Abstract
A life-threatening emaciation disease of unknown cause(s) is affecting the farming of olive flounders (Paralichthys olivaceus) and turbots (Scophthalmus maximus) on Jeju Island, Korea. As this is one of the major industries in the region, it is of great concern to local farmers trying to develop successful and sustainable aquaculture. We examined 16 olive flounders and one turbot cultured at three farms located in the southern part of Jeju Island, which manifested moderate to severe emaciation such as thinning of the body with notable appearance of bony ridges of the skull on heads. Fresh mucosal scrapings of the intestinal mucosa contained many myxosporean vegetative stages at various developments but not fully grown spores. Histological examination of gastrointestinal and other visceral organs revealed striking changes in the intestinal mucosa such as detachment and loss of the epithelium due to intensive parasitism of the myxosporean vegetative stages, accompanied by considerable leukocyte infiltration in the lamina propria, and at the final stage villus atrophy with no epithelial lining. Specific polymerase chain reaction using a pair of primers targeting a fragment of the 18S ribosomal RNA gene (rDNA) of Enteromyxum leei, a known pathogen causing myxosporean emaciation disease in a variety of cultured fish in Mediterranean countries and Japan, amplified 433-bp products in almost all diseased fish samples, particularly the gastrointestinal tract. Nearly the whole length of the 18S rDNA, 1672-bp long excluding primer-aligning sequences, of the present Korean isolate was comparable to those of E. leei isolates from Japan and Europe, particularly those from the former region. Taking the heavy load of various developmental stages of E. leei in the gastrointestinal mucosa into account, we ascribe the emaciation disease of the fish examined in the present study to this well-known myxosporean species and not to another unknown pathogen(s).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different myxosporean species (Myxozoa) cause a variety of lesions in marine and freshwater fishes depending mainly on their localizations and biological natures and/or host-related factors (Dyková and Lom 1988; Alvarez-Pellitero and Sitjà-Bobadilla 1993; Kent et al. 2001; Molnár 2002; Ali et al. 2007; Abdel-Ghaffar et al. 2008a, 2008b; Morsy et al. 2012; Hallett and Bartholomew 2012; Pote et al. 2012; Sitjà-Bobadilla and Palenzuela 2012). Myxosporean emaciation disease of cultured tiger puffers (Takifugu rubripes) emerged around 1995 in aquaculture farms in the western part of Japan, resulting in a devastating financial loss to the aquaculture industry (Tin Tun et al. 2000; Ogawa and Yokoyama 2001; Yanagida et al. 2008). The cause(s) of this disease in the cultured tiger puffers was identified as Enteromyxum leei and/or Sphaerospora fugu (syn. Leptotheca fugu), enteric myxosporean species with histozoic nature (Tin Tun et al. 2000, 2002; Ogawa and Yokoyama 2001; Yanagida et al. 2004, 2005). Mucosal parasitism of an excessive number of the vegetative myxosporean at different developmental stages (including sporoblasts) of these two species elicits epithelial changes such as degeneration, hyperplasia, partial detachment or complete exfoliation, and villus atrophy in the intestinal tract (Tin Tun et al. 2002). Affected fish suffer osmoregulation failure and malabsorption of nutrients (Ishimatsu et al. 2007), resulting in clinical manifestations of the disease such as anorexia, enophthalmia, bony ridges on the head, tapering or thinning of the body, cachexia, and death.
A wide host spectrum of E. leei is known, including more than 50 marine fish species (Diamant et al. 1994; Le Breton and Marques 1995; Diamant 1998; Kent 1999; Padrós et al. 2001; Yanagida et al. 2004, 2008; Yasuda et al. 2005; Sitjà-Bobadilla et al. 2007; Katharios et al. 2011, 2014; China et al. 2013) and experimental freshwater fish such as tiger barb (Puntius tetrazona), zebrafish (Danio rerio), oscar (Astronotus ocellatus), and Mozambique tilapia (Oreochromis mossambicus) (Diamant et al. 2006). Fish-to-fish transmission of E. leei vegetative stages exacerbates the spread of the disease in a contaminated water area or tank, as demonstrated by cohabitation, effluent, and oral exposure experiments (Diamant 1997, 1998; Yasuda et al. 2002; Yanagida et al. 2004; Sitjà-Bobadilla et al. 2007; China et al. 2013). Furthermore, the nature of autoinfection, in which the parasite proliferates in situ after invading the host (Diamant 1997; Redondo et al. 2004; Alvarez-Pellitero et al. 2008), subsequently leads to a fatal infection after a certain period of latent infection. Therefore, a disease outbreak has an enormous impact on aquaculture and aquarium industries worldwide (Rigos et al. 1999; Padrós et al. 2001; Palenzuela 2006; Montero et al. 2007; Rigos and Katharios 2010). Similar fish-to-fish transmission of Enteromyxum scophthalmi among turbots (Scophthalmus maximus) is also known to cause serious problems for the aquaculture industry (Redondo et al. 2002; Quiroga et al. 2006).
