Abstract
Three species of the Kudoid parasite (Myxozoa: Multivalvulida) were observed in the somatic muscle of Japanese parrotfish Calotomus japonicus caught off the coast of western Japan. All three species formed pseudocysts in myofibers and caused subclinical infections. The three Kudoa species were distinguished by spore morphology, as well as their 18S and 28S rDNA sequences. We identified a previously undescribed taxa Kudoa igami n. sp. with spores that were stellate with rounded peripheral edges and five to six polar capsules (prevalence 29.3 %). Kudoa igami n. sp. were morphologically most similar to Kudoa neothunni but were distinguishable by a more rounded shape in the apical view. Molecular analyses demonstrated that the K. igami n. sp. is closely related to Kudoa thalassomi; however, the similarity in the 28S rDNA sequence was <96 % and the spore morphology was different. We found Kudoa thalassomi in one sample (prevalence 2.4 %), which is a new host and geographical record for this species. Kudoa lateolabracis, which causes postmortem myoliquefaction in Chinese sea bass Lateolabrax sp. and olive flounder Paralichthys olivaceus was found in Japanese parrotfish (prevalence 41.5 %) for the first time, but did not cause myoliquefaction. We also expanded the host record for the brain-infecting Kudoa yasunagai (prevalence 94.1 %). In addition, an unidentified microsporidia was observed in the somatic muscle (prevalence 23.3 %).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kudoid myxozoan (Multivalvulida) are parasites of marine fish. To date, over 80 species have been described worldwide. Of these species, 17 species have been reported in Japan (Kent et al. 2001; Lom and Dyková 2006; Moran et al. 1999; Sato 2011; Yokoyama 2003). These parasites are important pathogens in aquaculture and capture fisheries due to their host pathology (e.g., spinal deformation caused by a brain-infecting Kudoa yasunagai), unsightly cyst formation in fish fillets (e.g., Kudoa amamiensis), and postmortem myoliquefaction (e.g., Kudoa thyrsites). These pathologies reduce the market value of fish and cause economic losses to the fishery industry. Furthermore, the public health effects of Kudoa are becoming a serious concern.
In recent years, food poisoning associated with the ingestion of fish infected with Kudoid myxozoan has been reported. Martinez de Velasco et al. (2008) reported that Kudoa sp. may induce an allergic reaction in some people. Through epidemiological analyses and animal testing, Kawai et al. (2012) found that Kudoa septempunctata in the olive flounder is a causative food-poisoning agent. In Japan, annually over 100 food-poisoning cases were identified as caused by K. septempunctata (Kawai et al. 2012; Iwashita et al. 2013). K. septempunctata infects the muscle tissue of flounder, but does not form cysts or cause myoliquefaction (Matsukane et al. 2010). The infection is subclinical and the pseudocysts cannot be detected by the naked eyes; therefore, infected fish are consumed and the millions of spores within the flesh go unnoticed. The discovery of the adverse effects of K. septempunctata on human health has rapidly increased the interest in Kudoa research. In Japan, three new Kudoa species that infect the somatic muscle of daily consumed fish have been described over the past few years (Matsukane et al. 2011; Yokoyama et al. 2012). These species, namely, Kudoa trachuri from Japanese jack mackerel Trachurus japonicus, Kudoa thunni from albacore Thunnus alalunga, and Kudoa ogawai from Pacific barrelfish Hyperoglyphe japonica form cysts and are relatively easy to detect. However, due to the subclinical nature of some Kudoa infections, many infections may be overlooked, and a potentially large number of species still to be found.
The Japanese parrotfish Calotomus japonicus (Scaridae) are widely distributed in the coastal areas of western Japan. These fish are not a major target species for fisheries, but are highly prized in some regions due to their white meat and delicate flavor. In southern Wakayama prefecture, Japanese parrotfish are locally called Igami (meaning “to snarl”; originated from their dentition that resembles a snarling dog) and considered a special fish for traditional New Year meals. The common manner of preparing Igami is to cook with soy sauce; however, Sashimi, fresh raw sliced fish meat, is also popular. During our recent survey, three species of Kudoid myxozoans were found in the trunk muscle of C. japonicus. In the present study, we aimed to identify these Kudoa parasites using morphological and molecular analyses.
