Abstract
The occurrence of Cryptosporidium and Giardia species in slaughter, sewage and river waters of the Qinghai Tibetan Plateau Area (QTPA), China, was investigated. A total of 456 samples were collected from different locations in the QTPA to study the contamination rates of Cryptosporidium spp. and Giardia via PCR and subsequent sequence analysis. Ten samples were Cryptosporidium positive, and 97 were Giardia positive, as confirmed by PCR amplification of the SSU rRNA gene. The percentages of positive Cryptosporidium and Giardia detection were 2.2% (10/456) and 21.3% (97/456), respectively. Cryptosporidium was detected in only sewage and river waters. Six species of Cryptosporidium were identified: Cryptosporidium hominis (n = 5), C. andersoni (n = 1), C. environmental (n = 1), C. struthionis (n = 1), C. canis (n = 1), and C. parvum (n = 1). G. duodenalis assemblage A was identified in almost all positive samples (n = 96), and one sample harboured G. duodenalis assemblage E. The results suggest that Cryptosporidium and Giardia species circulate through the aqueous environment and different hosts. Therefore, we strongly recommend that the local government and health authorities in China undertake control measures to reduce the contamination of water sources by these protozoa to protect the health of humans and animals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cryptosporidium and Giardia are the most common waterborne parasitic protozoa pathogens in many countries (Baldursson and Karanis 2011; Efstratiou et al. 2017; Karanis et al. 2007a). Good-quality water for human and animal consumption reduces the incidence of diseases. Cryptosporidium and Giardia species have received a considerable amount of attention worldwide over the past 40 years because they are the aetiological agents of water- and foodborne diseases (Ahmed and Karanis 2018a, b; Efstratiou et al. 2017; Mahmoudi et al. 2017; Rosado-García et al. 2017; Ryan et al. 2018). Cryptosporidium and Giardia are ubiquitous and responsible for cases of diarrhoea in humans, domestic and wild animals (Cacciò and Chalmers 2016; Garcia and Hayman 2016; Li et al. 2018; Plutzer and Karanis 2009, Plutzer et al. 2010). The dormant stage of Giardia duodenalis (syn. Giardia intestinalis, Giardia lamblia) is a cyst that measures 10.0–14.0 μm in length and 7.5–9.0 μm in width. These cysts are capable of surviving for 3 to 6 months in the environment outside of a host’s intestine (Plutzer et al. 2010). Oocysts of Cryptosporidium species are approximately 5.0–8.0 μm in diameter and represent the long-lasting form of the parasite (Ahmed and Karanis 2018a, b; Cacciò and Chalmers 2016; Plutzer and Karanis 2009; Upton and Current 1985). Oocysts are able to survive in the environment for 2 years. Because cysts and oocysts are highly stable in the environment and are resistant to disinfectants, it is difficult to control the risk of human exposure to contaminated water (Erickson and Ortega 2006; Karanis et al. 1996, 1998).
Domestic farm animals and wild animals are important sources of infection in humans, animals and the environment. Calves, yaks, sheep, other farm animals and wild animals play an important role in environmental contamination by excreting large quantities of (oo)cysts (Farizawati et al. 2005; Koehler et al. 2016; Li et al. 2018; Ma et al. 2014), which can contaminate the water sources used for humans and animals. Both Giardia and Cryptosporidium are orally ingested in humans and animals via contaminated drinking water, contaminated food, or direct contact. When a host becomes infected, (oo)cysts are released into the environment via faeces. Consequently, all surface water supplies used for drinking water are subject to parasitic contamination through human and animal excrements. When raw water is highly contaminated, the filtration barriers used to prepare drinking water may suffer from breakthrough events (Karanis et al. 1996, 1998). To minimise the risk of infections, the initial contamination level of raw water needs to be as low as possible. The Qinghai Tibetan Plateau area (QTPA) is located in northwestern China and is known as the “Three-River Source Area”, which is the source of three rivers (Changjiang River, Yellow River, and Lancangjiang River). Until recently, the main species and genotypes of Cryptosporidium and Giardia that circulate through the population (humans and animals) and their possible distribution in environmental samples in the QTPA have been poorly understood (Karanis et al. 2007b; Ma et al. 2014). Slaughterhouse, sewage and river waters are finally released into raw waters, which increases the risk of introducing parasites into raw water intended for drinking water preparation. Therefore, steps must be taken to protect the quality of the raw water supplies used to produce drinking water in provinces and megacities of China.