In Korea, where the olive flounder (Paralichthys olivaceus) is a major fish of the aquaculture industry (15.2 % of total marine culture products including seaweed and shellfish products and 48.1 % of finfish culture production) (Yoon 2008), emaciation disease of cultured olive flounders was first noticed in 2007 and, as yet, the cause of the disease has not been identified (Kim et al. 2011, 2015; Choi et al. 2012). Kim et al. (2011) reported that the pathophysiological changes in diseased olive flounders farmed on Jeju Island were comparable to those exhibited by cultured fish with myxosporean emaciation disease in Japan. Their most recent research (Kim et al. 2015) concluded that the cause of the emaciation disease was a myxosporean species with a morphological similarity to Enteromyxum species but genetically distinct from any known pathogens of emaciation disease.
In the present study, new representative samples—16 cultured olive flounders and one cultured turbot with emaciation disease—were collected from three farms on Jeju Island, and the possible cause of the disease was investigated with special reference to myxosporean pathogens.
Materials and methods
Fish samples and parasitological examination
On February 15 and 16, 2016, 16 olive flounders of various sizes (standard length, 15.2–36.7 cm [average ± standard deviation, 24.8 ± 7.4]), but with emaciation signs such as body thinning and bony head, were provided by three fish farms located in the southeastern part of Jeju Island, Korea. One turbot (standard length, 26.8 cm) with the same disease, cultured in one of these farms, was also examined. Cultured fish were kept in a flow-through system of land-based facilities using natural seawater of inshore waters 150 m from the coast.
After gross confirmation of external signs, fish were dissected and their viscera including the stomach, intestine, liver, gallbladder, kidney, and urinary bladder were removed. The intestine was divided into three equal segments (anterior, middle, and posterior parts) by length. Wet smears of fresh intestinal scrapings were checked by light microscopy. A piece of each organ mentioned above was individually stamped on a circle of Whatman™ FTA™ Classic Card (GE Healthcare Bio-Sciences Co., Piscataway, NJ, USA). Another piece of each organ was fixed in 10 % neutral-buffered formalin solution and/or 70 % alcohol. Formalin-fixed tissues were trimmed, dehydrated in a series of alcohols, cleared in xylene, and embedded in paraffin wax. Thin tissue sections, 6 μm in thickness, were stained with hematoxylin-eosin (HE).
Specimens collected in the present work were deposited in the Meguro Parasitological Museum, Tokyo, Japan, under collection nos. 21211–21215.
DNA extraction, polymerase chain reaction (PCR), and sequencing
Using a 2-mm Harris Uni-Core puncher (Whatman®; GE Healthcare Bio-Sciences Co.), two punches from each tissue-stamped circle of Whatman™ FTA™ Classic Card were placed in an Eppendorf tube. The DNA of each sample was extracted from these two punches using an Illustra™ tissue and cells genomicPrep Mini Spin Kit (GE Healthcare UK, Buckinghamshire, UK) according to the instructions of the manufacturer. PCR amplification of a fragment of the 18S ribosomal RNA gene (rDNA) was performed in a 20-μl volume containing a DNA polymerase, Blend Taq-Plus- (TOYOBO, Dojima Hama, Osaka, Japan), and either primer pair for E. leei (EL-F/EL-R), E. fugu (EF-F/EF-R), and S. fugu (LF-F/LF-R) according to Yanagida et al. (2005). As mentioned below, we modified the EL-R primer nucleotide sequence as follows: 5′-AGAAGCCAACGTATATGATTA-3′, with the removal of one “T” near the 3′-terminus (designated mEL-R). Using the primer pair EL-F and mEL-R, an additional PCR screening of the same sample set was performed. Subsequently, a combination of universal eukaryotic primers, forward Eurib1 and reverse Eurib2, was used to amplify almost the whole length of the 18S rDNA at once (Li et al. 2012). PCR products were purified using a FastGene Gel/PCR Extraction Kit (NIPPON Genetics Co., Tokyo, Japan) and sequenced directly as described previously (Li et al. 2012).
Since PCR products using the primer pair Eurib1 and Eurib2 were either a myxosporean rDNA sequence or host fish rDNA sequence depending on the ratio of parasite DNA to host DNA, new primers to detect exclusively myxosporean rDNA were designed with reference to three nucleotide sequences mentioned above, i.e., the rDNA sequences of E. leei, olive flounder, and turbot (Table 1). The PCR cycling protocol was 2 min at 94 °C, then 35 cycles of 30 s at 94 °C, 30 s at 63 °C, and 2 min at 72 °C, followed by a final extension at 72 °C for 7 min. When direct sequencing was not satisfactory, the purified PCR products were cloned into a plasmid vector, pTA2 (TArget Clone™; TOYOBO), and transformed into Escherichia coli JM109 (TOYOBO) according to the instructions of the manufacturer. Following propagation, the plasmid DNA was extracted using a FastGene Plasmid Mini Kit (NIPPON Genetics Co.) and inserts from multiple independent clones, at least three, were sequenced using universal M13 forward and reverse primers. The nucleotide sequences obtained in the present study are available from the DDBJ/EMBL/GenBank databases under the accession nos. LC155255–LC155258.
