Abstract
Kudoa neothunni is the first described Kudoa species having six shell valves and polar capsules, previously assigned to the genus Hexacapsula Arai and Matsumoto, 1953. Since its genetic analyses remain to be conducted, the present study characterizes the ribosomal RNA gene (rDNA) using two isolates from a yellowfin tuna (Thunnus albacares) with post-harvest myoliquefaction and a northern bluefin tuna (Thunnus thynnus) without tissue degradation. Spores of the two isolates localized in the myofiber of trunk muscles, forming pseudocysts, and showed typical morphology of K. neothunni with six equal-sized shell valves radially arranged in apical view: spores (n = 15) measuring 9.5–11.4 μm in width, 7.3–8.6 μm in suture width, 8.9–10.9 μm in thickness, and 7.3–7.7 μm in length; and polar capsules measuring 3.6–4.1 μm by 1.8–2.3 μm. In lateral view, the spores were pyramidal in shape without apical protrusions. Their 18S and 5.8S rDNA sequences were essentially identical, but variations in the ITS1 (62.4 % similarity across 757-bp length), ITS2 (66.9 % similarity across 599-bp length), and 28S (99.0 % similarity across 2,245-bp length) rDNA regions existed between the two isolates. On phylogenetic trees based on the 18S or 28S rDNA sequence, K. neothunni formed a clade with Kudoa spp. with more than four shell valves and polar capsules, particularly K. grammatorcyni and K. scomberomori. Semiquadrate spores of a kudoid species with four shell valves and polar capsules were detected from minute cysts (0.30–0.75 mm by 0.20–0.40 mm) embedded in the trunk muscle of a chub mackerel (Scomber japonicus) fished in the Sea of Japan. Morphologically, it resembled K. caudata described from a chub mackerel fished in the southeastern Pacific Ocean off Peru; however, it lacked filamentous projections on the shell valves of spores. Additionally, it morphologically resembled K. thunni described from a yellowfin tuna also fished in the Pacific Ocean; spores (n = 30) measuring 8.2–10.5 μm in width, 7.0–8.8 μm in thickness, and 6.1–6.8 μm in length; and polar capsule measuring 2.5–3.4 μm by 1.3–2.0 μm. The similarities of the 18S and 28S rDNA sequences between these two species were 98.5 % and 96.3 %, respectively. Simultaneously, the dimensions of cysts in the trunk muscle formed by K. thunni are clearly larger than those of the present species from a chub mackerel: 1.3–2.0 mm by 1.1–1.4 mm (n = 14) vs. 0.30–0.75 mm by 0.20–0.40 mm (n = 7), respectively. Thus, Kudoa scomberi n. sp. is proposed for this multivalvulid species found in the chub mackerel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kudoa neothunni Arai and Matsumoto, 1953 (Myxosporea: Multivalvulida) was first described from a yellowfin tuna (Thunnus albacares; syn. Neothunnus macropterus) with post-mortem myoliquefaction hauled from the Banda Sea and transported to the Tokyo Fish Market (Arai and Matsumoto 1953). The spores were packed in pseudocysts in the myofiber of trunk muscles, having six shell valves and polar capsules (SV/PC) arranged symmetrically. Due to this novel character and the archaic definition of the genus Kudoa at that time (Lom and Noble 1984), the new genus Hexacapsula was proposed based on this species (Arai and Matsumoto 1953). Similarly, the genera Pentacapsula (with five SV/PC) and Septemcapsula (with seven SV/PC) were added to the family Kudoidae in the order Multivalvulida (Naidenova and Zaika 1970; Hsieh and Chen 1984). When Whipps et al. (2003) described a new myxosporean species having 13 SV/PC, they placed it in the genus Kudoa, i.e., K. permulticapsula, because its 18S ribosomal RNA gene (rDNA) was closest to K. thyrsites and K. quadricornis, not forming a distinct clade from Kudoa spp. Subsequently, Whipps et al. (2004) demonstrated that the 18S rDNA of members classified in the genera Pentacapsula, Hexacapsula (with six SV/PC), and Septemcapsula rooted within a clade of Kudoa spp. with Unicapsula (Trilosporidae) as an outlier to these genera, and thus, proposed that all marine myxosporean parasites having four or more SV/PC should be classified in the genus Kudoa.
