Abstract
The Japanese Spanish mackerel (Scomberomorus niphonius; Perciformes: Scombridae) is widely distributed in the continental shelves of the northwestern Pacific Ocean around Japan, Sea of Japan, and East China Sea. In the present study, small, spindle-shaped myxosporean plasmodia (0.15–0.53 mm by 0.04–0.13 mm) were frequently encountered in the myofiber of trunk muscles of two Japanese Spanish mackerels; one fished in the Sea of Japan off western Japan and the other in the northwestern Pacific Ocean off southern Japan in the autumn of 2016. Isolated myxospores of Kudoa konishiae n. sp. (Myxosporea: Multivalvulida) from these two fish were stellate with six equal shell valves and polar capsules, 8.1–9.7 μm in width, 7.1–8.8 μm in thickness, and 7.1–8.8 μm in length. The polar capsules were teardrop-shaped, 2.7–4.7 μm by 1.2–2.5 μm. The lateral view of spores revealed a drawstring-pouch shape. The nucleotide sequences of the 18S and 28S ribosomal RNA gene (rDNA) were distinct from any recorded species. Phylogenetic trees demonstrated a close relationship of the present new species with Kudoa spp. with stellate spores with five or more shell valves/polar capsules, recorded in scombrid fishes. To clarify the phylogenetic relationships between three closely related species, i.e., Kudoa konishiae n. sp., Kudoa hexapunctata, and Kudoa neothunni, three mitochondrial DNA genes (cytochrome c oxidase subunit 1 gene (cox-1) and the small and large subunits of the ribosomal RNA gene (rns-rnl)) of two isolates of the new species, six isolates of K. hexapunctata, and 13 isolates of K. neothunni were sequenced. The interspecific and intraspecific variations of the newly obtained cox-1 and rns-rnl nucleotide sequences of K. hexapunctata, K. neothunni, and K. konishiae n. sp. were clarified for the first time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kudoa Meglitsch, 1947, is the largest genus in the order Multivalvulida (Myxozoa: Myxosporea), with more than 100 nominal species recorded to date (Moran et al. 1999; Lom and Dyková 2006; Eiras et al. 2014b; Sato and Kasai 2016). The genus is defined as myxosporeans with four or more shell valves (SV) and polar capsules (PC) which are equal in number (Whipps et al. 2003a, 2004; Lom and Dyková 2006). During the last two decades, introducing molecular genetic analyses into the process of specifically differentiating kudoids and other myxosporeans has enabled a more precise understanding of their biodiversity and biogeography. As discussed in a recent article from our laboratory (Kasai et al. 2017a), although a group of Kudoa spp. characterized by myxospores of unequal quadrangular pyramid shape and containing one large and three smaller SV/PC showed fairly close morphological resemblance to each other, at least nine species were differentiated by nucleotide sequencing of the ribosomal RNA gene (rDNA), i.e., K. thyrsites, K. minithyrsites, K. lateolabracis, K. megacapsula, K. whippsi, K. gunterae, K. cheilodipteri, K. parathyrsites, and K. akihitoi (Whipps et al. 2003b; Yokoyama et al. 2004; Yokoyama and Itoh 2005; Whipps and Kent 2006; Burger and Adlard 2010a; Heiniger et al. 2013; Kasai et al. 2016, 2017a). Despite the fact that different extents of nucleotide variation of the rDNA within a species can complicate the specific identification, phylogenetic critique of the phenotypical variation of a species facilitates our understanding of the real biodiversity, as previously demonstrated by numerous studies on a wide range of myxosporeans (Diamant et al. 2005; Burger et al. 2008; Burger and Adlard 2010b, 2011; Miller and Adlard 2012, 2013; Azevedo et al. 2014; Bartošová-Sojková et al. 2014; Fiala et al. 2015; Alama-Bermejo et al. 2016; Sekiya et al. 2016; Kato et al. 2017).
Up until the end of 2016, 25 Kudoa spp. and two Unicapsula spp. had been recorded from edible marine fish species in the natural seawater around Japan or popular imported marine fish consumed daily by Japanese people (Sato and Kasai 2016). This recent survey, however, highlights the need to record more myxosporeans from daily consumed marine fish. In the present study, we characterize morphologically and genetically a new kudoid species exhibiting stellate spores with six equal SV/PC, Kudoa konishiae n. sp., from the Japanese Spanish mackerel, Scomberomorus niphonius (Cuvier, 1832), purchased from fish markets in western Japan.
Materials and methods
Fish samples and parasitological examination
Whole bodies of eight individuals of the fish species Japanese Spanish mackerel were purchased from local fish markets in western Japan: two individuals fished on October 17, 2016, in the Sea of Japan off Abu-cho, Yamaguchi Prefecture, Japan, and six individuals fished on November 8 and 29, 2016, in the northwestern Pacific Ocean off Kagoshima Prefecture, Japan. The standard lengths and body weights of these eight individuals were 42–50 (46) cm and 0.78–1.02 (0.86) kg, respectively (range with the average in parentheses). Following transportation of the samples on ice, fish were cut open, and their gills and viscera were removed and examined under a dissection microscope. Filleted fish meats were examined on the day of arrival or frozen until examination. Thin slices of muscle fillets were pressed between two glass plates and examined under a dissection microscope to detect the presence of myxosporean cysts or pseudocysts.