Results
Necropsy of diseased fish
As shown in Fig. 1, diseased olive flounders and a turbot were thinned to various degrees, with evident bony heads. Gastrointestinal tracts were vacant, and the mucosa was hypertrophied with congestion or thinned depending on the progress of the disease. Instead of fatty livers with white/yellow coloration, the livers showed russet coloration probably due to limited nutrient supply. Wet smears of mucosal scrapings of the intestine, particularly the posterior part, contained numerous myxosporean vegetative stages, but no myxospores were detected in any of the specimens. In the urine of some olive flounders, several spores of Parvicapsula anisocaudata were found, as having been reported by Cho and Kim (2004).
Histological changes of diseased fish
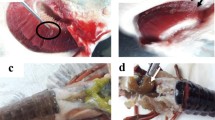
Histological lesions were localized mainly in the intestinal mucosa, and the degree of changes was variable in individual fish. As shown in Fig. 2, numerous myxosporean vegetative stages were observed intercellularly within the mucosal epithelium of the intestine, often with distinctive thickening and leukocyte infiltration in the lamina propria of affected intestinal villi. No developed spores were found, even in such an active phase of infection. The parasitized epithelium was hyperplastic, sloughed, and convoluted, and often no epithelial lining remained in the intestinal villi. In some more advanced fish cases, no mucosal epithelium was found throughout the intestine, with remarkable atrophy of intestinal villi. In addition, the mucosal epithelium of the stomach was sometimes invaded by the vegetative stages of the myxosporean. Parasitism of myxosporean species was not found in any other organs except for the epithelium of the urinary bladder. On the epithelial surface of the urinary bladder of a part of olive flounders and a turbot, several or numerous myxosporean sporoblasts of a coelozoic bivalvulid myxosporean, Myxodavisia sp., were found. A marked reduction of lipid droplets in the hepatocytes was noted.
Histopathological changes of the intestinal mucosa of olive flounders (a–e) and a turbot (f) with an active parasitism by Enteromyxum leei. Vegetative stages of E. leei localized in the epithelium, often hyperplastic, and leukocyte infiltration in the underlying lamina propria are striking. Parasitized epithelial layers are exfoliated and convoluted and detached from the underlying tissue. Scale bars on photographs are 100 μm (a), 50 μm (b and c), and 20 μm (d–f). HE staining
Genetic detection of myxosporeans in organs of diseased fish
PCR amplifications using three primer pairs to detect enteric myxosporeans, E. leei, E. fugu, and S. fugu, in cultured tiger puffers with myxosporean emaciation disease (Yanagida et al. 2005) were applied to DNA samples taken from 16 olive flounders and one turbot farmed on Jeju Island and diagnosed as manifesting the emaciation disease. A distinctive product of 433 bp for E. leei was amplified in most stomach, intestine, liver, gallbladder, kidney, and urinary bladder tissue samples (Table 2). No amplicons were generated in PCRs specific for E. fugu and S. fugu. Tissue DNA samples from affected fish with an active myxosporean infection (for example, fish with a hypertrophied intestine) had more PCR product in all organs examined by gel electrophoresis, while samples from diseased fish with thinned intestinal walls showed a positive reaction in a proportion of the gastrointestinal tract segments. Representative PCR products of various organs such as the stomach, different parts of the intestine, gallbladder, kidney, and urinary bladder were purified and sequenced. All 13 PCR products examined had an identical nucleotide sequence regardless of host fish species or organ. At this point in the study, we realized we needed to use a modified reverse primer (mEL-R) instead of the original one (EL-R) for nucleotide sequencing. PCRs with the primer pair Eurib1 and Eurib2 for the lower one third part of the intestine from an olive flounder and turbot with an active infection amplified 1712-bp products containing 40-bp-long primer-aligning parts. These two nucleotide sequences (DDBJ/EMBL/GenBank accession nos. LC155257 and LC155258, respectively) contained absolute lengths of E. leei-specific PCR products amplified by PCR using a primer pair of EL-F and EL-R or mEL-R. PCRs with the primer pair Eurib1 and Eurib2 of DNA samples taken from other tissues amplified nucleotide sequences of the 18S rDNA of host fish, i.e., olive flounder or turbot (DDBJ/EMBL/GenBank accession nos. LC155255 and LC155256, respectively). PCRs using newly designed primer pairs (Table 1) successfully amplified E. leei rDNA fragments in various organs including not only the intestine but also the liver, gallbladder, kidney, and urinary bladder. Table 3 compares several E. leei nucleotide sequences of the 18S rDNA from various fish hosts fished from the seawaters of the Far East (Korea and Japan) and the Mediterranean (Greece and Israel). Although the nucleotide at position 1566 appeared to be unique to the Korean isolates from olive flounders and a turbot, this nucleotide difference might be ascribed to an artificial one as discussed below. Most of the isolates contained several ambiguously resolved or polymorphic residues and no or only a few critical nucleotide differences of the 18S rDNA were found among deposited sequences of E. leei isolates from Japan and the Mediterranean (Table 3).