The slaughtering of millions of animals per year and processing of meats for human consumption generate large volumes of waste products, and most of these wastes are improperly managed in China and most countries of the world. Such activities result in the production of a high volume of animal-related waste materials, which are discharged into soils and raw water bodies. Environmental and health problems associated with this practice are not monitored and documented. Furthermore, large quantities of water are required during the production process to clean raw materials, machines and plants. Wastewater from butchers and slaughterhouses is heavily contaminated with fats and other organic substances, such as faeces and hair. Considerable quantities of solids from the intestines of animals are produced during slaughter.
We investigated the presence of Cryptosporidium spp. genotypes and Giardia assemblages, in slaughterhouse water, wastewater and river water samples obtained from different counties and locations of the QTPA, China, using PCR and sequencing.
Materials and methods
Site location and sampling
The water samples included natural river water, slaughterhouse water and sewage water collected from different locations in the QTPA. Domestic sewage water includes water from bathing, laundry, kitchens, shopping malls, sewage and washrooms. A total of 456 water samples were collected from different locations from 2015 to 2017, as shown in Tables 1, 2, and 3 and Fig. 1. In detail, 153 slaughterhouse water samples were collected from the Baide No. 1 and Baide No. 2 factories and Huangzhonghubang cattle farm, and 176 sewage water samples were collected from Huangyuan sewage, Nanchuan sewage, Xiushuiroad recycled water, a pharmaceutical factory and No. 2 sewage. The first factory is at the county level and does not have an existing rain sewage diversion method; thus, it processes all domestic sewage, industrial wastewater and storm water runoff. The last three factories are all at the city level are more advanced than the county-level factories. A total of 176 river water samples were obtained from several rivers. The Guoluo State River consists of small streams in pastoral areas and water without industrial processing, and this water is directly consumed by humans and animals (domestic and wild animals), which can easily cause cross-contamination. The Chahan River is situated in Datong County approximately 80 km from the centre of the city of Xining, and it is a large river that flows into the Heiquan reservoir, which is used by the residents of the city of Xining. The Baoku Village River is a large river that runs through Baoku village. Both the Chahan River and Baoku Village River serve as main drinking water sources. The Beichuan River and Xichuan River run through Xining and are used for irrigation for plants and trees. The Qilinwan River passes through the centre of Xining and mainly serves as a sightseeing location. The Ledu Tianlu water and Huzhu water are used as water for small ditches.
The total volume of each water sample collected was 20 l, and the samples were processed as previously described in Ma et al. (2014). The samples were filtered through a 13-mm diameter and 2-μm microfiltration membrane (Sterlitech, Kent, WA, USA) using negative pressure. Then, the filtered microporous membrane was placed on a glass board and gently washed three times with distilled water. When the filtering process was completed, all of the samples were collected in 50-mL centrifuge tubes and centrifuged for 15 min at 1500 r/min. After discarding the supernatant, a 5-mL sample was retained for purification. A fresh 15-mL sterile centrifuge tube was used; then, 1.096 Percoll solution (3 mL) followed by 1.056 Percoll solution (4 mL) were added. A 5-mL sample was centrifuged for 10 min at 2500 r/min (Ma et al. 2014). The final pellet was placed into storage tubes for DNA extraction.