When myxosporean plasmodia were detected, muscle slices were placed in physiological saline, and parasitized myofibers were carefully isolated with fine forceps. The release of myxospores from a pseudocyst in the myofiber was executed with fine forceps. Myxospores were observed using a microscope equipped with differential interference contrast imaging, photographed at a magnification of ×800, then transformed into photographs with Adobe® Photoshop® ver. 11.0 (Adobe Systems, San Jose, CA, USA). Photographs were then printed at a high magnification. Measurements were conducted on multiple printed photographs following the guidelines of Lom and Arthur (1989). All measurements are expressed in μm unless otherwise stated. Ranges with the means in parentheses are presented. Following removal of a portion of the myxospores for DNA extraction, the parasite was fixed in 10% neutral-buffered formalin solution and 70% ethanol solution. Specimens collected in the present work were deposited in the Meguro Parasitological Museum, Tokyo, Japan, under collection nos. 21377 and 21378.
For scanning electron microscopy, a portion of the formalin-fixed myxospores was washed three times in 0.2 M Na2HPO4-NaH2PO4 solution (PB), pH 7.8, and immersed in 2.5% glutaraldehyde in PB overnight. Following three washes in PB, the sample was post-fixed in 1% (w/v) osmium tetroxide in PB for 1 h. After washing three times in PB, the sample was dehydrated through a graded ethanol series, immersed in warmed t-butyl-alcohol, and cooled at 4 °C for 2 h. All processing was conducted using SEMpore® with 0.6-μm pore size (JEOL, Akishima, Tokyo, Japan) to reduce myxospore loss. The polycarbonate membrane of SEMpore® was then freeze-dried (model JFD-300; JEOL), mounted on stubs, and sputter-coated with gold-palladium at 200 Å (model JFC-1500; JEOL). Samples were examined using a scanning electron microscope (model JSM-6100; JEOL) at an accelerating voltage of 15 kV.
DNA extraction, polymerase chain reaction (PCR), and sequencing
Parasite DNA was extracted from kudoid plasmodia using an Illustra™ tissue and cells genomicPrep Mini Spin Kit (GE Healthcare UK, Buckinghamshire, UK) according to the instructions of the manufacturer. PCR amplification of overlapping fragments of the rDNA was performed in a 20-μl volume containing a DNA polymerase, Blend Taq-Plus- (TOYOBO, Dojima Hama, Osaka, Japan), and primers as described previously (Li et al. 2013; Kasai et al. 2015). The PCR products were purified using a FastGene Gel/PCR Extraction Kit (NIPPON Genetics Co., Tokyo, Japan) and sequenced directly. When direct sequencing was not satisfactory, the purified PCR products were cloned into the plasmid vector pTA2 (TArget Clone™; TOYOBO) and transformed into Escherichia coli JM109 (TOYOBO) according to the instructions of the manufacturer. Following propagation, the plasmid DNA was extracted using a FastGene Plasmid Mini Kit (NIPPON Genetics Co.), and inserts from multiple independent clones, at least three, were sequenced using universal M13 forward and reverse primers.
Further, molecular genetic characterization of kudoid isolates was conducted on the mitochondrial DNA, i.e., cytochrome c oxidase subunit 1 gene (cox-1) and the small and large subunits of the ribosomal RNA gene (rns-rnl). To this end, five plasmodia of two isolates (ABU and KGS) of the kudoid from two Japanese Spanish mackerel individuals and 22 plasmodia of ten yellowfin tunas (Thunnus albacares), six longtail tunas (Thunnus tonggol), and two Pacific bluefin tunas (Thunnus orientalis) were individually analyzed (Table 1). Detailed information on the tuna samples has been documented in a previous report (Kasai et al. 2017b). Amplification of two of the mitochondrial genes was attempted by using the primer pairs described by Takeuchi et al. (2016) for Kudoa septempunctata (cox1-F1 and cox1-R1 for the first round of PCR, followed by cox1-F2 and cox1-R3 for the second round of PCR; and similarly rnl-F1 and rnl-R1, followed by rnl-F2 and rnl-R2); however, this proved unsuccessful. Consequently, new primer pairs were designed using the online software Primer3web ver. 4.0.0 (Untergasser et al. 2012) and referring to a complete mitochondrial genome of K. hexapunctata (DDBJ/EMBL/GenBank accession no. LC009437; Takeuchi et al. 2015). The PCR cycling protocol was 3 min at 94 °C, then 35 cycles of 30 s at 94 °C, 30 s at 52 °C, and 60 s at 72 °C, followed by a final extension at 72 °C for 7 min. Primer pairs of Kudoa_Cox1-F3 and Kudoa_Cox1-R3, Kudoa_RnL-F3 and Kudoa_RnL-R3, and Kudoa_RnL-F4 and Kudoa_RnL-R4 (Table 2) were used to amplify long mitochondrial DNA sequences (the first pair for cox-1 and the second and third pairs for rns-rnl). When necessary, a second round of PCR using the primer pair Kudoa_Cox1-F4 and Kudoa_Cox1-R4 (Table 2) was conducted after the first round of PCR. Two primers, Kudoa_RnL-F5 and Kudoa_RnL-R5, were used only for sequencing, whereas the other primers mentioned above were used for both PCR amplification and amplicon sequencing.
The nucleotide sequences obtained in the present study are available from the DDBJ/EMBL/GenBank databases under the accession nos. LC316965 and LC316966 (rDNA), LC316989–LC316996 (cox-1), and LC316997–LC317003 (rns-rnl).