Discussion
First noticed in 2007, the recent emergence of emaciation disease in farmed olive flounders on Jeju Island, Korea, is of great concern to the local aquaculture industry (Choi et al. 2012; Kim et al. 2015). Kim et al. (2015) attributed the cause of the disease to a myxosporean pathogen and not to a viral or bacterial agent(s). Furthermore, they suggested a new myxosporean species as the possible cause. In the present study, however, we have demonstrated severe infection with E. leei in examined organs, particularly the intestine, of both diseased olive flounders and a diseased turbot exhibiting the same symptoms described by Kim et al. (2015). Although turbots cultured in the Mediterranean Sea are known to be seriously affected by emaciation disease caused by E. scophthalmi (Branson et al. 1999; Palenzuela et al. 2002; Redondo et al. 2002, 2004; Quiroga et al. 2006), the diseased turbot examined here was found to be infected by E. leei, similar to the infection in diseased olive flounders farmed in the same farm. This suggests a possible expansion of the disease to other cultured fish in aquaculture facilities using the same flow-through system of contaminated natural seawaters.
Kim et al. (2015) applied a PCR screening system to the diseased fish in Korea which used a primer pair (MM18Sf and MM18Sr) that produced a 1589-bp-long product of the 18S rDNA of European E. leei isolates (Palenzuela et al. 2002). They detected no amplification in all the DNA samples extracted from the intestine, thus excluding the possible involvement of E. leei as the cause of the emaciation disease of farmed olive flounders on Jeju Island. In our study, we instead applied a PCR screening system using a primer pair (EL-F and EL-R) which produced a 433-bp product of the 18S rDNA of E. leei (Yanagida et al. 2005). Using this system, we detected specific amplification of E. leei DNA in most of the examined tissues of affected fish (Table 2). Involvement of the gallbladder has been recorded in multiple marine fish hosts, e.g., Diplodus puntazzo (Le Breton and Marques 1995; Athanassopoulou et al. 1999; Cuadrado et al. 2008), Sparus aurata (Diamant et al. 2006; Fleurance et al. 2008), Epinephelus malabaricus (China et al. 2013), Sparisoma cretense (Katharios et al. 2014), and Chromis chromis (Özer et al. 2014). The presence of some E. leei spores in the urinary bladder of one of five examined S. aurata was recorded in the original description of the species by Diamant et al. (1994). In addition, a few trophozoites in the spleen and/or gill filaments of D. puntazzo were recorded by Alvarez-Pellitero et al. (2008). Trophozoites of a congener, E. scophthalmi, have also been observed in a blood vessel, suggesting dissemination of the myxosporean to the whole body (Redondo et al. 2002, 2004). Although E. leei showed its apparent tropism in the epithelium of the intestinal mucosa as assessed by our histological examinations, the same phenomenon of organ/tissue dissemination could easily apply to E. leei, thus explaining the positive PCR results in the liver and kidney presented here.
As mentioned above, a PCR screening system using a specific primer pair for E. leei, i.e., EL-F and EL-R, worked well for our purposes. However, after obtaining nucleotide sequences of the 433-bp-long 18S rDNA amplicons and 1712-bp-long amplicons generated by this system, it became evident that the original primer sequence of EL-R had a problem of mismatching to the 18S rDNA nucleotide sequence of E. leei. We therefore modified the nucleotide sequence of the original EL-R primer (mEL-R) and repeated the PCR screening of DNA samples using the new primer pair of EL-F and mEL-R. The results of this repeat screening with EL-F and mEL-R were almost identical to those of the original one with EL-F and EL-R; however, inclusion of mEL-R led to successful nucleotide sequencing. Of note, our finding of high but not complete PCR positivity or minimal PCR products of some samples taken from emaciation diseased fish (see Table 2) may be a consequence of complete loss of the intestinal mucosal epithelium (observed by histological examination) where E. leei would localize. Therefore, when conducting PCR screening for E. leei in emaciated moribund fish at the final stage of infection, we would recommend taking multiple DNA samples from different tissues.
Following nucleotide sequencing of almost the full length of the 18S rDNA of our Korean E. leei isolates, a nucleotide substitution at position 1566 (Table 3) was noted. However, this difference is ascribed to an inclusion of primer sequence in other deposited isolates in the DDBJ/EMBL/GenBank databases and not to the uniqueness of the Korean E. leei isolates. Apart from this difference, most of the isolates contained several ambiguously resolved or polymorphic residues and no or only a few critical nucleotide differences of the 18S rDNA were found among deposited sequences of E. leei isolates from Japan and the Mediterranean.