DNA extraction
Total genomic DNA was extracted from each water sample using a Stool DNA Kit (Qiagen, Germany) according to the manufacturer’s instructions, with the addition of 10 freeze-thaw cycles after the lysis buffer solution was added to rupture the (oo)cyst wall. Liquid nitrogen was used for freezing, and thawing was carried out at 85 °C in a thermostatic water tank (DK-8D, Shanghai YIHENG Technical Co., Ltd., China) (Ma et al. 2014). Finally, genomic DNA was concentrated by elution in 60 μl of AE buffer and stored at − 20 °C for PCR detection.
Molecular characterisation of Cryptosporidium spp. and Giardia spp.
Two-step nested PCR targeting the small subunit (SSU) rRNA gene of Cryptosporidium spp. was performed as previously described (Karanis et al. 2007b; Nichols et al. 2003). Briefly, the first PCR was carried out in a 50 μl reaction volume containing 2.5 μl of DNA, 2 μl of dNTP mix (10 mM of each dNTP), 5 μl of 10 × PCR buffer containing 1.5 mM MgCl2 (Qiagen), 0.5 μl of 5 U of HotStarTaq DNA Polymerase (Qiagen), 3 μl of 3 mM MgCl2 (Qiagen), 3 μl of bovine serum albumin (BSA, acetylated, 10 mg/mL) (Promega), 30 μl of PCR-grade water and 2 μl of each primer (10 μM) (forward, 5′-CAATTGGAGGGCAAGTCTGGTGCCAGC-3′; reverse, 5′-CCTTCCTATGTCTGGACCTGGTGAGT-3′) to generate approximately 655 to 667 bp depending on the species of Cryptosporidium. PCR amplification was performed under the following conditions: an initial heat-activation step at 95 °C for 15 min; 35 cycles of 94 °C for 45 s, 68 °C for 1 min, and 72 °C for 1 min; then, 72 °C for 10 min. The second PCR was performed in a 50.0-μl reaction volume containing 2.5 μl of the first PCR product, 5 μl of 10 × PCR buffer containing 1.5 mM MgCl2 (Qiagen), 3 μl of 3 mM MgCl2 (Qiagen), 2 μl of dNTP Mix (10 mM of each dNTP), 0.5 μl of 5 U of HotStarTaq DNA polymerase (Qiagen), 3 μl of BSA (acetylated, 10 mg/mL) (Promega), 30 μl of PCR-grade water and 2 μl of each of primer (10 μM) (forward, 5′-AAGCTCGTAGTTGGATTTCTG-3′; reverse, 5′-TAAGGTGCTGAAGGAGTAAGG-3′). PCR amplification was performed under conditions identical to those for the first PCR except primer annealing occurred at 60 °C for 1 min, and extension occurred at 72 °C for 45 s.
Semi-nested PCR targeting the 18S-rRNA gene locus of Giardia was carried out as previously described (Appelbee et al. 2003). Briefly, the first PCR was performed under conditions identical to those for the first PCR of Cryptosporidium, except that the primers and PCR programme were as follows. The primary primers were GiaF: 5′-AAG TGT GGT GCA GAC GGA CTC-3′ and GiaR: 5′-CTG CTG CCG TCC TTG GAT GT-3′; annealing occurred at 55 °C for 30 s, and the extension reaction occurred at 72 °C for 45 s to generate approximately 380-bp products. The secondary primers were RH11: 5′-CAT CCG GTC GAT CCT GCC-3′ and RH4: 5′-AGT CGA ACC CTG ATT CTC CGC CAG G-3′, the annealing occurred at 53 °C for 30 s, and the extension reaction occurred at 72 °C for 45 s to generate approximately 292-bp products. Positive and negative controls were included in each amplification reaction. The amplified PCR products were analysed using a WD-9413B gel imaging analysis system (Beijing Liuyi Biotechnology, China) following electrophoresis on a 1.5% agarose gel (Biowest Regular Agarose G-10, Gene Company) stained with ExRed nucleic acid electrophoresis dye (Beijing Zoman Biotechnology, China).
