holubova N, Zikmundova V, Limpouchova Z, Sak B, Konecny R, Hlaskova L, Rajsky D, Kopacz Z, McEvoy J, Kvac M (2019) Cryptosporidium proventriculi sp. n. (Apicomplexa: Cryptosporidiidae) in psittaciformes birds. Eur J Protistol 69:70–87
Hu S, Liu Z, Yan F, Zhang Z, Zhang G, Zhang L, Jian F, Zhang S, Ning C, Wang R (2017) Zoonotic and host-adapted genotypes of Cryptosporidium spp., Giardia duodenalis and Enterocytozoon bieneusi in dairy cattle in Hebei and Tianjin, China. Vet Parasitol 248:68–73
Ichikawa RS, Santana BN, Ferrari ED, do Nascimento IG, Nakamura AA, Nardi ARM, Meireles MV (2019) Detection and molecular characterization of Giardia spp. in captive psittaciformes in Brazil. Prev Vet Med 164: 10–12
Iijima Y, Itoh N, Phrompraphai T, Ito Y, Kimura Y, Kameshima S (2018) Molecular prevalence of Cryptosporidium spp. among companion birds kept in pet shops in Japan. Kor J Parasitol 56:281–285
Itagaki T, Kinoshita S, Aoki M, Itoh N, Saeki H, Sato N, Uetsuki J, Izumiyama S, Yagita K, Endo T (2005) Genotyping of Giardia intestinalis from domestic and wild animals in Japan using glutamate dehydrogenase gene sequencing. Vet Parasitol 133:283–287
Jellison KL, Distel DL, Hemond HF, Schauer DB (2004) Phylogenetic analysis of the hypervariable region of the 18S rRNA gene of Cryptosporidium oocysts in feces of Canada geese (Branta canadensis): evidence for five novel genotypes. Appl Environ Microbiol 70:452–458
Jian Y, Zhang X, Li X, Karanis G, Ma L, Karanis P (2018) Prevalence and molecular characterization of Giardia duodenalis in cattle and sheep from the Qinghai-Tibetan plateau area (QTPA), northwestern China. Vet Parasitol 250:40–44
Jin Y, Fei J, Cai J, Wang X, Li N, Guo Y, Feng Y, Xiao L (2017) Multilocus genotyping of Giardia duodenalis in Tibetan sheep and yaks in Qinghai, China. Vet Parasitol 247:70–76
Karanis P, Plutzer J, Halim NA, Igori K, Nagasawa H, Ongerth J, Liqing M (2007) Molecular characterization of Cryptosporidium from animal sources in Qinghai province of China. Parasitol Res 101:1575–1580
Kassa H, Harrington BJ, Bisesi MS (2004) Cryptosporidiosis: a brief literature review and update regarding Cryptosporidium in feces of Canada geese (Branta canadensis). J Environ Health 66(34–40):45
Koompapong K, Mori H, Thammasonthijarern N, Prasertbun R, Pintong AR, Popruk S, Rojekittikhun W, Chaisiri K, Sukthana Y, Mahittikorn A (2014) Molecular identification of Cryptosporidium spp. in seagulls, pigeons, dogs, and cats in Thailand. Paras21: 52
Kuhn RC, Rock CM, Oshima KH (2002) Occurrence of Cryptosporidium and Giardia in wild ducks along the Rio Grande River valley in southern New Mexico. Appl Environ Microbiol 68:161–165
Larki S, Alborzi A, Chegini R, Amiri R (2018) A preliminary survey on gastrointestinal parasites of domestic ducks in Ahvaz, Southwest Iran. Iran J Parasitol 13:137–144
Li J, Lin X, Zhang L, Qi N, Liao S, Lv M, Wu C, Sun M (2015) Molecular characterization of Cryptosporidium spp. in domestic pigeons (Columba livia domestica) in Guangdong Province, southern China. Parasitol Res 114:2237–2241
Li F, Wang H, Zhang Z, Li J, Wang C, Zhao J, Hu S, Wang R, Zhang L, Wang M (2016a) Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Beijing, China. Vet Parasitol 219:61–65
Li P, Cai J, Cai M, Wu W, Li C, Lei M, Xu H, Feng L, Ma J, Feng Y, Xiao L (2016b) Distribution of Cryptosporidium species in Tibetan sheep and yaks in Qinghai, China. Vet Parasitol 215:58–62
Liao C, Wang T, Koehler AV, Fan Y, Hu M, Gasser RB (2018) Molecular investigation of Cryptosporidium in farmed chickens in Hubei Province, China, identifies 'zoonotic' subtypes of C. meleagridis. Parasit Vectors 11: 484
Ma J, Cai J, Ma J, Feng Y, Xiao L (2014a) Occurrence and molecular characterization of Cryptosporidium spp. in yaks (Bos grunniens) in China. Vet Parasitol 202:113–118