Kim et al. (2015) succeeded to demonstrate fish-to-fish transmission of the emaciation disease from emaciated olive flounders to naïve olive flounders but not naïve red sea breams (Pagrus major) by cohabitation. This finding provided the authors with a basis to propose the uniqueness of the myxosporean pathogen causing the emaciation disease in farmed olive flounders on Jeju Island, Korea, as fish-to-fish transmission of E. leei from diseased olive flounders to naïve fish of the same species as well as naïve fish of different species (T. rubripes) was successfully established in an earlier study (Yasuda et al. 2005), based on a wide host spectrum of E. leei as mentioned above. Fish-to-fish transmission experiments are affected by various factors. A recent study (Estensoro et al. 2010) showed that peranal intubation provided a very uniform, reliable, and faster mode of transmission of E. leei than the commonly used transmission methods (cohabitation, exposure to infected effluent, and oral inoculation), which require long exposure times or give variable and unpredictable results. One of the major factors affecting transmission is the ambient temperature for fish exposed to E. leei. Several studies have established that this myxosporean actively proliferates in fish hosts kept at 20–25 °C, but it cannot proliferate in fish kept in water at or lower than 15 °C (Yanagida et al. 2006; Estensoro et al. 2010) and at or higher than 26 °C (China et al. 2013). In the former condition, it was proposed that E. leei remained under a latent infection within the fish, but in the latter condition, China et al. (2013) suggested the eradication of the pathogen within the fish body by temperature. Similarly, Redondo et al. (2002) reported that turbots infected with E. scophthalmi did not show any sign of disease when the water temperature was around 12–14 °C, but following transfer of the fish to water at 18 °C, their mortality increased to 86.6 %. The age of fish and incubation time are other important factors that affect the progress of the disease (prevalence and intensity) in fish exposed to pathogenic Enteromyxum spp. (Quiroga et al. 2006). Kim et al. (2015) exposed recipient fish under experimental cohabitation for only 2 weeks. Considering the length of the incubation time for Enteromyxum spp. to proliferate and spread to neighboring tissues, 2 weeks might be too short to conclude anything about the susceptibility of fish exposed to the myxosporean vegetative stages.
A single or a few primary foci established in the (gastro)intestinal mucosa proceed to an evident proliferation of Enteromyxum spp. in host fish. These foci are likely to extend from one spot to an adjacent spot (Fleurance et al. 2008), resulting in a patchy distribution of parasites in the intestinal lining during the early phase of infection (Alvarez-Pellitero et al. 2008; Fleurance et al. 2008; Katharios et al. 2014). Therefore, survivability of vegetative stages of Enteromyxum spp. in contaminated seawater is of great concern for the prevention of an initial establishment of the primary focus of the pathogen in one or a few fish in the flow-through system of land-based facilities and the control of the spread of the disease among the fish stock. Vegetative stages, including sporoblasts, of E. leei and E. scophthalmi have been reported to survive in seawater for up to 24 h (Redondo et al. 2003; Yokoyama et al. 2009), and a 1-h exposure to hyposalinity less than one-quarter of seawater salinity (8 ‰) significantly reduced the viability and infectivity of E. leei to susceptible fish (Yokoyama and Shirakashi 2007). Although the mechanical exclusion of Enteromyxum spp. by filtration of the natural seawater supply for land-based farming facilities has been demonstrated to be highly effective, it is speculated that the use of filtrated seawater in aquaculture farms as a routine prophylactic measure is not feasible due to the large water volumes involved (Quiroga et al. 2006).
As experienced by aquaculture farms in Mediterranean countries and Japan (Rigos et al. 1999; Tin Tun et al. 2002; Yasuda et al. 2005; Rigos and Katharios 2010), myxosporean emaciation disease due to E. leei or E. scophthalmi is one of the most threatening parasitic diseases in aquaculture, with the abandonment of farms in some sea areas. Considering that, in particular, the former species is known to have a wide spectrum of fish hosts, development of a new sterilization system(s) of natural seawater used for the flow-through system of land-based facilities for olive flounders and turbots is critical. Additionally, for the improvement of fish productivity, the initial introduction of non-infected healthy fish and the regular monitoring of their health are crucial.