Materials and methods
Fish sample
Japanese parrotfish were obtained from local fishermen in Wakayama prefecture, Japan. The fish were caught in 2012 and 2013 off the coast of Kushimoto (33.47, 135.78). Fish were kept live in stock tanks at the Wakayama prefectural fisheries experimental station until dissection. Thin slices of muscle tissue were flattened between two glass plates and checked for the presence of cysts and pseudocysts under a dissecting scope with transmitted light. The muscle tissues of infected fish were kept frozen at −20 °C for subsequent morphological and molecular analyses. Additionally, small segments (ca. 10 × 10 mm) of heavily infected muscle tissues were fixed in 10 % neutral buffered formalin for histological study. To investigate the infection prevalence by biopsy, a small portion of muscle tissue at the base of the tail of live fish (n = 32, mean body weight = 416.1 ± 212.8 g) were sampled with a 12 G syringe needle. These fish were kept in the tanks for several months and were subsequently released to the sea for other study purpose. Deceased fish were dissected and their brains were checked for Kudoa infection. A total of 41 fish were checked for Kudoa infection in somatic muscle, and of these, 17 fish were checked for brain infections.
Morphological and histological examination
Pseudocysts containing morphologically distinct myxospores were individually collected from the thawed muscle samples with a fine forceps. Wet-mounted preparations of squashed pseudocysts were observed under a light microscope for spore morphology. Photographs were taken at 1,000× magnification with a digital camera. Measurements were taken from 20 spores obtained from several pseudocysts using ImageJ (image processing program available at http://rsb.info.nih.gov/ij/). The spores were measured according to the guideline outlined by Adlard et al. (2005). Segments of formalin fixed muscle tissue were embedded in paraffin. Serial sections were cut at 4 μm, dewaxed and stained with haematoxylin and eosin (H&E) or Giemsa and eosin.
Molecular analyses
DNA from individual pseudocysts containing morphologically distinct myxospores was extracted using the QIAamp DNA Mini Kit (QIAGEN, Germany) according to the manufacturer’s instructions. When available, DNA samples for each Kudoa species were obtained from up to three individual fish. Partial small and large subunit ribosomal DNA (18S rDNA and 28S rDNA, respectively) were amplified by polymerase chain reaction (PCR) with the following primer pairs: 18e (5′-CTG GTT GAT CCT GCC AGT-3′)-Kud6R (5′-TCC AGT AGC TAC TCA TCG-3′) and Kud6F (5′-TCA CTA TCG GAA TGA ACG-3′)-18 g (5-GGT AGT AGC GAC GGG CGG CGTG-3 ) for 18S (Hillis and Dixon 1991; Whipps et al. 2003b), and Kt28S1F (5′-CAA GAC TAC CTG CTG AAC-3′), and 28S1R (5′-GTG TTT CAA GAC GGG TCG-3′) for 28S (Burger and Adlard 2010; Whipps et al. 2004). PCRs were carried out in a volume of 20 μl containing 0.1 μl of Takara ExTaqTM HS (5 U/μl; TaKaRa), 2.0 μl of 10× ExTaq Buffer, 1.6 μl of dNTP mixture (2.5 mM each), 0.6 μl of each primer (25 μM) and 1 μl of extracted DNA. Both PCRs were conducted with the following cycling program: initial denaturation of 95 °C for 2 min followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 4 min. PCR products were treated with illustra ExoStar (GE Healthcare) to remove excess primers and dNTPs and directly sequenced with BigDyeTM Terminator v3.1 in a 3500 DNA sequencer (Life Technologies, California, USA). The obtained sequences were compared with the available sequences in the GenBank database using a BLAST search at the National Center for Biotechnology Information (NCBI) website (http://blast.ncbi.nlm.nih.gov/).