As myxosporean spores are small and often possess only a few morphological features useful for differentiation, reliable species identification requires not only thorough morphological observations but also molecular analyses of rDNA sequences and other biological characterizations such as tissue tropism, host specificity, pathogenicity, and geographical distribution. To differentiate K. alliaria from K. rosenbuschi exhibiting a similar spore morphology and almost identical 18S rDNA sequences (99.8 % similarity across 1,680-bp length), Whipps and Diggles (2006) described that unlike the latter species, the former species was not associated with tissue degradation. Furthermore, Burger et al. (2007) found identical 18S rDNA sequences in two Kudoa isolates with distinct morphologies and clearly disparate host origins; K. yasunagai with seven polar capsules from Paralichthys olivaceus in Japan (Yokoyama et al. 2004) and Kudoa sp. with five and six SV/PC from sand whitings (Sillago ciliata) in Australia. They suggested that the 18S rDNA sequence was incapable of distinguishing some combinations of Kudoa spp. Later, however, Burger and Adlard (2010b) concluded that the molecular results indicated conspecificity rather than 18S rDNA analysis reaching its level of resolution.
Of 80 or more nominal Kudoa spp., approximately half have been genetically characterized using mainly 18S rDNA sequences (Burger and Adlard 2011; Sato 2011). Although genetically characterized Kudoa spp. face new taxonomic challenges as mentioned above, the species differentiation of Kudoa spp. known only by morphology is far more difficult, particularly when morphologically similar spores are detected from hosts other than the type host or fishes listed in the original description, or in geographically distant areas from the original record (Moran et al. 1999; Kent et al. 2001; Yokoyama 2003; Diamant et al. 2005; Whipps and Diggles 2006; Whipps and Kent 2006; Burger and Adlard 2010a, 2011).
In this study, we compared the rDNA sequences of two isolates of K. neothunni with/without post-harvest myoliquefaction in order to clarify the taxonomic relationship of these two isolates. Furthermore, a kudoid species having semiquadrate spores with four SV/PC from minute cysts in the trunk muscle of a chub mackerel (S. japonicus) fished in the Sea of Japan was examined morphologically and genetically, and compared with known species including K. caudata Kovaleva and Gaevskaya, 1983 described from the same host species but in the southeastern Pacific Ocean off Peru (Kovaleva and Gaevskaya 1983).
Materials and methods
Fish samples and parasitological examination
A frozen block of the trunk muscle of a northern bluefin tuna (T. thynnus) containing kudoid spores was provided by a Japanese-style restaurant located in Kanagawa Prefecture, Japan during the summer of 2010. The fish was fished from an unknown site in the Pacific Ocean. This fish did not show post-harvest myoliquefaction.
Thin slices of the frozen trunk muscle were placed in physiological saline and minced with fine forceps to release the parasite. The released spores were observed using a microscope equipped with differential interference contrast imaging, photographed at a magnification of ×400, and transformed into photographs with Adobe® Photoshop® ver. 11.0 (Adobe Systems, San Jose, California, USA). Photographs were then printed at a high magnification. Measurements were conducted on multiple printed photographs following the guidelines of Lom and Arthur (1989). All measurements are expressed in μm unless otherwise stated. Following removal of a portion of the spores for DNA extraction, the parasite was fixed in 10 % neutral-buffered formalin solution and used for scanning electron microscopy as previously described (Matsukane et al. 2010). Briefly, the preserved sample was washed three times in 0.2 M Na2HPO4-NaH2PO4-buffered solution, pH 7.8 (PB) and immersed in 2.5 % glutaraldehyde in PB overnight. After washing three times in PB, the specimen was post-fixed in 1 % (w/v) osmium tetroxide in PB for 1 h. Following three washes in PB, the sample was placed in a 1.5-ml tube then dehydrated through a graded ethanol series, immersed in warmed t-butyl-alcohol, and cooled in a refrigerator at 4 °C for 2 h. The sample was then freeze-dried in a freeze-drying device (model JFD-300; JEOL, Akishima, Tokyo, Japan), mounted on stubs, and sputter-coated with gold-palladium at 200 Å in an ion sputtering device (model JFC-1500; JEOL). Specimens were examined using a scanning electron microscope (model JSM-6100; JEOL) at an accelerating voltage of 15 kV.
