Abstract
Qinghai Province in northwest China is strongly influenced by agricultural activities and is an important source of food and drinking water. Here, we present findings regarding the occurrence and molecular epidemiology of Cryptosporidium and Giardia species based on a large-scale investigation of areas of Qinghai Province. The diagnosis and molecular detection of Cryptosporidium oocysts and Giardia cysts was carried out using immunofluorescence microscopy (IFT), whereas nested polymerase chain reaction (PCR) in fecal smears and water samples was used for the detection and molecular characterization of the species. In total, 561 samples (260 water samples and 301 fecal samples from animals) were collected and analyzed. Of the 260 water samples, 66 samples were Cryptosporidium-positive by IFT and 71 samples were positive by nested PCR; in addition, 39 samples were Giardia-positive by IFT and 40 samples were positive by nested PCR. Of the 301 fecal samples from animals, 98 samples were Cryptosporidium-positive by IFT and 61 samples were positive by nested PCR, whereas 52 samples were Giardia-positive by IFT and 31 samples were positive by nested PCR. We showed that the water supplies and animals investigated contained Cryptosporidium and Giardia (oo)cysts. Thus, we recommend that the Chinese Government and Chinese health authorities undertake control measures to protect the food and drinking water sources in Qinghai from these pathogenic protozoa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The occurrence of the (oo)cysts of Cryptosporidium and Giardia in agricultural water and other aquatic environments is a globally acknowledged public health problem. There are numerous documented reports of water-borne outbreaks of giardiasis and cryptosporidiosis throughout the world (Karanis et al. 2007a). At least 199 outbreaks resulting from the waterborne transmission of parasitic protozoa were reported worldwide during 2004–2010 (Baldursson and Karanis 2011). A widely used method for the detection of the (oo)cysts in environmental samples is the immunofluorescence assay (IFA), which is time-consuming and subject to variations in sensitivity. However, identifying the species infecting humans and animals is important in determining the epidemiology of the disease and the transmission routes of Cryptosporidium (Plutzer et al. 2007; 2008; Plutzer and Karanis 2009).
In many countries, including China, there are no requirements for testing surface water for the presence of these parasites. The numbers of clinical giardiasis and cryptosporidiosis cases are likely to be underestimated in China because of the lack of systematic methods for the diagnosis of these parasites. Molecular tools for the detection and characterization of Giardia and Cryptosporidium species have been widely applied in recent years, particularly for the detection and characterization of species from water supplies.
In the present study, we used IFT, polymerase chain reaction (PCR) assays for comparative detection of Giardia and Cryptosporidium (oo)cysts in environmental water samples from fecal samples and drinking water supplies in the Qinghai province of northwest China.
Materials and methods
General information on geography
Qinghai is located on the north-eastern part of the Tibetan Plateau. The Yellow River originates in the south of the province, whereas the Yangtze River and the Mekong have their sources in the south west. Qinghai is separated by the Riyue Mountain into pastoral and agriculture zones in the west and east. Located mostly on the Qinghai-Tibet plateau, the province has long been a melting pot for several ethnic groups, including the Han, Tibetans, Hui, Tu, Mongols, and Salars. Qinghai borders Gansu in the northeast, Xinjiang in the northwest, Sichuan in the southeast, and Tibet Autonomous Region in the southwest.
During the hot summer months, many tourists from the hot southern and eastern parts of China travel to Xining because its climate in July and August is relatively mild and comfortable, making the city an ideal summer retreat. Thousands of tourists use the seashore and rivers for swimming and camping activities. Because it is a popular tourist destination and the use of rivers, etc., is very popular, there is a need to understand the protozoan burden of the area.