Phylogenetic analysis
The sequences were edited using Geneious Pro version 7.0.4 (created by Biomatters, available from http://www.geneious.com/), and multiple alignments of each gene sequence were made using the program MAFFT (Katoh and Standley 2013) with the homologous sequences of other Kudoa species available on the GenBank database. For the data set of 18S and 28S sequences, Unicapsula sp. CMW-2003 and Unicapsula pyramidata were used as the outgroups, respectively, because the genus Unicapsula is sister to the genus Kudoa (Miller and Adlard 2013). The phylogenetic trees were generated by a maximum likelihood (ML) analysis using the RaxML algorithm (Stamatakis et al. 2008) on the CIPRES (Cyberinfrastructure for Phylogenetic Research) Portal (http://www.phylo.org/sub_sections/portal/) with the gamma model of rate heterogeneity and maximum likelihood search estimating the proportion of invariable site parameters. The robustness of the trees was tested by bootstrapping with 100 replicates. A Bayesian inference (BI) analysis was conducted using MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003). GTR + I + G was chosen as the best fit nucleotide substitution model for both the 18S and 28S data sets using AIC implemented in MrModeltest 2.3 (Nylander 2004) running on PAUP 4.0b (Swofford 2002). A Metropolis-coupled Markov chain Monte Carlo analysis was run for one million generations and the trees were sampled every 100 generations. The first 2,500 trees were discarded as burn-in. For all analyses, a 50 % majority rule consensus tree was constructed.
Results
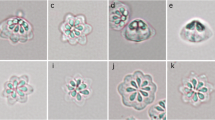
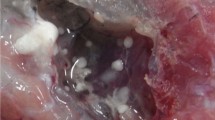
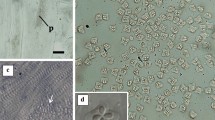
There were no visible cysts in the trunk muscle of C. japonicus; however, a number of pseudocysts were found when flattened muscle tissues were observed under a dissecting microscope. As many as several dozen pseudocysts were detected in 1 cm2 flattened tissue; however, the intensity of infection varied considerably among the individual fish. There were three morphologically distinct Kudoid myxospores among the pseudocysts and the partial sequences of the 18S (1,565–1,569 bp) and 28S rDNA (724–791 bp) were obtained from each. The first species was a stellate structure with four polar capsules, one larger than the other three (Fig. 1). The myxospores resembled K. lateolabracis, though the spores were larger than those previously reported in the Chinese sea bass Lateolabrax sp. (Table 1; Yokoyama et al. 2004). The 18S rDNA sequence (AB844442) from this isolate was identical to that of the K. lateolabracis obtained from Chinese sea bass (AY382606), and the 28S sequence (AB844445) was 99.7 % similar to that of K. lateolabracis obtained from olive flounder (JQ309928); therefore, we identify this species as K. lateolabracis. The prevalence of K. lateolabracis in the C. japonicas samples was 41.5 % (17/41).
The second species had six or seven equal spore valves (ratio 29.8:70.2 %, n = 84), each containing a single polar capsule (Fig. 1). The spore dimensions overlapped with isolates of K. thalassomi obtained from Bengal sergeant Abudefduf bengalensis and moon wrasse Thalassoma lunare described by Burger and Adlard (2011). However, the spores of the present isolate were generally larger than those of K. thalassomi in the original description provided by Adlard et al. (2005; Table 2). The 18S rDNA sequence (AB844443) from the present isolate was 100 % identical to that of K. thalassomi from moon wrasse and the 28S sequence (AB84446) shared 97.4–99.1 % sequence identity with the 24 isolates of K. thalassomi available in the database. This species was identified as K. thalassomi, and it had a prevalence rate of 2.4 % (1/41).