Phylogenetic analysis
For phylogenetic analysis, the newly obtained rDNA nucleotide sequences of the Japanese Spanish mackerel Kudoa sp. in the present study and related Kudoa sequences retrieved from the DDBJ/EMBL/GenBank databases were aligned using the CLUSTAL W multiple alignment program (Thompson et al. 1994), with subsequent manual adjustment. The accession numbers of the sequences analyzed in the present study are given in the figures showing phylogenetic trees. Regions judged to be poorly aligned and characters with a gap in any sequence were excluded from subsequent analyses; 1371 characters, of which 368 were variable, remained for subsequent analysis for the 18S rDNA of the majority of kudoids forming either cysts or pseudocysts (70 taxa), 1401 characters, of which 169 were variable, for the 18S rDNA of mainly pseudocyst-forming kudoids (42 taxa), and 564 characters, of which 243 were variable, for the 28S rDNA of mainly pseudocyst-forming kudoids (46 taxa). Maximum likelihood (ML) analysis was performed with the program PhyML as described previously (Matsukane et al. 2010; Li et al. 2013). Three Unicapsula spp. (Myxosporea: Multivalvulida; DDBJ/EMBL/GenBank accession nos. AB971675, AB971677, and AB971679) recorded in Japan, Vietnam, and Australia (Miller and Adlard 2013; Tomochi et al. 2014) were used as an outgroup for the construction of the ML phylogenetic tree based on the 18S rDNA of the majority of kudoids. To increase the resolution of the ML phylogenetic trees, kudoid species forming cysts for plasmodia, which usually position near the root of, but form separate clades from, the vast majority of Kudoa spp. in phylogenetic trees based on the rDNA, were omitted from the analyses, and selected species forming pseudocysts were phylogenetically analyzed.
Similarly, partial mitochondrial DNA sequences of cox-1 and rnl were aligned; 435 characters, of which 106 were variable, and 709 characters, of which 137 were variable, remained for subsequent analyses for cox-1 and rnl, respectively. ML phylogenetic trees were constructed with multiple K. septempunctata sequences (three cox-1 haplotypes and two rnl haplotypes; Takeuchi et al. 2016) as an outgroup for each gene.
Results
Incidence of kudoid infection in the Japanese Spanish mackerel
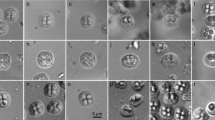
Two Japanese Spanish mackerels, one from the Sea of Japan off Abu-cho, Yamaguchi Prefecture, and the other from the northwestern Pacific Ocean off Kagoshima Prefecture, were infected with a myxosporean species forming pseudocysts in the myofiber of trunk muscles. Stellate myxospores isolated from the plasmodia had six equal SV/PC which were radially arranged in apical view and drawstring-pouch-shaped in lateral view with teardrop-shaped PC (Figs. 1e–h, and 2). Since the isolated myxospores are unique in morphology and distinct from any of the known Kudoa spp. as shown later in the present study, a new species, Kudoa konishiae n. sp., is erected for this parasite as follows.
Photographs of plasmodia (a–d) and fresh myxospores (e–h) of Kudoa konishiae n. sp. from Scomberomorus niphonius. Myxospores are shown in apical (e, f) and lateral (g, h) view. All photographs of each set (a–d or e–h) are at the same magnification, with the scale shown on the rightmost photograph of each line (d, h)
Description
Kudoa konishiae n. sp. (Myxosporea: Multivalvulida)
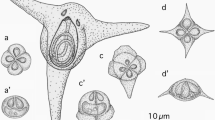
Small, club-shaped plasmodia with blunt ends or spindle-shaped plasmodia, 0.15–0.42 (0.28) mm by 0.07–0.12 (0.09) mm (n = 8) or 0.22–0.53 (0.34) mm by 0.04–0.13 (0.08) mm (n = 8) in the two host fish, forming pseudocysts in the myofiber of trunk muscles, polysporic and synchronized spore development. Myxospores stellate with six equal SV/PC in apical view, without SV ornamentation and apical projection, demonstrated by scanning electron microscopy round peripheral expansion of SV with distinct suture lines between them in apical view. In lateral view, myxospores drawstring-pouch in shape, PC teardrop-like, were occupying apical half of myxospores. Coils of polar filament were not seen in wet preparations. The spores were having dimensions of (n = 20): width 8.1–9.7 (9.0), thickness 7.2–8.8 (8.2), sutural thickness 6.7–7.5 (7.1), length 7.1–7.9 (7.5), PC length 2.7–4.2 (3.6), and PC width 1.2–1.8 (1.5).
Nucleotide sequences of the 18S and 28S rDNA, 1561, and 743 bp in length, respectively, were obtained for five plasmodia from two fish samples fished in the Sea of Japan (three plasmodia of ABU isolate) and northwestern Pacific Ocean (two plasmodia of KGS isolate). The nucleotide sequences of each region were identical for all plasmodia (DDBJ/EMBL/GenBank accession nos. LC316965 and LC316966). Partial nucleotide sequences of the mitochondrial genes cox-1 (437 bp) and rns-rnl (1367 bp) were similarly obtained for each isolate and were absolutely identical to each other (DDBJ/EMBL/GenBank accession nos. LC316996 and LC317003).
Taxonomic summary
Host
Scomberomorus niphonius (Cuvier, 1832), Japanese Spanish mackerel (Actinopterygii: Perciformes: Scombridae).
Locality
Sea of Japan, off Abu-cho, Yamaguchi Prefecture, Japan. In addition, northwestern Pacific Ocean off Kagoshima Prefecture, Japan.
Site of infection
Pseudocysts in the myofiber of somatic muscles.
Materials deposited
Hapantotype no. 21377, Meguro Parasitological Museum, Tokyo, Japan (ABU isolate). Paratype no. 21378 in the same museum (KGS isolate).