Ma L, Sotiriadou I, Cai Q, Karanis G, Wang G, Wang G, Lu Y, Li X, Karanis P (2014b) Detection of Cryptosporidium and Giardia in agricultural and water environments in the Qinghai area of China by IFT and PCR. Parasitol Res 113:3177–3184
Ma L, Zhang X, Jian Y, Li X, Wang G, Hu Y, Karanis P (2019) Detection of Cryptosporidium and Giardia in the slaughterhouse, sewage and river waters of the Qinghai Tibetan plateau area (QTPA), China. Parasitol Res 118:2041–2051
Majewska AC, Graczyk TK, Slodkowicz-Kowalska A, Tamang L, Jedrzejewski S, Zduniak P, Solarczyk P, Nowosad A, Nowosad P (2009) The role of free-ranging, captive, and domestic birds of Western Poland in environmental contamination with Cryptosporidium parvum oocysts and Giardia lamblia cysts. Parasitol Res 104:1093–1099
McEvoy JM, Giddings CW (2009) Cryptosporidium in commercially produced turkeys on-farm and post slaughter. Lett Appl Microbiol 48:302–306
McRoberts KM, Meloni BP, Morgan UM, Marano R, Binz N, Eriandsen SL, Halse SA, Thompson RC (1996) Morphological and molecular characterization of Giardia isolated from the straw-necked ibis (Threskiornis spinicollis) in Western Australia. J Parasitol 82:711–718
Medema G (1999) Cryptosporidium and Giardia: new challenges to the water industry. University of Utrecht, Dissertation
Mi R, Wang X, Li C, Huang Y, Zhou P, Li Z, Lei M, Cai J, Chen Z (2013) Prevalence and genetic characterization of Cryptosporidium in yaks in Qinghai Province of China. PLoS One 8:e74985
Nakamura AA, Meireles MV (2015) Cryptosporidium infections in birds--a review. Rev Bras Parasitol Vet 24:253–267
Nakamura AA, Simoes DC, Antunes RG, da Silva DC, Meireles MV (2009) Molecular characterization of Cryptosporidium spp. from fecal samples of birds kept in captivity in Brazil. Vet Parasitol 166:47–51
Ng J, Pavlasek I, Ryan U (2006) Identification of novel Cryptosporidium genotypes from avian hosts. Appl Environ Microbiol 72:7548–7553
Oates SC, Miller MA, Hardin D, Conrad PA, Melli A, Jessup DA, Dominik C, Roug A, Tinker MT, Miller WA (2012) Prevalence, environmental loading, and molecular characterization of Cryptosporidium and Giardia isolates from domestic and wild animals along the Central California coast. Appl Environ Microbiol 78:8762–8772
Oliveira BCM, Ferrari ED, da Cruz Panegossi MF, Nakamura AA, Corbucci FS, Nagata WB, Dos Santos BM, Gomes JF, Meireles MV, Widmer G, Bresciani KDS (2017a) First description of Cryptosporidium parvum in carrier pigeons (Columba livia). Vet Parasitol 243:148–150
Oliveira BCM, Nagata WB, Arana DG, Ferreira GC, Sitton HA, de Oliveira MRF, Meireles MV (2017b) Cryptosporidium baileyi in wild captive psittacines in Brazil. Vet Parasitol Reg Stud Reports 10:154–156
Pavlasek I (1993) The black-headed gull (Larus ridibundus L.), a new host for Cryptosporidium baileyi (Apicomplexa: Cryptosporidiidae). Vet Med (Praha) 38:629–638
Petersen HH, Jianmin W, Katakam KK, Mejer H, Thamsborg SM, Dalsgaard A, Olsen A, Enemark HL (2015) Cryptosporidium and Giardia in Danish organic pig farms: seasonal and age-related variation in prevalence, infection intensity and species/genotypes. Vet Parasitol 214:29–39
Plutzer J, Karanis P (2009) Genetic polymorphism in Cryptosporidium species: an update. Vet Parasitol 165:187–199
Plutzer J, Tomor B (2009) The role of aquatic birds in the environmental dissemination of human pathogenic Giardia duodenalis cysts and Cryptosporidium oocysts in Hungary. Parasitol Int 58:227–231
Plutzer J, Torokne A, Karanis P (2010) Combination of ARAD microfibre filtration and LAMP methodology for simple, rapid and cost-effective detection of human pathogenic Giardia duodenalis and Cryptosporidium spp. in drinking water. Lett Appl Microbiol 50:82–88