References
Abdel-Ghaffar F, Abdel-Baki AS, Bayoumy EM, Bashtar A-R, Al-Quraishy S, Morsy KS, Alghamdy A, Mehlhorn H (2008a) Light and electron microscopic study on Henneguya suprabranchiae Landsberg, 1987 (Myxozoa: Myxosporea) infecting Oreochromis niloticus, a new host record. Parasitol Res 103:609–617
Abdel-Ghaffar F, Ali MA, Al Quraishy S, Entzeroth R, Abdel-Baki AS, Al Farraj S, Bashtar A-R (2008b) Zschokkella helmii n.sp. (Myxozoa: Myxosporea), a new parasite of marble spinefoot Siganus rivulatus (Forsskal 1775), Red Sea, Egypt: light and transmission electron microscopy. Paasitol Res 102:183–192
Ali MA, Abdel-Baki AS, Abdel-Ghaffar F (2007) Zschokkella egyptica n. sp. (Myxosporea : Bivalvulaida) infecting the gallbladder of the eel catfish Plotosus lineatus Thunberg, 1787 and the freckled goatfish Upeneus tragula Richardson, 1846 in the Red Sea, Egypt. Parasitol Res 100:625–628
Alvarez-Pellitero P, Sitjà-Bobadilla A (1993) Pathology of Myxosporea in marine fish culture. Dis Aquat Org 17:229–238
Alvarez-Pellitero P, Palenzuela O, Sitjà-Bobadilla A (2008) Histopathology and cellular response in Enteromyxum leei (Myxozoa) infections of Diplodus puntazzo (Teleostei). Parasitol Int 57:110–120
Athanassopoulou F, Prapas T, Rodger H (1999) Diseases of Puntazzo puntazzo Cuvier in marine aquaculture systems in Greece. J Fish Dis 22:215–218
Branson E, Riaza A, Alvarez-Pellitero P (1999) Myxosporean infection causing intestinal disease in farmed turbot, Scophthalmus maximus (L.), (Teleostei: Scophthalmidae). J Fish Dis 22:395–399
China M, Nakamura H, Hamakawa K, Tamaki E, Miwa S, Meng F, Yokoyama H (2013) Occurrence of the myxosporean emaciation disease caused by Enteromyxum leei in cultured Malabar grouper Epinephelus malabaricus. Fish Pathol 48:88–96 (in Japanese with English summary)
Cho JB, Kim KH (2004) Light and electron microscopical observations of Parvicapsula anisocaudata (Myxosporea: Parvicapsulidae) from urinary system of cultured olive flounder, Paralichtys olivaceus. J Fish Pathol 17:179–189
Choi HS, Jun LJ, Kim SM, Jeong HD, Kim YK, Lim H, Yeo I, Jeong JB (2012) Clinical features of fish with pathogens isolated from emaciated olive flounder Paralichthys olivaceus. J Fish Pathol 25:67–76 (in Korean with English summary)
Cuadrado M, Marques A, Diamant A, Sitjà-Bobadilla A, Palenzuela O, Alvarez- Pellitero P, Padrós F, Crespo S (2008) Ultrastructure of Enteromyxum leei (Diamant, Lom, & Dyková) (Myxozoa), an enteric parasite infecting gilthead sea bream (Sparus aurata) and sharpsnout sea bream (Diplodus puntazzo). J Eukaryot Microbiol 55:178–184
Diamant A (1997) Fish-to-fish transmission of a marine myxosporean. Dis Aquat Org 30:99–105
Diamant A (1998) Red drum Sciaenops ocellatus (Sciaenidae), a recent introduction to Mediterranean mariculture, is susceptible to Myxidium leei (Myxosporea). Aquaculture 162:33–39
Diamant A, Lom J, Dyková I (1994) Myxidium leei n. sp., a pathogenic myxosporean of cultured sea bream Sparus aurata. Dis Aquat Org 20:137–141
Diamant A, Ram S, Paperna I (2006) Experimental transmission of Enteromyxum leei to freshwater fish. Dis Aquat Org 72:171–178
Dyková L, Lom J (1988) Review of pathogenic myxosporeans in intensive culture of carp (Cyprinus carpio) in Europe. Folia Parasitol 35:289–307
Estensoro I, Redondo MJ, Alvarz-Pellitero P, Sitjà-Bobadilla A (2010) Novel horizontal transmission route for Enteromyxum leei (Myxozoa) by anal intubation of gilthead sea bream Sparus aurata. Dis Aquat Org 92:51–58
Fleurance R, Sauvegrain C, Marques A, Le Breton A, Guereaud C, Cherel Y, Wyers M (2008) Histopathological changes caused by Enteromyxum leei infection in farmed sea bream Sparus aurata. Dis Aquat Org 79:219–228
Hallett SL, Bartholomew JL (2012) Myxobolus cerebralis and Ceratomyxa shasta. In: Woo PTK, Buchmann K (eds) Fish parasites: pathobiology and protection. CAB International, Oxford, pp 131–162
Ishimatsu A, Hayashi M, Nakane M, Sameshima M (2007) Pathophysiology of cultured tiger puffer Takifugu rubripes suffering from the myxosporean emaciation disease. Fish Pathol 42:211–217