The third species was found in 29.3 % (12/41) of sampled fish and was described as Kudoa igami n. sp. In most cases, one species of Kudoa was identified in an individual fish, but there was one case of multiple infections in which all three Kudoa species were detected from a single individual. It has to be noted, however, that we only checked a limited number of pseudocyst from each fish, so the actual prevalence of multiple infections is most likely much higher. Histologically, pseudocysts develop intracellularly in myofibers (Fig. 2). Neither host response to the parasite nor liquefaction of neighboring muscle tissue was observed. No postmortem myoliquefaction was evident in any samples. In addition to three muscle-infecting Kudoa, we found K. yasunagai (94.1 %, 16/17) and an unidentified microsporidia (23.3 %, 7/30) in the brain and somatic muscle of C. japonicus, respectively. There was no apparent deformation or abnormal behavior in the fish infected with K. yasunagai. An unidentified microsporidia was found as pseudocysts within the myofibers of somatic muscle. The spores were ellipsoidal in shape and 2.81 ± 0.14 × 4.99 ± 0.56 μm in size.
Description
Kudoa igami n. sp. (Myxozoa: Multivalvulida)
Mature spores are hexaradiate in the apical view, with typically six equal spore valves each containing one polar capsule (Figs. 3 and 4). The number of spore valves and polar capsules rarely reached 5, and the ratio was 81:19 for six and five valves, respectively. The peripheral edge of the spore valves is rounded and lacking projections. The polar capsules are convergent and pyriform in shape. In the side view, the spores are elliptical with a slightly flat bottom. The mean ± SD (range) spore thickness and width is 9.1 ± 0.3 (8.5–9.6) μm and 9.9 ± 0.4 (9.2–10.8) μm, respectively, and the suture width, spore length, polar capsule length, and polar capsule width were 7.9 ± 0.5 (7.0–9.1) μm, 6.8 ± 0.3 (6.2–7.3) μm, 2.5 ± 0.3 (2.1–3.3) μm, and 1.7 ± 0.2 (1.4–2.1) μm (Table 2), respectively:
Type host: Calotomus japonicus, Japanese parrot fish (Scaridae) |
Type locality: The coast off Wakayama Prefecture, Japan |
Site of infection: Skeletal musculature |
Type-material: Syntype specimens (Methylene blue-stained smear of spores), Accession number MPM Coll No. 20938, Meguro Parasitological Museum, Tokyo, Japan. |
Etymology: Species name refers to the Japanese local name of the type host |
Japanese name: Igami-kudoa (“Igami” is a local name for Japanese parrotfish in Wakayama Prefecture) |
Prevalence of infection: 29.3 % (12 out of 41) |
Remarks
Of the seven described Kudoa species with six or seven spore valves, the K. igami n. sp. spore dimensions are most closely related to Kudoa neothunni obtained from yellowfin tuna Thunnus albacares (Table 2). However, the ratios of suture width/spore width for the former and the latter species are 0.79 and 0.645, respectively. This means that the spore shape of the former is rather round, while that of the latter is pointed. K. neothunni is also different from the present species because it causes myoliquefaction of the host fish. K. igami n. sp. is distinct from both Kudoa grammatorcyni and Kudoa scomberomori in having larger spore width and smaller polar capsule size relative to valve size. K. igami n. sp. is distinct from all isolates of K. thalassomi because of its smaller spore width and the rounded peripheral edge of its valves. K. igami n. sp. superficially resembles Kudoa lethrini and K. yasunagai, but the latter two species infects brain tissue. K. igami n. sp. is distinguishable from K. septempunctata in having smaller spore size and polar capsule. A BLAST search revealed that the nucleotide sequence of the 18S rDNA (AB844444) was most similar to K. thalassomi (AY302738) with 99.3 % sequence identity. The 28S rDNA sequence (AB844447, AB844448) were also most similar to K. thalassomi, but the sequence identity was less than 96 %. The ML and BI analyses of the tree topologies inferred from the 18S and 28S rDNA sequences were similar, with only slight differences (data not shown), and clearly demonstrated that K. igami n. sp. is a sister species to K. thalassomi. These parasites cluster with brain-infecting species and K. septempunctata (Figs. 5 and 6).