Sample sizes and sites
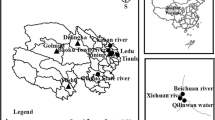
During 2011 and 2012, a total of 561 samples were collected, including 260 water samples and 301 fecal samples. For each sample, information was collected about its location, river name, and size (Fig. 1; Table 1). There are no seasonal differences in sampling. Fecal samples were collected from domesticated animals (including yak, sheep, goat, cattle, horse, donkey, and dog) on farms (including national and private) and some wild animals, such as the Tibetan antelope, pika, and sokhor. The location, animal species, and size of each sample are shown in Fig. 1, and Tables 2 and 3. All the samples were collected from the south and east parts of Qinghai Province where the land is used for farming. The interaction of humans, animals, and the environment with Cryptosporidium and Giardia enable these organisms to complete their life circle. Therefore, it is crucial to understand the prevalence of Cryptosporidium and Giardia in this region.
Distribution of the sites of sample collection in Qinghai Province. All samples were collected from the south and east parts of Qinghai Province, which are the main farming regions. In addition, the Three River Source area is important to downstream areas for farming and drinking water supplies. Black triangles indicate the main rivers sampled
Sample collection method
Water samples
At each sampling point, a 20-L water sample was collected and divided in two equal portions; 10 L was used for IFT, and 10 L was analyzed using molecular methods. The water was collected in sterile plastic tanks by the sample containers dropped into the water and transferred to the laboratory for parasitological analysis. The samples were analyzed in the laboratory of the Academy of Animal Sciences in Xining in the Department of Infectious Diseases.
Fecal samples
Fecal material from each animal was collected in labeled Eppendorf cups (2 × 1.5 mL); duplicate samples were placed in 1.5 mL cups, capped, and then immediately placed on ice and transported to the laboratory. In the laboratory, the fecal samples were preserved in 2.5 % potassium dichromate and kept at 4 °C until use. In total, 301 fresh fecal samples were collected during 2011 and 2012 from different animal species (Tables 2 and 3).
(Oo)cyst concentration
Water samples
The (oo)cyst concentration in the investigated environmental water samples was carried out following a modified version of the methodology of Karanis et al. (2006). Briefly, 10 L of river water was filtered through membrane filters (diameter 142 mm) with a pore size of 1.2 μm by means of a vacuum device. After filtration, the filters were washed at least three times with 15 mL 0.1 % Tween 80. The eluate and the wash solutions were collected in sterile 50-mL conical tubes and centrifuged at 1,500 × g for 15 min. The supernatant was removed, and the pellet (1–2 mL, depending on the water turbidity) was subjected to sucrose flotation (Karanis et al. 2006, 2007a).
Microscopic examination
Microscopic examinations using immunofluorescence (CRYPTO CEL, Cellabs Pty, Brookvale, Australia) were performed on all samples after discontinuous sucrose gradient purification and diethyl-ether/phosphate-buffered saline (2:1 v/v) biphasic concentration as described previously (Karanis et al. 2006, 2007a).
Detection and enumeration of (oo)cysts by IFT
A 25-μl aliquot of each suspension was placed on a glass-well microscope slide for the enumeration of Cryptosporidium oocysts and Giardia cysts. The samples were mounted onto slides, fixed with methanol, and then stained with fluorescing iso-thiocyanate-conjugated anti-Cryptosporidium spp. and anti-Giardia spp. monoclonal antibodies obtained from Cellabs Biotechnology (Australia). Each slide was scanned completely for cysts and oocysts by using an immunofluorescence microscope (Olympus BHF-T). Organisms that met the criteria for Giardia cysts and Cryptosporidium oocysts (based on size, shape, and fluorescence under a total magnification of × 200 to × 400) were labeled as (oo)cysts.
Detection of (oo)cysts by PCR
DNA extraction
DNA extraction was performed for all water and fecal samples. DNA from the water samples was extracted from seeded water and concentrations of river water samples using a QIAamp water Mini Kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer’s instructions, with the addition of ten freeze–thaw cycles, as described previously (Plutzer et al. 2008, 2009). DNA from the fecal samples was extracted using a QIAamp DNA Stool Mini Kit (Qiagen GmbH) according to the manufacturer’s instructions.