Sequencing and phylogenetic analysis
Direct sequencing of the positive PCR products was performed by the BEIJING GENEWIZ Company (Beijing, China). To confirm their genotypes, the sequences were processed by Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) and compared with references using BLAST analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi) alignment to reference sequences in the GenBank database to identify the Cryptosporidium and Giardia species. Phylogenetic trees of Cryptosporidium and Giardia species were constructed in MEGA 5.05 using the neighbour-joining (NJ) method, which was calculated by the Jukes-Cantor model with 1000 bootstrap replicates.
Results
A total of 456 water samples were collected from different locations in the QTPA, China (Fig. 1; Tables 1, 2 and 3) from 2015 to 2017 to study the occurrence of Cryptosporidium spp. and Giardia assemblages/genotypes via PCR and sequencing analysis. Among the samples, 10 were Cryptosporidium positive, and 97 were Giardia positive as confirmed by PCR amplification, and the percentages of positive detection were 2.2% (10/456) and 21.3% (97/456), respectively. In detail, the results showed that the percentages of positive detection of Cryptosporidium and Giardia species in river water, slaughterhouse water and sewage water were 2.4% (3/127) and 20.5% (26/127), 0% (0/153) and 11.1% (17/153) and 4.0% (7/176) and 30.7% (54/176), respectively, as shown in Tables 1, 2 and 3. Therefore, Cryptosporidium was not detected in slaughterhouse water, and positive samples were collected from river water and sewage. The Cryptosporidium-positive samples were identified as C. hominis (n = 5), C. andersoni (n = 1), C. environmental (n = 1), C. struthionis (n = 1), C. canis (n = 1) and C. parvum (n = 1). The G. duodenalis-positive samples were identified as G. duodenalis assemblage A (n = 96), with one sample containing G. duodenalis assemblage E.
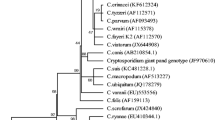
All the nucleotide sequences of Cryptosporidium and Giardia showed similarity (above 98%) with a query coverage of 98% (the lowest query was 97%, the highest query was 100%). The nucleotide sequences identified in our study were deposited in the GenBank database under the accession numbers MH206220-MH206229 and MH206230-MH206239, respectively. The phylogenetic analysis employing the NJ method indicated that all 18S rRNA gene and SSU rRNA representative sequences from the Cryptosporidium and Giardia species generated in the present study formed well-defined clusters with their respective reference sequences (Figs. 2 and 3).
Discussion
The gastrointestinal parasites Cryptosporidium and Giardia are common in humans, animals and the environment. These parasites are public health problems in many countries and particularly in remote areas. The present study was carried out to investigate the occurrence of Cryptosporidium and Giardia species and provide sound data on the species and genotypes of the two pathogenic protozoa in water sources from slaughterhouse waters, wastewater and river waters in the QTPA.
The results of our study confirmed the detection of Cryptosporidium in river water and sewage samples but not slaughterhouse waters, while Giardia species were present in all three types of water samples. Five samples were positive for C. hominis; the other Cryptosporidium species, C. andersoni, C. environmental, C. struthionis, C. canis and C. parvum, were single observations. Ninety-six samples were positive for G. duodenalis assemblage A, and only one sample with G. duodenalis assemblage E was found. The results indicate that water sources in the investigated areas are contaminated with Cryptosporidium and Giardia (oo) cysts and therefore can act as contamination sources for human and animal infections in the area. The overall detection rate of Cryptosporidium spp. in the investigated water was 2.2%. Unexpectedly, no Cryptosporidium-positive samples were obtained from the slaughterhouse water samples. Despite the low numbers of positive samples, the results indicated that C. hominis was the dominant species in the water samples in the QTPA. All Cryptosporidium species that were found (five C. hominis, one C. andersoni, and one C. parvum) represent human and animal pathogenic species.