Phylogram of Kudoa spp. inferred from the Bayesian analysis using 18S rDNA data set. The values at each node are posterior probabilities (percent). The species names are followed by the corresponding GenBank accession numbers, site of infection and valve numbers. B brain, C connective tissue, H heart, O ovary, I intestine, T trunk muscle, M multiple site
Phylogram of Kudoa spp. inferred from Bayesian analysis using 28S rDNA data set. The values at each node are posterior probabilities (percent). The species names are followed by the corresponding GenBank accession numbers, site of infection and valve numbers. B brain, C connective tissue, H heart, O ovary, I intestine, T trunk muscle, M multiple site
Discussions
The importance of molecular information in the identification of myxozoans has been well recognized with the increasing 18S and 28S (and also some internal transcribed spacers) rDNA sequences datasets. Although morphology is the foundation of taxonomic classification, phenotypical similarities between species and intraspecific morphological variation among Kudoid myxozoans make it difficult to distinguish species solely by morphology (Burger and Adlard 2010; Heiniger and Adlard 2012; Heiniger et al. 2013). A combination of spore morphology and genetic analyses of K. igami n. sp. showed that it is distinct from known Kudoa species. The rDNA phylogenic analysis constantly placed K. igami n. sp. as sister to K. thalassomi, another muscle infecting Kudoa with six or seven polar capsules. Phylogenetic analysis based on both 28S and 18S rDNA sequences showed that Kudoa spp. with more than five pular capsules made two robust monophyletic groups, indicating that the number of spore valves is an important evolutionary characteristics in the phylogeny of previously reported Kudoa spp. (Burger and Adlard 2010; Fiala 2006; Matsukane et al. 2010; Whipps et al. 2003a; Whipps and Kent 2006).
To date, seven Kudoa spp., including K. igami n. sp., possessing six polar capsules have been identified from the somatic muscle of marine fish (Li et al. 2013). All of these species form pseudocysts, except K. scomberomori, for which neither cysts nor pseudocysts were evident (Adlard et al. 2005). Of these species, only K. neothunni is known to induce postmortem myoliquefaction in the yellowfin tuna T. albacares, and infections with other species are subclinical. Subclinical infection seems to be the dominant characteristic of muscle-infecting Kudoa with six or more polar capsules. In addition to spore morphology and genetic characteristics, the formation of cysts or pseudocysts and the induction of myoliquefaction are important taxonomic characteristics; therefore, this information should be included in the description of Kudoa spp.
The tree topologies of the 18S and 28S rDNA data sets were different, especially in K. ogawai and Kudoa paniformis. K. ogawai was first described in the somatic muscle of the Pacific barrelfish in Japan, and the phylogenetic analyses based on the 18S and 28S rDNA sequences demonstrated that K. ogawai is a sister to all Kudoa species (Yokoyama et al. 2012). In the present study, however, K. paniformis was a sister to all Kudoa species when the 28S rDNA data set was used. Similar topological discrepancy has recently been shown in the phylogenetic analyses using Unicapsula and Kudoa species (Miller and Adlard 2013). This observation may be due to the use of short fragments of 28S rDNA.
Despite the relatively high K. lateolabracis infection prevalence (41.5 %), we did not observe postmortem myoliquefaction in the flesh of Japanese parrotfish. Yokoyama et al. (2004) reported that 15 % (3/20) of cultured Chinese sea bass exhibited postmortem myoliquefaction due to K. lateolabracis. We do not know the reason for this difference, but this phenomenon has been observed for other Kudoa species. For instance, Kudoa megacapsula induces myoliquefaction in red barracuda Sphyraena pinguis, but not in yellowtail Seriola quinqueradiata (Yokoyama and Itoh 2005; Yokoyama et al. 2006). One possible explanation for this difference is the development stage of the parasite. It has been shown in other Kudoa spp. that only pre-sporogonic components in developing plasmodia produce proteolytic enzymes (Patashnik et al. 1982; Stehr and Whitaker 1969). It may be possible that all K. lateolabracis found in the present study are mature, though the encapsulation by thick collagenous layer that occurs in K. megacapsula obtained from yellowtail was not evident. In addition, the difference may arise from the infection intensity. Although we did not quantify the infection intensity in this study, we detected only a few pseudocysts in the flattened flesh; therefore, the parasite density may be too low to induce muscle liquefaction. It is possible that liquefaction only occurs in heavily infected fish.