PCR for Cryptosporidium and Giardia
Cryptosporidium
A nested PCR was performed to detect Cryptosporidium oocysts. PCR primers that had been previously described were used to amplify the 825-bp fragment of C. parvum 18S RNA (Karanis et al. 2007a, c). Both PCRs were performed in standard mixtures of 50 μL containing 1.25 μL primer mixtures (200 nmol of each primer), 8 μL of 200 mM dNTP mix (200 mM of each dNTP) (Finnzymes, Espoo, Finland), 1× PCR buffer containing 5 μL 1.5 mM MgCl2 (Qiagen GmbH), 3 μL 3 mM MgCl2 (Qiagen GmbH), 0.5 μL 2.5 U HotstarTaq DNA polymerase (Qiagen GmbH), 2 μL bovine serum albumin (BSA; acetylated, 10 mg/mL) (Promega, Madison, WI, USA), 3 μL DNA, and 27.25 μL distilled water.
For primary PCR, the amplification reactions were run according to the following PCR program: An initial step at 94 °C for 3 min; 35 cycles of 94 °C for 45 s, 55 °C for 45 s, and 72 °C for 1 min; then 72 °C for 7 min and a final hold at 4 °C. For secondary PCR, each reaction was prepared as for primary PCR, but F2 and R2 primers were used and the following PCR program was run: 94 °C, 3 min; 35 cycles of 94 °C for 45 s, 58 °C for 45 s, and 72 °C for 1 min; then 72 °C for 7 min and a final hold at 4 °C.
Giardia
Semi-nested PCR was performed using the primers published by Read et al. (2004) to amplify a 432-bp fragment of the Giardia glutamate dehydrogenase gene. A nested PCR was performed in a standard mixture of 25 μL containing 200 nmol of each primer, 0.2 mM dNTP, 1.5 mM MgCl2, 2.5 U Taq DNA polymerase (Qiagen), and 2 μL BSA (10 mg/mL) plus 10× Perkin-Elmer PCR buffer. The templates were subjected to an initial denaturation at 94 °C for 2 min followed by 35 cycles of 94 °C for 2 min, 55 °C for 10 s, 72 °C for 30 s and a final extension at 72 °C for 5 min. A secondary PCR was run using the following conditions: An initial step at 96 °C for 4 min, followed by 35 amplification cycles of 94 °C for 20 s, 53 °C for 20 s, 72 °C for 40 s and a final extension at 72 °C for 5 min. PCR products were electrophoresed on a 1.5 % agarose gel containing ethidium bromide (0.6 mg/mL) and visualized using a Gel Doc device.
Results
Water samples
The study determined that most of the water samples contained high concentrations of Cryptosporidium oocysts and low concentrations of Giardia cysts (Tables 1). Cryptosporidium oocysts were more prevalent (66 out of 260 water samples; 98 out of 301 fecal samples) compared with Giardia cysts (39 out of 260 water samples; 52 out of 301 fecal samples) based on the IFA method. The nested PCR results showed that 71 out of 260 water samples and 61 out of 301 fecal samples were Cryptosporidium positive, whereas 40 out of 260 water samples and 31 out of 301 fecal samples were Giardia-positive.
The highest Cryptosporidium positive rate was Daotangh River with a rate of 75 % (Table 1). The lowest Cryptosporidium rate was the Nanmenxia reservoir (10.0 %). In terms of Giardia, the highest positive rate was the Nanmenxia wetland (40.0 %) (Table 1). No Giardia cysts have been detected in any of the samples from the nine rivers in the present study (Table 1).