A previous study reported a Cryptosporidium detection rate of 27.3% in environmental water samples in Qinghai Province by microscopy, but no molecular characterisation was included (Ma et al. 2014). The results from other studies (see Table 4) in China showed that the prevalence of Cryptosporidium spp. ranged between 16.7% and 82.7% in water samples. Ten Cryptosporidium species or genotypes, including C. muris, C. meleagridis, C. parvum, C. hominis, C. canis, C. felis, C. baileyi, C. suis-like, rat genotype I and rat genotype IV, were found in urban wastewater treatment plant effluents in Shanghai, China, as reported by Ma et al. (2016). Twenty (40%) out of 50 effluent samples were positive for Cryptosporidium spp. based on PCR or PMA-PCR results. Oocysts were detected by immunofluorescence test (IFT) microscopy in 31 (62%) of the 50 samples, indicating high prevalence densities of the oocysts in these waters. Cryptosporidium oocysts were found in 86.4% of 66 water samples, with high concentrations in treated effluent (Xiao et al. 2013). Furthermore, Cryptosporidium infections have been reported in domestic animals, e.g., 14.4% in young cattle and 5.2% in young sheep (Zhang et al. 2018b), 28.5% in yaks and 12.3% in Tibetan sheep (Li et al. 2016), 24.2% in yaks (Mi et al. 2013) and 7.8% in wild animals (Zhang et al. 2018a).
The overall detection rate of Giardia in the investigated waters during the present study was 21.3%, as determined by PCR. The results indicated that G. duodenalis assemblage A was the dominant type in the water samples in the QTPA. Seventeen (11%) slaughterhouse water samples were G. duodenalis positive. G. duodenalis assemblage E was detected in only one slaughterhouse water sample. G. duodenalis assemblage A is a pathogenic assemblage for humans. In other studies, the prevalence of Giardia spp. was from 15% to 98%, as reported by Ma et al. (2014). Giardia cysts were found in 65.2% of 66 water samples, with high concentrations in treated effluent (Xiao et al. 2013). An analysis of the gdh locus by Ma et al. (2016) showed that 90% (45/50) of the samples from the effluents of wastewater treatment plants in Shanghai were positive for G. duodenalis, including sub-types and human pathogenic assemblages A and B. In addition, G. duodenalis infections have been reported in domestic animals, e.g. 10% in young cattle (Jian et al. 2018), 12.3% in yaks, 13% in sheep, 3.9% in goats, 6.4% in cattle and 7.7% in donkeys in Qinghai Province (Ma et al. 2014).
Sewage treatment plant influent samples usually contained high numbers of (oo)cysts of Cryptosporidium and Giardia, and this has also been reported in different provinces in China. A comprehensive overview of the detection rates of Cryptosporidium and Giardia in water samples in China is presented in Tables 4 and 5; Table 4 for Cryptosporidium (Feng et al. 2011; Hu et al. 2014; Huang et al. 2017; Li et al. 2012; Ma et al. 2016; Xiao et al. 2012, 2013, 2017, 2018) and Table 5 for Giardia (Huang et al. 2017; Li et al. 2012; Liu et al. 2011; Ma et al. 2016; Xiao et al. 2017, 2018).
Companies in the slaughter industry require a large amount of water as a component of their products. The Baide company (Baide No. 1 and Baide No. 2) is a large slaughter company that has slaughtering production lines and represents the largest beef and mutton livestock trading, slaughtering and processing company in a well-known market in the northwest provinces of China, which is also one of the country’s largest halal beef and mutton livestock markets. Many cattle, yaks, beef cattle, obsolete cows, sheep and goats are transported to this market. During the slaughter of animals and the processing of meat products for human consumption, the gastrointestinal contents of the animals are occasionally discharged into soils and water bodies or released to wastewater treatment plants. The Xining sewage factory is located at an appropriate distance from the residential area and forms part of the rain sewage diversion mode. The Xining sewage factory is used only to dispose of domestic sewage and industrial wastewater (no storm water runoff), and more advanced processing systems are used in Xining than in more technically deficient districts. The Huangyuan sewage factory is situated in an agriculture and animal husbandry binding area near an economically deficient area and has low-level processing systems. Industrial waste waters must be treated before they can be discharged into public sewage treatment plants. For direct discharge into bodies of water, extensive cleaning is necessary through special sewage treatment plants.