Katharios P, Rigos G, Divanach P (2011) Enteromyxum leei (Myxozoa), a lethal intruder of tropical pet fish: first case in humphead wrasse, Cheilinus undulates (Rüppell, 1835). J Exot Pet Med 20:138–143
Katharios P, Kokkari C, Sterioti A, Smyrli M, Kalatzis PG (2014) Enteromyxum leei infection in parrotfish, Sparisoma cretense: histopathological, morphological and molecular study. Vet Parasitol 199:136–143
Kent ML (1999) A myxozoan resembling Myxidium leei in the anemone fish Amphiprion frenatus from the Pacific Ocean. Bull Eur Assoc Fish Pathol 19:42–43
Kent ML, Andree KB, Bartholomew JB, El-Matbouli M, Desser SS, Devlin RH, Feist SW, Hedrick RP, Hoffman RW, Khattra J, Hallett SL, Lester JG, Longshaw M, Palenzuela O, Siddall ME, Xiao C (2001) Recent advances in our knowledge of the Myxozoa. J Eukaryot Microbiol 48:395–413
Kim YK, Jeong JB, Lee MK, Park SI, Park MA, Choe MK, Yeo IK (2011) Pathophysiology of olive flounder Paralichthys olivaceus suffering from emaciation. J Fish Pathol 24:11–18 (in Korean with English summary)
Kim SM, Jun LJ, Park MA, Jeong HD, Jeong JB (2015) Characterization of the myxosporean parasie isolated from emaciated olive flounders Paralichthys olivaceus on Jeju Island. Korean J Fish Aquat Sci 48:337–345 (in Korean with English summary)
Le Breton A, Marques A (1995) Occurrence of an histozoic Myxidium infection in two marine cultured species: Puntazzo puntazzo C. and Pagrus major. Bull Eur Assoc Fish Pathol 15:210–212
Li Y-C, Sato H, Kamata Y, Ohnishi T, Sugita-Konish Y (2012) Three novel myxobolid species of genera Henneguya and Myxobolus (Myxosporea: Bivalvulida) from marine fish in Japan. Parasitol Res 111:819–826
Montero FE, Cuadrado M, Padrós F, Crespo S, Raga JA (2007) Cryptocaryon irritans and Enteromyxum leei, two threats for the culture of Diplodus puntazzo in the Mediterranean. Bull Eur Assoc Fish Pathol 27:242–249
Molnár K (2002) Differences between the European carp (Cyprinus carpio carpio) and the coloured carp (Cyprinus carpio haematopterus) in susceptibility to Thelohanellus nikolskii (Myxosporea) infection. Acta Vet Hung 50:51–57
Morsy K, Abdel-Ghaffar F, Bashtar AR, Mehlhorn H, Al Quraishy S, Abdel-Gaber R (2012) Morphology and small subunit ribosomal DNA sequence of Henneguya suprabranchiae (Myxozoa), a parasite of the catfish Clarias gariepinus (Clariidae) from the River Nile, Egypt. Parasitol Res 111:1423–1435
Ogawa K, Yokoyama H (2001) Emaciation disease of cultured tiger puffer Takifugu rubripes. Bull Natul Res Inst Aquacult, Suppl 5:65–70
Özer A, Öztürk T, Özkan H, Çam A (2014) First report of Enteromyxum leei (Myxozoa) in the Black Sea in a potential reservoir host Chromis chromis. Fish Pathol 49:57–60
Padrós F, Palenzuela O, Hispano C, Tosas O, Zarza C, Crespo S, Alvarez-Pellitero P (2001) Myxidium leei (Myxozoa) infections in aquarium-reared Mediterranean fish species. Dis Aquat Org 47:57–62
Palenzuela O (2006) Mixozoan infections in Mediterranean mariculture. Parassitologia 48:27–29
Palenzuela O, Redondo MJ, Álvarez-Pellitero P (2002) Description of Enteromyxum scophthalmi gen. nov., sp. nov. (Myxozoa), an intestinal parasite of turbot (Scophthalmus maximus L.) using morphological and ribosomal RNA sequence data. Parasitology 124:369–379
Pote LMW, Khoo L, Griffin M (2012) Henneguya ictaluri. In: Woo PTK, Buchmann K (eds) Fish parasites: pathobiology and protection. CAB International, Oxford, pp 177–192
Quiroga MI, Redondo MJ, Sitjà-Bobadilla A, Palenzuela O, Riaza A, Macías A, Vázquez S, Perez A, Nieto JM, Alvarez-Pellitero P (2006) Risk factors associated with Enteromyxum scophthalmi (Myxozoa) infection in cultured turbot, Scophthalmus maximus. Parasitology 133:433–442
Redondo MJ, Palenzuela O, Riaza A, Macias Á, Álvarz-Pellitero P (2002) Experimental transmission of Enteromyxum scophthalmi (Myxozoa), an enteric parasite of turbot Scophthalmus maximus. J Parasitol 88:482–488
Redondo MJ, Palenzuela O, Alvarz-Pellitero P (2003) In vitro studies on viability and proliferation of Enteromyxum scophthalmi (Myxozoa), an enteric parasite of cultured turbot Scophthalmus maximus. Dis Aquat Org 55:133–144