In most previous cases (if not all), K. lateolabracis was detected in fish fillet exhibiting myoliquefaction. There may be high incidence of “hidden infection” in which a low number of K. lateolabracis is present in the tissue, but does not result in myoliquefaction. In addition, differences in the parasite strain may affect the occurrences of myoliquefaction. Recent study have demonstrated that K. neothunni induces postmortem myoliquefaction in yellowfin tuna, while Pacific bluefin tuna T. orientalis that are infected with different genotype of K neotunni show no muscle liquefaction (Li et al. 2013). They were originally considered the same species and share 99.9 and 99.3 % similarity in 18S and 28S rDNA, respectively but recently recognized as two different species (Yokoyama et al. 2014). In the present study, the K. lateolabracis spore size was larger than the original description and there was a 0.3 % difference in the 28S rDNA from the isolate obtained in the liquefied olive flounder. These differences in the parasite may explain the differences in myoliquefaction. A difference in the host fish may also be a possible explanation; however, there is no logical reason to explain why K. lateolabracis would induce myoliquefaction in Chinese sea bass and olive flounder but not in Japanese parrotfish. To understand the observed phenomena, we need further studies to investigate the host/parasite interaction, the precise underlying mechanism and the spore density threshold of K. lateolabracis required to induce postmortem myoliquefaction.
K. thalassomi has previously only been reported in Australia (Adlard et al. 2005; Burger and Adlard 2011). The present study expands the known geographical distribution of these organisms to the northern hemisphere and has added C. japonicus as a new host. K. thalassomi has a broad host range, with 19 previously known fish hosts representing six different fish families (Burger and Adlard 2011). K. thalassomi has already been reported in other Scarid, such as Scarus flavipectoralis; therefore, it is not surprising that C. japonicus is also a host. In general, parasites with broad host ranges tend to have wider geographical distribution. For example, a cosmopolitan myxozoan, K. thyrsites, a possibly species complex, has been reported in 37 fish species belonging to 18 families from all around the globe (Burger and Adlard 2011 and references within). K. thalassomi may also have a similarly wide distribution. However, known hosts for K. thalassomi are coral reef fish or coastal fish, which tend to show very little migration. It would be interesting to further investigate the host and geographical range of K. thalassomi to understand the relationship between the degree of host specificity and distribution of kudoid myxozoa.
The three Kudoa spp. observed in the somatic muscle of C. japonicus in this study caused subclinical infections. These infections can easily be overlooked, and our knowledge of kudoid myxozoa is most likely only the tip of the iceberg. Molecular detection of the parasite is useful, but it may not provide detail regarding the important characteristics, such as tissue localization (cyst/pseudocyst) and myoliquefaction. We believe close observation of the tissue and the parasite is critical for understanding the nature of infection; this information should be accompanied with molecular information. The discovery of new Kudoa species will influence the fishery industry and may also impact public health, as is the case in food poisoning by K. septempunctata. We expect a rapid increase in the knowledge of Kudoa species over the next several years.