Prevalence
One of two individuals fished in the Sea of Japan, or one of six individuals in the northwestern Pacific Ocean in the autumn of 2016. In each fish individual, 13–28 (15.8) pseudocysts/g muscle or 4–7 (4.7) pseudocysts/g muscle.
Etymology
The species is named in honor of Prof. Yoshiko Sugita-Konishi, Azabu University, Kanagawa, Japan, who is a former director of the Division of Microbiology, National Institute of Health Sciences, Japan, and played a crucial role in identifying the cause of food poisoning due to unknown cause(s) after consumption of fresh fish fillets, currently known as “Kudoa food poisoning.”
Remarks
Four Kudoa spp. have previously been described from Scomberomorus spp., i.e., Kudoa crumena in Atlantic Spanish mackerel (S. maculatus) (Iversen and Van Meter 1967), K. permulticapsula and K. scomberomori in narrow-barred Spanish mackerel (S. commerson) (Whipps et al. 2003a; Adlard et al. 2005), and Kudoa sp. in Serra Spanish mackerel (S. brasiliensis) (Eiras et al. 2014a). Except for K. scomberomori which has stellate myxospores with six equal SV/PC, the three other species are distinct from the present new species in myxospore morphology, having four or 13 SV/PC (Iversen and Van Meter 1967; Whipps et al. 2003a; Eiras et al. 2014a). Although myxospores of K. scomberomori, K. neothunni, K. hexapunctata, and K. grammatorcyni are stellate with six SV/PC, those of K. konishiae n. sp. are morphologically different in terms of spore dimensions (see Table 3) and the degree of peripheral SV expansion (rounded edge vs. pointed edge). Furthermore, scanning electron microscopy revealed a greater relative spore height (spore length against spore thickness or width) compared with the other kudoids displaying stellate myxospores with six SV/PC (Fig. 2). The morphological uniqueness of the present new species from known kudoids is supported by molecular genetic analyses of the 18S and 28S rDNA and mitochondrial DNA genes (cox-1 and rnl) as discussed below.
Phylogenetic analyses of the rDNA
Nucleotide sequences of the 18S and 28S rDNA, 1561 and 743 bp long, respectively, were obtained for K. konishiae n. sp. The closest 18S rDNA nucleotide sequence was K. permulticapsula (AY078429) with 99.23% (1548/1560) identity and one nucleotide insertion/deletion (indel), followed by K. neothunni, K. hexapunctata, K. grammatorcyni, and K. scomberomori with 99.04% (1546/1561) or 98.98% (1545/1561) identity. Other Kudoa spp. had 98.78% (1541/1560) or lower identities with frequent nucleotide indels. The closest 28S rDNA nucleotide sequence was K. neothunni with 95.81% (709/740) identity and three indels, followed by K. hexapunctata with 94.73% (701/740) identity and three indels. The 28S rDNA nucleotide sequences of other Kudoa spp. showed frequent indels and lower identities with that of K. konishiae n. sp. Regarding molecular rDNA homology with Kudoa spp. with deposited nucleotide sequences, K. konishiae n. sp. was positioned at the root or near the root of clades of Kudoa spp. with stellate myxospores with five to seven (mainly six) SV/PC from scombrid fish (confined to the genera Grammatorcynus, Scomberomorus, and Thunnus at present) in the phylogenetic trees based on the 18S and 28S rDNA nucleotide sequences (Figs. 3 and 4).
ML phylogenetic trees based on the 18S rDNA sequence of the majority of cyst- and pseudocyst-forming Kudoa spp. (a), or representative Kudoa spp. forming pseudocysts (b) which correspond to species in the phylogenetic branch boxed in the tree (a). On the right side of the tree (b), the number of spore valves and polar capsules per myxospore, and parasite localization are shown. Abbreviations of tissue preference are CN, central nervous system; OV, ovary and eggs; TM, trunk muscle; and VM, visceral membrane. Gray bars on the rightmost edge indicate two different clades of Kudoa spp. with five or more shell valves / polar capsules
ML phylogenetic tree based on the 28S rDNA sequence of representative Kudoa spp. forming pseudocysts. On the right side of the tree, the number of spore valves and polar capsules per myxospore, and parasite localization are shown as in Fig. 3. Additional abbreviations of tissue preference are VO, visceral organs; and SO, systemic organs. Similarly as in Fig. 3, gray bars on the rightmost edge indicate two different clades of Kudoa spp. with five or more shell valves/polar capsules
Phylogenetic analyses of the mitochondrial DNA genes (cox-1 and rnl)
Partial cox-1 nucleotide sequences of two isolates of K. konishiae n. sp. were successfully obtained by nested PCR using the primer pair of Kudoa_Cox1-F4 and Kudoa_Cox1-R4 after the first round of PCR using primers Kudoa_Cox1-F3 and Kudoa_Cox1-R3. The length of the resultant cox-1 nucleotide sequences was 437 bp. Partial cox-1 nucleotide sequences of K. neothunni and K. hexapunctata were obtained by standard PCR using the primer pair of Kudoa_Cox1-F3 and Kudoa_Cox1-R3. Six types of 711-bp long cox-1 nucleotide sequences were obtained for these two species: two types for K. neothunni and four types for K. hexapunctata. Intraspecific nucleotide variation of cox-1 sequences of K. neothunni was found at five nucleotide sites (99.30% or higher molecular genetic identities), and that of K. hexapunctata was found at six nucleotide sites (99.16% or higher molecular genetic identities). A maximum of 97.32% nucleotide sequence identity was found between these two species. All of the 237-amino acid sequences translated from the different cox-1 nucleotide sequences of K. neothunni and K. hexapunctata were absolutely identical, suggesting that the nucleotide substitutions occurred predominantly at the third nucleotide of codons. The maximum cox-1 nucleotide identities of K. konishiae n. sp. with K. neothunni and K. hexapunctata were 87.87% (384/437) or 88.56% (387/437), respectively, with translated amino acid identities of 92.47% (135/146), demonstrating substantial genetic distances of K. konishiae n. sp. from these closely related species and illustrated by the phylogenetic tree based on the cox-1 nucleotide sequences (Fig. 5a).