Qi M, Zhou H, Wang H, Wang R, Xiao L, Arrowood MJ, Li J, Zhang L (2015) Molecular identification of Cryptosporidium spp. and Giardia duodenalis in grazing horses from Xinjiang, China. Vet Parasitol 209:169–172
Qi M, Zhang Z, Zhao A, Jing B, Guan G, Luo J, Zhang L (2019) Distribution and molecular characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi amongst grazing adult sheep in Xinjiang, China. Parasitol Int 71:80–86
Rao JR, Cherie Millar B, Moore JE (2009) Avian influenza, migratory birds and emerging zoonoses: unusual viral RNA, enteropathogens and Cryptosporidium in poultry litter. Biosci Hypotheses 2:363–369
Reboredo-Fernandez A, Ares-Mazas E, Caccio SM, Gomez-Couso H (2015) Occurrence of Giardia and Cryptosporidium in wild birds in Galicia (Northwest Spain). Parasitol 142:917–925
Ren M, Wu F, Wang D, Li LY, Chang JJ, Lin Q (2019) Molecular typing of Cryptosporidium species identified in fecal samples of yaks (Bos grunniens) of Qinghai Province, China. J Parasitol 105:195–198
Richter D, Wiegand-Tripp G, Burkhardt E, Kaleta EF (1994) Natural infections by Cryptosporidium sp. in farm-raised ducks and geese. Avian Pathol 23:277–286
Ryan U (2010) Cryptosporidium in birds, fish and amphibians. Exp Parasitol 124:113–120
Ryan U, Caccio SM (2013) Zoonotic potential of Giardia. Int J Parasitol 43:943–956
Ryan U, Xiao L, Read C, Zhou L, Lal AA, Pavlasek I (2003) Identification of novel Cryptosporidium genotypes from the Czech Republic. Appl Environ Microbiol 69:4302–4307
Ryan U, Fayer R, Xiao L (2014) Cryptosporidium species in humans and animals: current understanding and research needs. Parasitol 141:1667–1685
Rzymski P, Slodkowicz-Kowalska A, Klimaszyk P, Solarczyk P, Poniedzialek B (2017) Screening of protozoan and microsporidian parasites in feces of great cormorant (Phalacrocorax carbo). Environ Sci Pollut Res Int 24:9813–9819
Santana BN, Kurahara B, Nakamura AA, da Silva CV, Ferrari ED, da Silva GS, Nagata WB, Meireles MV (2018) Detection and characterization of Cryptosporidium species and genotypes in three chicken production systems in Brazil using different molecular diagnosis protocols. Prev Vet Med 151:73–78
Shemshadi B, Ranjbar-bahadori S, Faghihzadeh-gorji S (2014) Occurrence of parasitic protozoa in wild waterfowl in southern coastal Caspian Sea lagoons. Iran J Vet Med 8:261–267
Shemshadi B, Ranjbar-bahadori S, Delfan-abazari M (2016) Prevalence and intensity of parasitic infection in domestic ducks (Anas platyrhynchas) in Gilan Province, Northern Iran. Compar Clin Pathol 26:165–167
Smith HV, Brown J, Coulson JC, Morris GP, Girdwood RW (1993) Occurrence of oocysts of Cryptosporidium spp. in Larus spp. gulls. Epidemiol Infect 110:135–143
Squire SA, Yang R, Robertson I, Ayi I, Ryan U (2017) Molecular characterization of Cryptosporidium and Giardia in farmers and their ruminant livestock from the coastal Savannah zone of Ghana. Infect Genet Evol 55:236–243
Una MM, Paul TM, Lihua X, Josef L, Irshad S, Shane R, Peter OD, Robin G, Allan M, Ronald F, Byron LB, Altaf AL (2001) Molecular and phylogenetic characterisation of Cryptosporidium from birds. Int J Parasit 31:289–296
Wang R, Jian F, Sun Y, Hu Q, Zhu J, Wang F, Ning C, Zhang L, Xiao L (2010) Large-scale survey of Cryptosporidium spp. in chickens and Pekin ducks (Anas platyrhynchos) in Henan, China: prevalence and molecular characterization. Avian Pathol 39:447–451
Wang R, Wang F, Zhao J, Qi M, Ning C, Zhang L, Xiao L (2012) Cryptosporidium spp. in quails (Coturnix coturnix japonica) in Henan, China: molecular characterization and public health significance. Vet Parasitol 187:534–537
Wang G, Wang GP, Li XP, Ma LQ, Karanis G, Christodoulou-Vafeiadou E, Karanis P (2017) Detection of Giardia duodenalis assemblage E infections at the Tibetan Plateau Area: yaks are suitable hosts. Acta Trop 169:157–162