Fecal samples
Table 2 summarizes the information on the fecal samples relating to location, sample size, and detection results. Of the 301 fecal samples, 98 were Cryptosporidium-positive by IFA and 61 were positive by nested PCR, whereas 52 samples were Giardia-positive by IFA and 31 samples were positive by nested PCR. All the fecal samples were taken from areas that are known for their farming of cattle, yak, sheep, goat, and other domestic animals. The highest Cryptosporidium positive rate was from Menyuan county (46.15 %) by both IFA and PCR, whereas the lowest rate was 10.71 %, from Qilian county. Menyuan county also had the highest rate of detection of Giardia (38.46 %). The lowest positive rate of Giardia was found in Gangcha and Zeku counties, with the IFT and nested PCR detection methods, respectively. Two out of the 18 samples from Gangcha county were Giardia-positive by IFT, but not by nested PCR. From the 23 samples collected from Zeku county, IFT detected no Giardia, whereas four samples were Giardia-positive using nested PCR.
The fecal samples varied in composition, with those animals harboring the parasites usually being watery and containing mucus. In addition, blood or leukocytes in such samples were occasionally observed.
Table 3 summarizes the results of fecal sample by animal species. The 301 fecal samples were collected from ten animal species, including both domestic and wild animals, although the latter were hard to sample. Although the sample sizes differed among species, the Cryptosporidium-positive rates were almost the same, at approximately 30 % by IFT, whereas the results detected by nested PCR varied from 14.29 % to 27.27 % (Table 3). No Giardia infections were found in the seven Tibetan antelope samples with either IFT or nested PCR (Table 3).
Discussion
Waterborne diseases occur worldwide, and outbreaks caused by the contamination of community water systems have the potential to cause disease in large numbers of consumers. In addition to outbreaks caused by contaminated potable water, there are outbreaks caused following the accidental ingestion of recreational waters. Rapid and effective monitoring methods are needed at drinking-water facilities to determine the occurrence of (oo)cysts in source and treated water. The development of such methods would lead to better decisions concerning the public health risks and treatment associated with water sources (Karanis et al. 2007a, b, c; Baldursson and Karanis 2011).
In our study, the microscopic identification of (oo)cysts was based on IFA analysis, which showed that Cryptosporidium was more prevalent than Giardia. The number of positive results may have differed because the samples were collected at different times, seasons, or locations between 2011 and 2012.
However, oocysts of Cryptosporidium are smaller (approx. 4–6 μm) and are able to change shape, which enables them to squeeze through the pores of membrane filters during vacuum pump filtration (Mayer and Palmer 1996). Therefore, the filtration techniques might be more efficient for the detection of Giardia cysts (approx. 8–12 μm) than of Cryptosporidium oocysts.
More frequent detection of Giardia cysts in untreated waters relies on the assumption that giardiasis is more widespread and occurs with greater intensity than cryptosporidiosis. Other studies reported this trend in surface waters in other countries (Plutzer et al. 2007; Carmena et al. 2007) too.
The concentration of (oo)cysts in surface water is usually low, being reported from different countries as ranging from 1 to 18 (oo)cysts/10 L (Robertson and Gjerde 2001; Hanninen et al. 2005). In The Netherlands, a higher concentration of (oo)cysts in surface water has been detected. The average concentration, corrected with the average recovery efficiency, was 4.5 and 5.4 oocysts/L and 22 and 95 cysts/L in the rivers Rhine and Meuse, respectively (Medema and Schijven 2001). Natural surface water from rivers and reservoirs in northern Spain have been found with concentrations that reached 1,767 Cryptosporidium oocysts and >25,000 Giardia cysts per 100 L (Carmena et al. 2007). Surface water in Italy was contaminated with 0–5 Cryptosporidium oocysts and 8× 101 to 6 × 103 Giardia cysts per L (Briancesco and Bonadonna 2005). Cryptosporidium oocysts in surface waters draining a livestock farm on a Warwickshire (UK) estate showed a median concentration of 0.48 oocysts/L (Bodley-Tickell et al. 2002). In a study of water supplies in southern Russia and Bulgaria, 16 out of 166 samples (9.6 %) were positive for Giardia and 30 (18.1 %) were positive for Cryptosporidium (Karanis et al. 2006). Despite that the detection methodologies are different, it is difficult to make comparisons in the results of the various studies. However, in our study, we do not provide oocyst concentrations (number of oocysts per liter); we provide only the positivity rate, and this was very high according to our results.