References
Adlard RD, Bryant MS, Whipps CM, Kent ML (2005) Multivalvulid myxozoans from eastern Australia: three new species of Kudoa from scombrid and labrid fishes of the Great Barrier Reef, Queensland, Australia. J Parasitol 91(5):1138–1142
Arai Y, Matsumoto K (1953) On a new sporozoa, Hexacapsula neothunni gen. et sp. nov., from the muscle of yellowfin tuna, Neothunnus macropterus. Bull Jap Soc Sci Fish 18:293–299
Burger MAA, Adlard RD (2010) Phenotypic variation in a significant spore character in Kudoa (Myxosporea: Multivalvulida) species infecting brain tissue. Parasitology 137(12):1759–1772
Burger MAA, Adlard RD (2011) Low host specificity in the Kudoidae (Myxosporea: Multivalvulida) including seventeen new host records for Kudoa thalassomi. Folia Parasitol 58(1):1–16
Fiala I (2006) The phylogeny of Myxosporea (Myxozoa) based on small subunit ribosomal RNA gene analysis. Int J Parasitol 36(14):1521–1534
Heiniger H, Adlard RD (2012) Host specificity and local infection dynamics of Kudoa leptacanthae n. sp (Multivalvulida: Kudoidae) from the pericardial cavity of two Zoramia spp. (Perciformes: Apogonidae) at Lizard Island lagoon, Queensland, Australia. Parasitol Int 61(4):697–706
Heiniger H, Cribb TH, Adlard RD (2013) Intra-specific variation of Kudoa spp. (Myxosporea: Multivalvulida) from apogonid fishes (Perciformes), including the description of two new species, K. cheilodipteri n. sp and K. cookii n. sp., from Australian waters. Syst Parasitol 84(3):193–215
Hillis DM, Dixon MT (1991) Ribosomal DNA: molecular evolution and phylogenetic inference. Q Rev Biol 66(4):411–453
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17(8):754–755
Iwashita Y et al (2013) Food poisoning associated with Kudoa septempunctata. J Emerg Med 44(5):943–945
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30(4):772–780
Kawai T et al (2012) Identification of Kudoa septempunctata as the causative agent of novel food poisoning outbreaks in Japan by consumption of Paralichthys olivaceus in raw fish. Clin Infect Dis 54(8):1046–1052
Kent ML et al (2001) Recent advances in our knowledge of the Myxozoa. J Eukaryot Microbiol 48(4):395–413
Li YC, Sato H, Tanaka S, Ohnishi T, Kamata Y, Sugita-Konishi Y (2013) Characterization of the ribosomal RNA gene of Kudoa neothunni (Myxosporea: Multivalvulida) in tunas (Thunnus spp.) and Kudoa scomberi n. sp in a chub mackerel (Scomber japonicus). Parasitol Res 112(5):1991–2003
Lom J, Dyková I (2006) Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol 53(1):1–36
Martinez de Velasco G, Rodero M, Cuellar C, Chivato T, Mateos JM, Laguna R (2008) Skin prick test of Kudoa sp. antigens in patients with gastrointestinal and/or allergic symptoms related to fish ingestion. Parasitol Res 103(3):713–715
Matsukane Y, Sato H, Tanaka S, Kamata Y, Sugita-Konishi Y (2010) Kudoa septempunctata n. sp (Myxosporea: Multivalvulida) from an aquacultured olive flounder (Paralichthys olivaceus) imported from Korea. Parasitol Res 107(4):865–872
Matsukane Y, Sato H, Tanaka S, Kamata Y, Sugita-Konishi Y (2011) Kudoa iwatai and two novel Kudoa spp., K. trachuri n. sp. and K. thunni n. sp. (Myxosporea: Multivalvulida), from daily consumed marine fish in western Japan. Parasitol Res 108(4):913–926
Miller TL, Adlard RD (2013) Unicapsula species (Myxosporea: Trilosporidae) of Australian marine fishes, including the description of Unicapsula andersenae n. sp. in five teleost families off Queensland, Australia. Parasitol Res 112(8):2945–2957
Moran JDW, Whitaker DJ, Kent ML (1999) A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture 172(1–2):163–196
Nylander JAA (2004) MrModeltest v2. Program distributed by the author. Uppsala University, Evolutionary Biology Centre