Wang G, Wang G, Li X, Zhang X, Karanis G, Jian Y, Ma L, Karanis P (2018a) Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in 1-2-month-old highland yaks in Qinghai Province, China. Parasitol Res 117:1793–1800
Wang H, Zhang Y, Wu Y, Li J, Qi M, Li T, Wang J, Wang R, Zhang S, Jian F, Ning C, Zhang L (2018b) Occurrence, molecular characterization, and assessment of zoonotic risk of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi in pigs in Henan, Central China. J Eukaryot Microbiol 65:893–901
Wang K, Gazizova A, Wang Y, Zhang K, Zhang Y, Chang Y, Cui Y, Zhang Y, Zhang S, Zhang L (2019) First detection of Cryptosporidium spp. in migratory whooper swans (Cygnus cygnus) in China. Microorg 8: 6
Xiang L, Guo F, Yu Y, Parson LS, LaCoste L, Gibson A, Presley SM, Peterson M, Craig TM, Rollins D, Fedynich AM, Zhu G (2017) Multiyear survey of coccidia, Cryptosporidia, Microsporidia, Histomona, and Hematozoa in wild quail in the rolling plains ecoregion of Texas and Oklahoma, USA. J Eukar Microbiol 64:4–17
Xiao L, Sulaiman IM, Ryan UM, Zhou L, Atwill ER, Tischler ML, Zhang X, Fayer R, Lal AA (2002) Host adaptation and host-parasite co-evolution in Cryptosporidium: implications for taxonomy and public health. Int J Parasitol 32:1773–1785
Yao QX, Zhang XX, Chen K, Ma JG, Zheng WB, Xu XQ, Zhu XQ (2017) Prevalence and genetic characterization of Cryptosporidium infection in Java sparrows (Lonchura oryzivora) in Northern China. Biomed Res Int 2017:2318476
Zhang XX, Zhang NZ, Zhao GH, Zhao Q, Zhu XQ (2015) Prevalence and genotyping of Cryptosporidium infection in pet parrots in North China. Biomed Res Int 2015:549798
Zhang X, Jian Y, Li X, Ma L, Karanis G, Karanis P (2018a) The first report of Cryptosporidium spp. in Microtus fuscus (Qinghai vole) and Ochotona curzoniae (wild plateau pika) in the Qinghai-Tibetan Plateau area, China. Parasitol Res 117:1401–1407
Zhang X, Jian Y, Li X, Ma L, Karanis G, Qigang C, Karanis P (2018b) Molecular detection and prevalence of Cryptosporidium spp. infections in two types of domestic farm animals in the Qinghai-Tibetan Plateau Area (QTPA) in China. Parasitol Res 117:233–239
Zhang HJ, Song JK, Wu XM, Li YH, Wang Y, Lin Q, Zhao GH (2019) First report of Giardia duodenalis genotypes in Zangxiang pigs from China. Parasitol Res 118:2305–2310
Zhong Z, Tu R, Ou H, Yan G, Dan J, Xiao Q, Wang Y, Cao S, Shen L, Deng J, Zuo Z, Ma X, Zhou Z, Liu H, Yu S, Ren Z, Hu Y, Peng G (2018) Occurrence and genetic characterization of Giardia duodenalis and Cryptosporidium spp. from adult goats in Sichuan Province, China. PLoS One 13:e0199325
Zhou L, Kassa H, Tischler ML, Xiao L (2004) Host-adapted Cryptosporidium spp. in Canada geese (Branta canadensis). Appl Environ Microbiol 70:4211–4215
Ziegler PE, Wade SE, Schaaf SL, Stern DA, Nadareski CA, Mohammed HO (2007) Prevalence of Cryptosporidium species in wildlife populations within a watershed landscape in southeastern New York state. Vet Parasitol 147:176–184
Acknowledgements
We are grateful to the staff Yubang He, Yonglin Wu, and Yuansheng Hou working in Qinghai Lake National Nature Reserve Administration for their assistance with collecting the samples.
Funding
The research was supported by the National Natural Science Foundation of China (no. 32060804), the Young and Middle-Aged Research Funding Project from Qinghai University (2017-QNY-1) and the One Thousand Talents Plan (P. Karanis) of the Chinese Government (no. WQ2013630172).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Julia Walochnik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jian, Y., Zhang, X., Li, X. et al. Occurrence of Cryptosporidium and Giardia in wild birds from Qinghai Lake on the Qinghai-Tibetan Plateau, China. Parasitol Res 120, 615–628 (2021). https://doi.org/10.1007/s00436-020-06993-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06993-w