Companies that process meat and poultry require advanced processes for biological wastewater treatment consisting of multiple closed systems with no open basins. For example, Nanchuan sewage, Xiushui road recycled water, the pharmaceutical factory and No. 2 sewage are all at the city level, which is more advanced than the county level. Huangyuan sewage is a county-level sewage service and had a 28.7% percentage of G. duodenalis detection. Therefore, the wastewater in the Huangyauan sewage factory is expected to be more contaminated with Cryptosporidium and Giardia than the water from the Xining sewage factory (all of the sewage water samples were collected from sewage pipe inlets).
Chinese drinking and wastewater treatment still need to be improved, and some studies have confirmed the presence of both pathogens in animals, humans and water supplies, as indicated above. The current discharge standard of pollutants from municipal wastewater treatment plants in China uses the faecal coliform count as a microbiological indicator and is not focused on detecting Cryptosporidium and Giardia in drinking water. Even if raw wastewater is well treated, the reuse of wastewater treatment plant effluents for irrigation, recreation or surface water recharge can be a potential public health problem (Xiao et al. 2018). Cryptosporidium and Giardia (oo)cysts in wastewater treatment effluents may contaminate fresh produce, thus causing foodborne outbreaks of diarrhoeal illnesses (Ahmed and Karanis 2018a).
The presence of these protozoa in the investigated samples emphasises the importance of protecting the water sources in China, because these water sources have no riparian vegetation or adequate protective structures and are situated near the ground close to grazing areas. Due to the negative results of Cryptosporidium in samples from slaughterhouses, there is no clear evidence on the level at which intestinal cryptosporidial infections in cattle and other slaughtered animals exist in these slaughterhouses. However, slaughterhouse workers in China are constantly exposed to unhygienic working environments and gastrointestinal parasites. Improved human and slaughterhouse surveillance would provide any missing data that is needed to control the spread of such parasites in areas of high endemicity. The findings above demonstrate the potential risk of infection by pathogenic strains of the Cryptosporidium and Giardia species/genotypes/assemblages for people and animals living in the study area. Moreover, zoonotic Cryptosporidium and Giardia species/assemblages were identified in the investigated water samples, providing evidence on the cycle of infections among humans, animals and water sources. Environmental and health problems associated with improper waste management put the health of humans and animals at high risk. There is a need for local governments and health authorities in China to undertake control measures and reduce the contamination of water sources by these two protozoa to protect the health of animals and humans in China.
Change history
14 November 2019
The authors of this article would like to state that <Emphasis Type="Italic">C.</Emphasis> environmental is not a species, but rather a group of un-identified <Emphasis Type="Italic">Cryptosporidium</Emphasis> isolates from the environment. It is referred to in the literature as <Emphasis Type="Italic">Cryptosporidium</Emphasis> environmental sequence and not as a species.