Several studies have shown that nested PCR appears to be more sensitive than microscopy for the detection of Cryptosporidium and Giardia in water samples (Jiang et al. 2005; Nichols et al. 2003). In our study, only five samples out of 20 that were Cryptosporidium-positive by IFA and one out of 20 samples that were Giardia-positive by IFA were also positive by PCR. This could be because of the high concentration of PCR inhibitors or the presence of empty oocysts in the investigated water samples, which would prevent their detection by molecular-based methods (Karanis et al. 2006, 2007c; Jiang et al. 2005). Additionally, these results might be the result of a low concentration of DNA and the uneven distribution of template DNA, especially in samples containing low numbers (1–3) of oocysts (Smith and Nichols 2010).
Although, in our study, IFA was a more sensitive technique than the nested PCR, PCR can be used to determine the species and genotype of an isolate. The discrepancies caused by IFA-positive–PCR-negative results could also result from the tendency of the IFA reagents to cross-react with non-target organisms, such as algae, or from the inhibitory effects on PCR enzymes caused by interfering substances, such as humic acids. It is important to note that, because of the specificity of the targeting to actual genetic sequences that are unique to a given organism, the PCR method is less likely to show cross-reactivity. This provides it with a distinct advantage over the IFA method. At present, empty oocysts cannot be detected by molecular-based methods, which leads to underestimation of (oo)cyst contamination (Karanis et al. 2006; Smith and Nichols 2010).
As mentioned above, at present, each method has its own advantages and disadvantages; therefore, depending on the aim and the design of the study, a combination of more detection methods is likely to be best.
In our study, only new information on the contamination of rivers by the pathogenic protozoa Cryptosporidium and Giardia in Qinghai province and Xining City is provided. Furthermore, the study reveals for the first time that Guoluo and Yushu prefectures, which provide water for irrigation, are heavily contaminated by Cryptosporidium and Giardia. Among the samples collected from the Three River source region (i.e., source of the Yangtze River, the Yellow River, and Lancang River), both parasites were detected by at least one of the IFA or PCR methods.
The presence of Giardia and Cryptosporidium in aquatic ecosystems in Qinghai makes it imperative to develop prevention strategies for water and food safety. Human incidences of giardiasis and cryptosporidiosis and prevalence-based studies provide baseline data so that risk factors associated with waterborne and foodborne transmission can be identified. However, this type of information is not yet available in China. Standardized methods are required to maximize public health surveillance, and documentation of outbreaks will provide better insight into the public health impact of waterborne pathogenic protozoa such as Giardia and Cryptosporidium. National statistics on outbreaks linked to contaminated water are not available for China. We and others have shown that these pathogens are in recreational and drinking water in China (Karanis et al. 2007a; Baldursson and Karanis 2011). However, in China, there are no requirements for testing surface waters for the presence of these parasites, and the detection of Giardia and Cryptosporidium and information on the prevalence of these organisms in water is scarce. The present work will contribute to filling this gap and functioning as a platform for future research. More systematic studies and further monitoring of these pathogens in China are required.