Redondo MJ, Palenzuela O, Alvarz-Pellitero P (2004) Studies on transmission and life cycle of Enteromyxum scophthalmi (Myxozoa), an enteric parasite of turbot Scophthalmus maximus. Folia Parasitol 51:188–198
Rigos G, Katharios P (2010) Pathological obstacles of newly-introduced fish species in Mediterranean mariculture: a review. Rev Fish Biol Fisheries 20:47–70
Rigos G, Christophilogiannis P, Yiagnisi M, Andriopoulou A, Koutsodimou M, Nengas I, Alexis M (1999) Myxosporean infections in Greek mariculture. Aquacult Int 7:361–364
Sitjà-Bobadilla A, Palenzuela O (2012) Enteromyxum species. In: Woo PTK, Buchmann K (eds) Fish parasites: pathobiology and protection. CAB International, Oxford, pp 163–176
Sitjà-Bobadilla A, Diamant A, Palenzuela O, Alvarez-Pellitero P (2007) Effect of host factors and experimental conditions on the horizontal transmission of Enteromyxum leei (Myxozoa) to gilthead sea bream, Sparus aurata L., and European sea bass, Dicentrarchus labrax (L.). J Fish Dis 30:243–250
Tun T, Yokoyama H, Ogawa K, Wakabayashi H (2000) Myxosporeans and their hyperparasitic microsporeans in the intestine of emaciated tiger puffer. Fish Pathol 35:145–156
Tun T, Ogawa K, Wakabayashi H (2002) Pathological changes induced by three myxosporeans in the intestine of cultured tiger puffer, Takifugu rubripes (Temminck and Schlegel). J Fish Dis 25:63–72
Yanagida T, Nomura Y, Kimura T, Fukuda Y, Yokoyama H, Ogawa K (2004) Molecular and morphological redescriptions of enteric myxozoans, Enteromyxum leei (formerly Myxidium sp. TP) and Enteromyxum fugu comb. n. (syn. Myxidiium fugu) from cultured tiger puffer. Fish Pathol 39:137–143
Yanagida T, Freeman MA, Nomura Y, Takami I, Sugihara Y, Yokoyama H, Ogawa K (2005) Development of a PCR-based method for the detection of enteric myxozoans causing the emaciation disease of cultured tiger puffer. Fish Pathol 40:23–28
Yanagida T, Sameshima M, Nasu H, Yokoyama H, Ogawa K (2006) Temperature effects on the development of Enteromyxum spp. (Myxozoa) in experimentally infected tiger puffer, Takifugu rubripes (Temminck & Schlegel). J Fish Dis 29:561–567
Yanagida T, Plaenzuela O, Hirae T, Tanaka S, Yokoyama H, Ogawa K (2008) Myxosporean emaciation disease of cultured red sea bream Pagrus major and spotted knifejaw Oplegnathus punctatus. Fish Pathol 43:45–48
Yasuda H, Ooyama T, Iwata K, Tun T, Yokoyama H, Ogawa K (2002) Fish-to-fish transmission of Myxidium spp. (Myxozoa) in cultured tiger puffer suffering from emaciation disease. Fish Pathol 37:29–33
Yasuda H, Ooyama T, Nakamura A, Iwata K, Palenzuela O, Yokoyama H (2005) Occurrence of the myxosporean emaciation disease caused by Enteromyxum leei in cultured Japanese flounder Paralichthys olivaceus. Fish Pathol 40:175–180
Yokoyama H, Shirakashi S (2007) Evaluation of hyposalinity treatment on infection with Enteromyxum leei (Myxozoa) using anemonefish Amphiprion spp. as experimental host. Bull Eur Assoc Fish Pathol 27:74–78
Yokoyama H, Kageyama M, Yanagida T, Ogawa K (2009) Seawater survival of Enteromyxum leei (Myxozoa) evaluated by in vitro viability and in vivo infectivity assays. Fish Pathol 44:172–177
Yoon GH (2008) Aquaculture in Korea. Aquacult News 34:16–17
Acknowledgments
We are indebted to Ms. Ji-Hyeon Ahn, Daebong LS, Ltd. for her Korean–Japanese translation. This study was supported in part by Grant-in-Aid for Scientific Research 2015 from the Towa Foundation for Food Science and Research (HS), by Grant-in-Aid for International Collaboration Research in Asia 2016 from the Heiwa Nakajima Foundation, and by JSPS KAKENHI Grant Number 15K07722.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sekiya, M., Setsuda, A., Sato, H. et al. Enteromyxum leei (Myxosporea: Bivalvulida) as the cause of myxosporean emaciation disease of farmed olive flounders (Paralichthys olivaceus) and a turbot (Scophthalmus maximus) on Jeju Island, Korea. Parasitol Res 115, 4229–4237 (2016). https://doi.org/10.1007/s00436-016-5200-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5200-5