ML phylogenetic trees based on the partial mitochondrial gene sequences (a, cox-1 and b, rnl) of representative Kudoa spp. with six or more spore valves/polar capsules per myxospore. Parasite names are followed by host fish species, isolation localities, and DDBJ/EMBL/GenBank accession numbers. To the far right, the haplotype names of mitochondrial genes of K. septempunctata, defined by Takeuchi et al. (2016), are shown for reference
PCR using the primer pair of Kudoa_RnL-F3 and Kudoa_RnL-R3 amplified a 1367-bp long mitochondrial DNA sequence of K. konishiae n. sp. containing an almost complete small subunit ribosomal RNA gene and an approximately 2/3 portion of the 5′-terminus area of the large subunit ribosomal RNA gene. Similarly, a 1368-bp nucleotide sequence of K. neothunni (13 isolates) and 1370-bp long nucleotide sequences of K. hexapunctata (six isolates) were newly obtained. No intraspecific variation was found throughout the length of the 1368-bp rns-rnl nucleotide sequences of K. neothunni, whereas intraspecific variation of the same mitochondrial DNA region of K. hexapunctata was demonstrated at nine nucleotide sites; however, the nucleotide sequence identity between different isolates was never lower than 99.49%. Interspecific variation between K. neothunni and K. hexapunctata was found at 52 nucleotide sites (including two indels), resulting in approximately 95% identities. Both K. neothunni and K. hexapunctata showed approximately 80% nucleotide sequence identities with K. konishiae n. sp. The phylogenetic relationships of these three species along with K. septempunctata based on the partial rnl nucleotide sequences are shown in Fig. 5b.
Discussion
When Whipps et al. (2004) redefined the genus Kudoa Meglitsch, 1947, as myxosporeans having four or more SV/PC, there were only six Kudoa spp. with more than four SV/PC: K. neurophila, K. cutanea, K. muscularis, K. shulmani, K. neothunni, and K. yasunagai. Currently, such Kudoa spp. have increased in number to 18 (Sato and Kasai 2016), and many but not all of them have been genetically characterized based on the rDNA nucleotide sequences (Grossel et al. 2003, 2005; Whipps et al. 2003a, 2004; Adlard et al. 2005; Gunter et al. 2006; Burger et al. 2007; Burger and Adlard 2010b, 2011; Matsukane et al. 2010; Meng et al. 2011; Miller and Adlard 2012; Li et al. 2013; Yokoyama et al. 2014; Shirakashi et al. 2014; Kasai et al. 2017b). In Figs. 3 and 4 showing the phylogenetic trees based on either the 18S or 28S rDNA nucleotide sequence, Kudoa spp. with more than four SV/PC were exclusively confined to two clades: the first clade containing muscle-preferring Kudoa spp. with mainly six SV/PC (exceptionally five or 13 SV/PC), such as K. permulticapsula, K. monodactyli, K. grammatorcyni, K. scomberomori, K. neothunni, K. hexapunctata, and the present new species K. konishiae n. sp.; the other clade containing Kudoa spp. with fluctuating numbers of SV/PC (between five and nine) even within a species, localized in the central nervous system or trunk muscles, such as K. neurophila, K. lethrini, K. yasunagai, K. chaetodoni, K. lemniscati, K. prunusi, K. septempunctata, K. thalassomi, and K. igami. Host fish for the former clade belonged exclusively to Scombrinae (Perciformes: Scombridae), whereas those for the latter clade were varied.
Recently, mitochondrial DNA genes (cox-1 and rnl) of myxosporeans were analyzed for the first time to biogeographically characterize K. septempunctata isolated from olive flounders (Paralichthys olivaceus), farmed in Japan and Korea, or fished from the natural waters around Japan (Takeuchi et al. 2016). Takeuchi et al. (2016) differentiated three genotypes based on single nucleotide polymorphisms at seven cox-1 and two rnl nucleotide sites, i.e., ST1 genotype (cox1-1 and rnl-1), ST2 genotype (cox1-2 and rnl-2), and ST3 genotype (cox1-3 and rnl-2), and suggested an almost exclusive prevalence of ST1 and ST2 genotypes in Japan and ST3 genotype in Korea. This molecular strategy to identify different parasite populations was applied to the ABU and KGS isolates of K. konishiae n. sp. from Japanese Spanish mackerels fished in the Sea of Japan and northwestern Pacific Ocean, respectively, since S. niphonius is thought to be divided into two ecological stocks, East China Sea (China) stock and Seto Inland Sea (Japan) stock (Shui et al. 2009), and it is possible that the two aforementioned isolates might originate from different spawning places of the host fish. The analyses were conducted together with multiple isolates of K. hexapunctata and K. neothunni reported in our previous work (Kasai et al. 2017b), which show close morphological and phylogenetic resemblances to the new species.