References
Baldursson S, Karanis P (2011) Waterborne transmission of protozoan parasites: review of worldwide outbreaks: an update 2004–2010. Water Res 45:6603–6614
Bodley-Tickell AT, Kitchen SE, Sturdee AP (2002) Occurrence of Cryptosporidium in agricultural surface waters during an annual farming cycle in lowland UK. Water Res 36:1880–1886
Briancesco R, Bonadonna L (2005) An Italian study on Cryptosporidium and Giardia in wastewater, fresh water and treated water. Environ Monit Assess 104:445–457
Carmena D, Aguinagalde X, Zigorraga C, Fernández-Crespo JC, Ocio JA (2007) Presence of Giardia cysts and Cryptosporidium oocysts in drinking water supplies in northern Spain. J Appl Microbiol 102:619–629
Hanninen ML, Horman A, Rimhanen-Finne R, Vahtera H, Malmberg S, Herve S (2005) Monitoring of Cryptosporidium and Giardia in the Vantaa river basin, southern Finland. Int J Hyg Environ Health 208:163–171
Jiang J, Alderisio KA, Singh A, Xiao L (2005) Development of procedures for direct extraction of Cryptosporidium DNA from water concentrates and for relief of PCR inhibitors. Appl Environ Microbiol 71:1135–1141
Karanis P, Sotiriadou I, Kartashev V, Kourenti C, Tsvetkova N, Stojanova K (2006) Occurrence of Giardia and Cryptosporidium in water supplies of Russia and Bulgaria. Environ Res 102:260–271
Karanis P, Kourenti C, Smith H (2007a) Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health 5:1–38
Karanis P, Plutzer J, Halim NA, Igori K, Nagasawa H, Ongerth J, Liqing M (2007b) Molecular characterization of Cryptosporidium from animal sources in Qinghai province of China. Parasitol Res 101:1575–1580
Karanis P, Thekisoe O, Kiouptsi K, Ongerth J, Igarashi I, Inoue N (2007c) Development and preliminary evaluation of a loop-mediated isothermal amplification procedure for sensitive detection of Cryptosporidium oocysts in fecal and water samples. Appl Environ Microbiol 73:5660–5662
Mayer CL, Palmer CJ (1996) Evaluation of PCR, nested PCR, and fluorescent antibodies for detection of Giardia and Cryptosporidium species in wastewater. Appl Environ Microbiol 62:2081–2085
Medema GJ, Schijven JF (2001) Modelling the sewage discharge and dispersion of Cryptosporidium and Giardia in surface water. Water Res 35:4307–4316
Nichols RAB, Campbell BM, Smith HV (2003) Identification of Cryptosporidium spp. oocysts in U.K. still natural mineral waters and drinking waters using a modified nested PCR-RFLP assay. Appl Environ Microbiol 69:4183–4189
Plutzer J, Karanis P (2009) Rapid identification of Giardia duodenalis by loop-mediated isothermal amplification (LAMP) from fecal and environmental samples and comparative findings by PCR and Real-Time PCR methods. Parasitol Res 104:1527–1533
Plutzer J, Tako MH, Marialigeti K, Torokne A, Karanis P (2007) First investigations into the prevalence of Cryptosporidium and Giardia spp. in Hungarian drinking water. J Water Health 5:573–584
Plutzer J, Karanis P, Domokos K, Torokne A, Marialigeti K (2008) Detection and characterisation of Giardia and Cryptosporidium in Hungarian raw, surface and sewage water samples by IFT, PCR and sequence analysis of the SSUrRNA and GDH genes. Int J Hyg Environ Health 211:524–533
Plutzer J, Torokne A, Karanis P (2009) Combination of ARAD microfibre filtration and LAMP methodology for simple, rapid and cost-effective detection of human pathogenic Giardia duodenalis and Cryptosporidium spp. in drinking water. Lett Appl Microbiol 50:82–88
Read CM, Monis PT, Thompson RC (2004) Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infect Genet Evol 4:125–130
Robertson LJ, Gjerde B (2001) Occurrence of Cryptosporidium oocysts and Giardia cysts in raw waters in Norway. Scand J Public Health 29:200–207
Smith HV, Nichols RA (2010) Cryptosporidium: detection in water and food. Exp Parasitol 124:61–79
Acknowledgments
We would like to express our appreciation to the Chinese Government for financial support. This work was supported by Grant 2009-Z-48 from The Ministry of Agriculture of the People's Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, L., Sotiriadou, I., Cai, Q. et al. Detection of Cryptosporidium and Giardia in agricultural and water environments in the Qinghai area of China by IFT and PCR. Parasitol Res 113, 3177–3184 (2014). https://doi.org/10.1007/s00436-014-3979-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3979-5