Patashnik M, Groninger HS Jr, Barnett H, Kudo G, Koury B (1982) Pacific Whiting, Merluccius productus. Abnormal muscle texture caused by myxosporidian-induced proteolysis. Mar Fish Rev 44:1–12
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574
Sato H (2011) Biology of the myxozoa, a newly recognized parasitic pathogen causing food poisoning. Yamaguchi. J Vet Med 38:1–26
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57(5):758–771
Stehr C, Whitaker DJ (1969) Host–parasite interaction of the myxosporean Kudoa paniformis (Kabata & Whitaker 1981) and Kudoa thyrsites (Gilchrist 1924) in the muscle of the Pacific whiting, Merluccius productus (Ayres): an ultrastructural study. J Fish Dis 9:505–517
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony, version 4.0b10. Sinauer Associates, Massachusetts
Whipps CM, Kent ML (2006) Phylogeography of the cosmopolitan marine parasite Kudoa thyrsites (Myxozoa: Myxosporea). J Eukaryot Microbiol 53(5):364–373
Whipps CM, Adlard RD, Bryant MS, Kent ML (2003a) Two unusual myxozoans, Kudoa quadricornis n. sp (Multivalvulida) from the muscle of goldspotted trevally (Carangoides fulvoguttatus) and Kudoa permulticapsula n. sp (Multivalvulida) from the muscle of Spanish mackerel (Scomberomorus commerson) from the Great Barrier Reef, Australia. J Parasitol 89(1):168–173
Whipps CM, Adlard RD, Bryant MS, Lester RJ, Findlay V, Kent ML (2003b) First report of three Kudoa species from eastern Australia: Kudoa thyrsites from mahi mahi (Coryphaena hippurus), Kudoa amamiensis and Kudoa minithyrsites n. sp. from sweeper (Pempheris ypsilychnus). J Eukaryot Microbiol 50(3):215–219
Whipps CM et al (2004) Phylogeny of the multivalvulidae (Myxozoa : Myxosporea) based on comparative ribosomal DNA sequence analysis. J Parasitol 90(3):618–622
Yokoyama H (2003) A review: gaps in our knowledge on myxozoan parasites of fishes. Fish Pathol 38(4):125–136
Yokoyama H, Itoh N (2005) Two multivalvulid myxozoans causing postmortem myoliquefaction: Kudoa megacapsula n. sp from red barracuda (Sphyraena pinguis) and Kudoa thyrsites from splendid alfonso (Beryx splendens). J Parasitol 91(5):1132–1137
Yokoyama H, Whipps CM, Kent ML, Mizuno K, Kawakami H (2004) Kudoa thyrsites from Japanese flounder and Kudoa lateolabracis n. sp from chinese sea bass: causative myxozoans of post-mortem myoliquefaction. Fish Pathol 39(2):79–85
Yokoyama H, Yanagida T, Takemaru I (2006) The first record of Kudoa megacapsula (Myxozoa : Multivalvulida) from farmed yellowtail Seriola quinqueradiata originating from wild seedlings in South Korea. Fish Pathol 41(4):159–163
Yokoyama H, Yanagida T, Shirakashi S (2012) Kudoa ogawai n. sp (Myxozoa: Multivalvulida) from the trunk muscle of Pacific barrelfish Hyperoglyphe japonica (Teleostei: Centrolophidae) in Japan. Parasitol Res 110(6):2247–2254
Yokoyama H, Suzuki J, Shirakashi S (2014) Kudoa hexapunctata n. sp. (Myxozoa: Multivalvulida) from the somatic muscle of Pacific bluefin tuna Thunnus orientalis and re-description of K. neothunni in yellowfin tuna T. albacares. Parasitol Int 63:571–579
Acknowledgments
We would like to thank the Wakayama Prefectural Fisheries Experimental Station staff for storing the fish and helping with sampling. This research was supported in part by the SATREPS program of the Japan Science and Technology Agency (JST) to SS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shirakashi, S., Yamane, K., Ishitani, H. et al. First report of Kudoa species in the somatic muscle of the Japanese parrotfish Calotomus japonicus (Scaridae) and a description of Kudoa igami
, n. sp. (Myxozoa: Multivalvulida).
Parasitol Res 113, 2515–2524 (2014). https://doi.org/10.1007/s00436-014-3901-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3901-1