As shown in Fig. 5, both the cox-1 and rnl nucleotide sequences of mitochondrial DNA clearly separated the three different species with six SV/PC in stellate spores. Furthermore, unique K. neothunni spores with seven SV/PC recently isolated from the longtail tuna in the East China Sea (Kasai et al. 2017b) were demonstrated to be conspecific with the species with six SV/PC prevalent in the yellowfin tuna in the northwestern Pacific Ocean around the Philippines. This was ascertained from the finding of no or only a few nucleotide differences in the 18S, 5.8S, and 28S rDNA but substantial nucleotide differences in the internal transcribed regions (ITS1 and ITS2) of the rDNA (Kasai et al. 2017b). As far as examined here, no genetically distinct populations of any of these Kudoa species (K. hexapunctata, K. neothunni, or K. konishiae n. sp.) were noted by host fish or locality (sea area). Incorporation of the nucleotide sequencing of mitochondrial genes for closely related kudoid organisms could provide further insights into their phylogenetic or biogeographical relatedness after speciation and geographical dispersal of different species or intraspecific unique phenotype(s).
References
Adlard RD, Bryant MS, Whipps CM, Kent ML (2005) Multivalvulid myxozoans from eastern Australia: three new species of Kudoa from scombrid and labrid fishes of the Great Barrier Reef, Queensland, Australia. J Parasitol 91(5):1138–1142. https://doi.org/10.1645/GE-368R.1
Alama-Bermejo G, Jirků M, Kodádková A, Pecková H, Fiala I, Holzer AS (2016) Species complexes and phylogenetic lineages of Hoferellus (Myxozoa, Cnidaria) including revision of the genus: a problematic case for taxonomy. Parasites Vectors 9(1):13. https://doi.org/10.1186/s13071-015-1265-8
Azevedo C, Rocha S, Motos P, Motos E, Oliveira E, Al-Quraishy S, Csal G (2014) Morphological and phlogeny of Henneguya jocu n. sp. (Myxosporea: Myxobolidae), infecting the gills of the marine fish Lutjanus jocu. Eur J Protistol 50:185–193
Bartošová-Sojková P, Hrabcová M, Pecková H, Patra S, Kodádková A, Jurajda P, Tyml T, Holzer AS (2014) Hidden diversity and evolutionary trends in malacosporean parasites (Cnidaria: Myxozoa) identified using molecular phylogenetics. Int J Parasitol 44(8):565–577. https://doi.org/10.1016/j.ijpara.2014.04.005
Burger MAA, Adlard RD (2010a) Four new species of Kudoa Meglitsch, 1947 (Myxosporea: Multivalvulida) from Australia with recommendations for species descriptions in the Kudoidae. Parasitology 137(05):793–814. https://doi.org/10.1017/S0031182009991557
Burger MAA, Adlard RD (2010b) Phenotypic variation in a significant spore character in Kudoa (Myxosporea: Multivalvulida) species infecting brain tissue. Parasitology 137(12):1759–1772. https://doi.org/10.1017/S0031182010000673
Burger MAA, Adlard RD (2011) Low host specificity in the Kudoidae (Myxosporea: Multivalvulida) including seventeen new host records for Kudoa thalassomi. Folia Parasitol 58(1):1–16. https://doi.org/10.14411/fp.2011.001
Burger MAA, Cribb TH, Adlard RD (2007) Patterns of relatedness in the Kudoidae with descriptions of Kudoa chaetodoni n. sp. and K. lethrini n. sp. (Myxosporea: Multivalvulida). Parasitology 134(05):669–681. https://doi.org/10.1017/S0031182006001995
Burger MA, Brames AC, Adlard RD (2008) Wildlife as reservoirs for parasites infecting commercial species: host specificity and redescription of Kudoa amamiensis from teleost fish in Australia. J Fish Dis 31(11):835–844. https://doi.org/10.1111/j.1365-2761.2008.00958.x
Diamant A, Ucko M, Paperna I, Colorni A, Lipshitz A (2005) Kudoa iwatai (Myxosporea: Multivalvulida) in wild and cultured fish in the red sea: redescription and molecular phylogeny. J Parasitol 91:1175–1189
Eiras JC, Lima JTAX, Cruz CF, Saraiva A (2014) A note on the infection of Scomberomorus brasiliensis (Osteichthyes, Scombridae) by Kudoa sp. (Myxozoa: Multivalvulida). Braz J Biol 73 (suppl) 74(3 suppl 1):S164–S166. https://doi.org/10.1590/1519-6984.23712
Eiras JC, Saravia A, Cruz C (2014) Synopsis of the species of Kudoa Meglitsch, 1947 (Myxozoa: Myxosporea: Multivalvulida). Syst Parasitol 87: 153–180
Fiala I, Hlavničková M, Kodádková A, Freeman MA, Bartošová-Sojková P, Atkinson SD (2015) Evolutionary origin of Ceratonova shasta and phylogeny of the marine myxosporean lineage. Mol Phylogenet Evol 86:75–89
Grossel GW, Dykova I, Handlinger J, Munday BL (2003) Pentacapsula neurophila sp. n. (Multivalvulida) from the central nervous system of striped trumpeter, Latris lineata (Forster). J Fish Dis 26(6):315–320. https://doi.org/10.1046/j.1365-2761.2003.00459.x