References
Ahmed SA, Karanis P (2018a) An overview of methods/techniques for the detection of Cryptosporidium in food samples. Parasitol Res 117:629–653
Ahmed SA, Karanis P (2018b) Comparison of current methods used to detect Cryptosporidium oocysts in stools. Int J Hyg Environ Health S1438-4639(17):30469–30468
Appelbee AJ, Frederick LM, Heitman TL, Olson ME (2003) Prevalence and genotyping of Giardia duodenalis from beef calves in Alberta, Canada. Vet Parasitol 112:289–294
Baldursson S, Karanis P (2011) Waterborne transmission of protozoan parasites: a review of worldwide outbreaks - an update 2004-2010. Water Res 45:6603–6614
Cacciò SM, Chalmers RM (2016) Human cryptosporidiosis in Europe. Clin Microbiol Infect 22:471–480
Efstratiou A, Ongerth J, Karanis P (2017) Evolution of monitoring for Giardia and Cryptosporidium in water. Water Res 123:96–112
Erickson MC, Ortega YR (2006) Inactivation of protozoan parasites in food, water, and environmental systems. J Food Prot 69:2786–2808
Farizawati S, Lim YA, Ahmad RA, Fatimah CT, Siti-Nor Y (2005) Contribution of cattle farms towards river contamination with Giardia cysts and Cryptosporidium oocysts in Sungai Langat Basin. Trop Biomed 22:89–98
Feng Y, Zhao X, Chen J, Jin W, Zhou X, Li N, Wang L, Xiao L (2011) Occurrence, source, and human infection potential of Cryptosporidium and Giardia spp. in source and tap water in Shanghai, China. Appl Environ Microbiol 77:3609–3616
Garcia RJC, Hayman DT (2016) Origin of major infectious disease in vertebrates: the timing of Cryptosporidium evolution and its hosts. Parasitol 143:1683–1690
Hu Y, Feng Y, Huang C, Xiao L (2014) Occurrence, source, and human infection potential of Cryptosporidium and Enterocytozoon bieneusi in drinking source water in Shanghai, China, during a pig carcass disposal incident. Environ Sci Technol 48:14219–14227
Huang C, Hu Y, Wang L, Wang Y, Li N, Guo Y, Feng Y, Xiao L (2017) Environmental transport of emerging human-pathogenic Cryptosporidium species and subtypes through combined sewer overflow and wastewater. Appl Environ Microbiol 83(16):pii: e00682–pii: e00617
Jian Y, Zhang X, Li X, Karanis G, Ma L, Karanis P (2018) Prevalence and molecular characterization of Giardia duodenalis in cattle and sheep from the Qinghai-Tibetan plateau area (QTPA), northwestern China. Vet Parasitol 250:40–44
Karanis P, Kourenti C, Smith H (2007a) Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health 5:1–38
Karanis P, Plutzer J, Halim NA, Igori K, Nagasawa H, Ongerth J, Liqing M (2007b) Molecular characterization of Cryptosporidium from animal sources in Qinghai province of China. Parasitol Res 101:1575–1580
Karanis P, Schoenen D, Seitz HM (1996) Giardia and Cryptosporidium in backwash water from rapid sand filters used for drinking water production. Zentralbl Bakteriol 284:107–101
Karanis P, Schoenen D, Seitz HM (1998) Distribution and removal of Giardia and Cryptosporidium in water supplies in Germany. Water Sci Technol 37:9–18
Koehler AV, Haydon SR, Jex AR, Gasser RB (2016) Cryptosporidium and Giardia taxa in fecal samples from animals in catchments supplying the city of Melbourne with drinking water (2011 to 2015). Parasit Vectors 9:315
Li K, Shahzad M, Zhang H, Jiang X, Mehmood K, Zhao X, Li J (2018) Socio-economic burden of parasitic infections in yaks from 1984 to 2017 on Qinghai Tibetan Plateau of China-A review. Acta Trop 183:103–109
Li N, Xiao L, Wang L, Zhao S, Zhao X, Duan L, Guo M, Liu L, Feng Y (2012) Molecular surveillance of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi by genotyping and subtyping parasites in wastewater. PLoS Negl Trop Dis 6:e1809
Li P, Cai J, Cai M, Wu W, Li C, Lei M, Xu H, Feng L, Ma J, Feng Y, Xiao L (2016) Distribution of Cryptosporidium species in Tibetan sheep and yaks in Qinghai, China. Vet Parasitol 215:58–62