Grossel GW, Handlinger J, Battaglene S, Munday BL (2005) Diagnostic polymerase chain reaction assay to detect Kudoa neurophila (Myxozoa: Multivalvulida) in a marine finfish hatchery. Dis Aquat Org 64(2):141–149. https://doi.org/10.3354/dao064141
Gunter NL, Cribb TH, Whipps CM, Adlard RD (2006) Characterization of Kudoa monodactyli n. sp. (Myxosporea: Multivalvulida) from the muscle of Monodactylus argenteus (Teleostei: Monodactylidae) from Moreton Bay, Queensland, Australia. J Eukaryot Microbiol 53(5):374–378. https://doi.org/10.1111/j.1550-7408.2006.00115.x
Heiniger H, Cribb TH, Adlard RD (2013) Intra-specific variation of Kudoa spp. (Myxosporea: Multivalvulida) from apogonid fishes (Perciformes), including the description of two new species, K. cheilodipteri n. sp. and K. cookii n. sp., from Australian waters. Syst Parasitol 84(3):193–215. https://doi.org/10.1007/s11230-012-9400-9
Iversen ES, Van Meter NN (1967) A new myxosporidian (Sporozoa) infecting the Spanish mackerel. Bull Mar Sci 17:268–273
Kasai A, Li Y-C, Setsuda A, Mafie E, Sato H (2015) Genetic characterization of Kudoa iwatai and Kudoa trachuri in commercial marine fish (Platycephalus sp. and Trachurus japonicus) for human consumption. Jpn J Vet Parasitol 14:22–30
Kasai A, Li Y-C, Mafie E, Sato H (2016) New host records of monacanthid fish for three Kudoa spp. (K. septempunctata, K. thyrsites, and K. shiomitsui) prevalent in the olive flounder (Paralichthys olivaceus), with the description of K. parathyrsites n. sp. from a black scraper (Thamnaconus modestus). Parasitol Res 115(7):2741–2755. https://doi.org/10.1007/s00436-016-5023-4
Kasai A, Setsuda A, Sato H (2017) Morphological and genetic characterization of Kudoa whippsi (Myxosporea: Multivalvulida) from Cheilodactylus zonatus in the western Pacific Ocean off Japan, and two new Kudoa spp. (K. akihitoi n. sp. and K. empressmichikoae n. sp.) from Acanthogobius hasta in the Sea of Ariake, Japan. Parasitol Res 116:647–659
Kasai A, Tsuduki H, Jimenez LA, Li Y-C, Tanaka S, Sato H (2017) Incidence of three Kudoa spp., K. neothunni, K. hexapunctata, and K. thunni (Myxosporea: Multivalvulida), in Thunnus tunas distributed in the western Pacific Ocean. Parasitol Res 116(4):1137–1150. https://doi.org/10.1007/s00436-016-5369-7
Kato E, Kasai A, Tomochi H, Li Y-C, Sato H (2017) Four Myxobolus spp. (Myxosporea: Bivalvulida) from the gill lamellae of common carp (Cyprinus carpio) and Japanese silver crucian carp (Carassius langsdorfii) in the western part of Japan, with the description of three new species (M. tanakai n. sp., M. paratoyamai n. sp., and M. ginbuna n. sp.) Parasitol Res 116:2427–2441
Li Y-C, Sato H, Tanaka S, Ohnishi T, Kamata Y, Sugita-Konishi Y (2013) Characterization of the ribosomal RNA gene of Kudoa neothunni (Myxosporea: Multivalvulida) in tunas (Thunnus spp.) and Kudoa scomberi n. sp. in a chub mackerel (Scomber japonicus). Parasitol Res 112(5):1991–2003. https://doi.org/10.1007/s00436-013-3357-8
Lom J, Arthur JR (1989) A guideline for the preparation of species descriptions in Myxosporea. J Fish Dis 12(2):151–156. https://doi.org/10.1111/j.1365-2761.1989.tb00287.x
Lom J, Dyková I (2006) Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol 53(1):1–36. https://doi.org/10.14411/fp.2006.001
Matsukane Y, Sato H, Tanaka S, Kamata Y, Sugita-Konishi Y (2010) Kudoa septempunctata n. sp. (Myxosporea: Multivalvulida) from an aquacultured olive flounder (Paralichthys olivaceus) imported from Korea. Parasitol Res 107(4):865–872. https://doi.org/10.1007/s00436-010-1941-8
Meng F, Yokoyama H, Shirakashi S, Grabner D, Ogawa K, Ishimaru K, Sawada Y, Murata O (2011) Kudoa prunusi n. sp. (Myxozoa: Multivalvulida) from the brain of Pacific bluefin tuna Thunnus orientalis (Temminck & Schlegel, 1844) cultured in Japan. Parasitol Int 60(1):90–96. https://doi.org/10.1016/j.parint.2010.11.006
Miller TL, Adlard RD (2012) Brain infecting kudoids of Australia’s coral reefs, including a description of Kudoa lemniscati n. sp. (Myxosporea: Kudoidae) from Lutjanus lemniscatus (Perciformes: Lutjanidae) off Ningaloo Reef, western Australia. Parasitol Int 61(2):333–342. https://doi.org/10.1016/j.parint.2012.01.002
Miller TL, Adlard RD (2013) Unicapsula species (Myxosporea: Trilosporidae) of Australian marine fishes, including the description of Unicapsula andersenae n. sp. in five teleost families off Queensland, Australia. Parasitol Res 112(8):2945–2957. https://doi.org/10.1007/s00436-013-3467-3
Moran JDW, Whitaker DJ, Kent ML (1999) A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture 172(1-2):163–196. https://doi.org/10.1016/S0044-8486(98)00437-2
Sato H, Kasai A (2016) Kudoa species (Myxozoa: Myxosporea: Multivalvulida) recorded in Japan or its surrounding natural waters (1930-2016). Jpn J Vet Parasitol 15: 111–138 (in Japanese with English summary)
Sekiya M, Setsuda A, Sato H, Song K, Han J-K, Kim G-J, Yeo IK (2016) Enteromyxum leei (Myxosporea: Bibalvulida) as the cause of myxosporean emaciation disease of farmed olive flounders (Paralichthys olivaceus) and a turbot (Scophthalmus maximus) on Jeju Island, Korea. Parasitol Res 115(11):4229–4237. https://doi.org/10.1007/s00436-016-5200-5
Shirakashi S, Yamane K, Ishitani H, Yanagida T, Yokoyama H (2014) First report of Kudoa species in the somatic muscle of the Japanese parrotfish Calotomus japonicus (Scaridae) and a description of Kudoa igami n. sp. (Myxozoa: Multivalvulida). Parasitol Res 113:2515–2524
Shui B-N, Han Z-Q, Gao T-X, Miao Z-Q, Yanagimoto T (2009) Mitochondrial DNA variation in the East China Sea and Yellow Sea populations of Japanese Spanish mackerel Scomberomorus niphonius. Fish Sci 75(3):593–600. https://doi.org/10.1007/s12562-009-0083-3
Takeuchi F, Sekizuka T, Ogasawara Y, Yokoyama H, KamikawaR IY, Nozaki T, Sugita-Konishi Y, Ohnishi T, Kuroda M (2015) The mitochondrial genomes of a myxozoan genus Kudoa are extremely divergent in Metazoa. PLoS One 10(7):e0132030. https://doi.org/10.1371/journal.pone.0132030
Takeuchi F, Ogasawara Y, Kato K, Sekizuka T, Nozaki T, Sugita-Konishi Y, Ohnishi T, Kuroda M (2016) Genetic variants of Kudoa septempunctata (Myxozoa: Multivalvulida), a flounder parasite causing foodborne disease. J Fish Dis 39(6):667–672. https://doi.org/10.1111/jfd.12395
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680. https://doi.org/10.1093/nar/22.22.4673
Tomochi H, Li YC, Tran BT, Yanagida T, Sato H (2014) Three Unicapsula species (Myxosporea: Trilosporidae) of Asian marine fishes, including the description of Unicapsula setoensis n. sp. in the yellowfin goby (Acanthogobius flavimanus) from the Inland Sea of Japan. Parasitol Res 113(10):3807–3816. https://doi.org/10.1007/s00436-014-4048-9
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40(15):e115. https://doi.org/10.1093/nar/gks596
Whipps CM, Kent ML (2006) Phylogeography of the cosmopolitan marine parasite Kudoa thyrsites (Myxozoa: Myxosporea). J Eukaryot Microbiol 53(5):364–373. https://doi.org/10.1111/j.1550-7408.2006.00114.x
Whipps CM, Adlard RD, Bryant MS, Kent ML (2003a) Two unusual myxozoans, Kudoa quadricornis n. sp. (Multivalvulida) from the muscle of goldspotted trevally (Carangoides fulvoguttatus) and Kudoa permulticapsula n. sp. (Myltivalvulida) from the muscle of Spanish mackerel (Scomberomorus commerson) from the Great Barrier Reef, Australia. J Parasitol 89(1):168–173.
Whipps CM, Adlard RD, Bryant MS, Lester RJG, Findlay V, Kent ML (2003b) First report of three Kudoa species from eastern Australia: Kudoa thyrsites from mahi mahi (Coryphaena hippurus), Kudoa amamiensis and Kudoa minithyrsites n. sp. from sweeper (Pempheris ypsilychnus). J Eukaryot Microbiol 50(3):215–219. https://doi.org/10.1111/j.1550-7408.2003.tb00120.x
Whipps CM, Grossel G, Adlard RD, Yokoyama H, Bryant MS, Munday BL, Kent ML (2004) Phylogeny of the Multivalvulidae (Myxozoa: Myxosporea) based on comparative ribosomal DNA sequence analysis. J Parasitol 90(3):618–622. https://doi.org/10.1645/GE-153R
Yokoyama H, Itoh N (2005) Two multivalvulid myxozoans causing postmortem myoliquefaction: Kudoa megacapsula n. sp. from red barracuda (Sphyraena pinguis) and Kudoa thyrsites from splendid alfonso (Beryx splendens). J Parasitol 91(5):1132–1137. https://doi.org/10.1645/GE-548R.1
Yokoyama H, Whipps CM, Kent M, Mizuno K, Kawakami H (2004) Kudoa thyrsites from Japanese flounder and Kudoa lateolabracis n. sp. from Chinese sea bass: causative myxozoans of post-mortem myoliquefaction. Fish Parasitol 39:79–85
Yokoyama H, Suzuki J, Shirakashi S (2014) Kudoa hexapunctata n. sp. (Myxozoa: Multivalvulida) from the somatic muscle of Pacific bluefin tuna Thunnus orientalis and re-description of K. neothunni in yellowfin tuna T. albacares. Parasitol Int 63(4):571–579. https://doi.org/10.1016/j.parint.2014.03.006
Funding
This study was supported in part by Grant-in-Aid for Scientific Research 2017 from The Towa Foundation for Food Science and Research (HS), and JSPS KAKENHI Grant Number 15K07722.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Christopher Whipps
Rights and permissions
About this article
Cite this article
Sakai, H., Kato, E., Sakaguchi, S. et al. Morphological and molecular genetic characterization of Kudoa konishiae n. sp. (Myxosporea: Multivalvulida) in the muscle of Japanese Spanish mackerel (Scomberomorus niphonius). Parasitol Res 117, 893–904 (2018). https://doi.org/10.1007/s00436-018-5770-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5770-5