Liu A, Ji H, Wang E, Liu J, Xiao L, Shen Y, Li Y, Zhang W, Ling H (2011) Molecular identification and distribution of Cryptosporidium and Giardia duodenalis in raw urban wastewater in Harbin, China. Parasitol Res 109:913–918
Ma L, Sotiriadou I, Cai Q, Karanis G, Wang G, Wang G, Lu Y, Li X, Karanis P (2014) Detection of Cryptosporidium and Giardia in agricultural and water environments in the Qinghai area of China by IFT and PCR. Parasitol Res 113:3177–3184
Ma J, Feng Y, Hu Y, Villegas EN, Xiao L (2016) Human infective potential of Cryptosporidium spp., Giardia duodenalis and Enterocytozoon bieneusi in urban wastewater treatment plant effluents. J Water Health 14:411–423
Mahmoudi MR, Ongerth JE, Karanis P (2017) Cryptosporidium and cryptosporidiosis: the Asian perspective. Int J Hyg Environ Health 220:1098–1109
Mi R, Wang X, Li C, Huang Y, Zhou P, Li Z, Lei M, Cai J, Chen Z (2013) Prevalence and genetic characterization of Cryptosporidium in yaks in Qinghai province of China. PLoS One 8:e74985
Nichols RA, Campbell BM, Smith HV (2003) Identification of Cryptosporidium spp. oocysts in United Kingdom non-carbonated natural mineral waters and drinking waters by using a modified nested PCR-restriction fragment length polymorphism assay. Appl Environ Microbiol 69:4183–4189
Plutzer J, Karanis P (2009) Genetic polymorphism in Cryptosporidium species: an update. Vet Parasitol 165:187–199
Plutzer J, Ongerth J, Karanis P (2010) Giardia taxonomy, phylogeny, and epidemiology: facts and open questions. Int J Hyg Environ Health 213:321–333
Rosado-García FM, Guerrero-Flórez M, Karanis G, Hinojosa MDC, Karanis P (2017) Water-borne protozoa parasites: the Latin American perspective. Int J Hyg Environ Health 220:783–798
Ryan U, Hijjawi N, Xiao L (2018) Foodborne cryptosporidiosis. Int J Parasitol 48:1–12
Upton SJ, Current WL (1985) The species of Cryptosporidium (Apicomplexa: Cryptosporidiidae) infecting mammals. J Parasitol 71:625–629
Xiao S, An W, Chen Z, Zhang D, Yu J, Yang M (2012) Occurrences and genotypes of Cryptosporidium oocysts in river network of southern-eastern China. Parasitol Res 110:1701–1709
Xiao G, Qiu Z, Qi J, Chen JA, Liu F, Liu W, Luo J, Shu W (2013) Occurrence and potential health risk of Cryptosporidium and Giardia in the Three Gorges Reservoir, China. Water Res 47:2431–2445
Xiao S, Yin P, Zhang Y, Hu S (2017) Occurrence of Cryptosporidium and Giardia and the relationship between protozoa and water quality indicators in swimming pools. Korean J Parasitol 55:129–135
Xiao S, Yin P, Zhang Y, Zhao X, Sun L, Yuan H, Lu J, Hu S (2018) Occurrence, genotyping, and health risk of Cryptosporidium and Giardia in recreational lakes in Tianjin, China. Water Res 141:46–56
Zhang X, Jian Y, Li X, Ma L, Karanis G, Karanis P (2018a) The first report of Cryptosporidium spp. in Microtus fuscus (Qinghai vole) and Ochotona curzoniae (wild plateau pika) in the Qinghai-Tibetan plateau area, China. Parasitol Res 117:1401–1407
Zhang X, Jian Y, Li X, Ma L, Karanis G, Qigang C, Karanis P (2018b) Molecular detection and prevalence of Cryptosporidium spp. infections in two types of domestic farm animals in the Qinghai-Tibetan plateau area (QTPA) in China. Parasitol Res 117:233–239
Acknowledgments
We acknowledge the funding support from the One Thousand Talents Plan of the Chinese Government (NO. WQ2013630172).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Julia Walochnik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, L., Zhang, X., Jian, Y. et al. Detection of Cryptosporidium and Giardia in the slaughterhouse, sewage and river waters of the Qinghai Tibetan plateau area (QTPA), China. Parasitol Res 118, 2041–2051 (2019). https://doi.org/10.1007/s00436-019-06330-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06330-w