Another K. neothunni isolate was obtained from a yellowfin tuna (T. albacares), 36 kg in body weight, which was fished in the southwestern Pacific Ocean near Mindanao Island, Philippines (approximate sea position at 7–8N, 131E) during 15–20 October 2011 and landed at Naha, Okinawa Prefecture, Japan on 5 November 2011. This fish showed post-harvest myoliquefaction. The kudoid isolate from this fish was examined under a light microscopy as above.
A fresh fillet of a chub mackerel (S. japonicus) with several macroscopic but minute white spots was obtained from a local fish market near Yamaguchi University on 11 February 2011. This fish was fished in the Sea of Japan off Nagasaki Prefecture, Japan, and was examined parasitologically as described above.
Specimens collected in the present work were deposited in the National Museum of Nature and Science, Tokyo, Japan under specimen numbers NSMT-Pr320–Pr330.
DNA extraction, polymerase chain reaction (PCR), and sequencing
Parasite DNA was extracted from kudoid spore-rich samples using an Illustra™ tissue and cells genomicPrep Mini Spin Kit (GE Healthcare UK, Buckinghamshire, UK) according to the instructions of the manufacturer. PCR amplification of overlapping fragments of the rDNA was performed in a 20-μl volume containing a DNA polymerase, Blend Taq-Plus (TOYOBO, Dojima Hama, Osaka, Japan), and primers (listed in Table 1). The PCR cycling protocol was 3 min at 94 °C, then 40 cycles of 45 sec at 94 °C, 1 min at 64 °C or 62 °C (see Table 1), and 1 min at 72 °C, followed by a final extension at 72 °C for 7 min. The PCR products were purified using a High Pure PCR Cleanup Micro Kit (Roche Diagnostics GmbH, Mannheim, Germany), and sequenced directly using the primers listed in Table 1. When direct sequencing was not satisfactory, the purified PCR products were cloned into a plasmid vector, pTA2 (TArget Clone™; TOYOBO), and transformed into Escherichia coli JM109 (TOYOBO) according to the instructions of the manufacturer. Following propagation, the plasmid DNA was extracted using a NucleoSpin® Plasmid kit (MACHEREY-NAGEL GmbH, Düren, Germany) and inserts from multiple independent clones, at least three, were sequenced using universal M13 forward and reverse primers.
Phylogenetic analysis
For phylogenetic analysis, the newly obtained 18S and 28S rDNA sequences of Kudoa spp. in the present study (DDBJ/EMBL/GenBank accession nos. AB693040–AB693049) and Kudoa-related sequences retrieved from the DDBJ/EMBL/GenBank databases were aligned using the CLUSTAL W multiple alignment program (Thompson et al. 1994), with subsequent manual adjustment. The accession numbers of the sequences analyzed in the present study are given in the figures showing phylogenetic trees. Regions judged to be poorly aligned and characters with a gap in any sequences were excluded from subsequent analyses (Smythe et al. 2006); 1,341 characters, of which 341 were variable, remained for subsequent analysis for 18S rDNA, and 506 characters, of which 286 were variable, remained for subsequent analysis for 28S rDNA. Maximum likelihood (ML) analysis was performed with the program PhyML (Guindon and Gascuel 2003; Dereeper et al. 2008) provided on the “phylogeny.fr” website (http://www.phylogeny.fr/). The probability of inferred branch was assessed by the approximate likelihood-ratio test (aLRT), an alternative to the non-parametric bootstrap estimation of branch support (Anisimova and Gascuel 2006). Unicapsula sp. (Trilosporidae, Multivalvulida; DDBJ/EMBL/GenBank accession nos. AY302725 and AY302727) was used as an outgroup for the construction of ML phylogenetic trees.
Results
Morphological observations
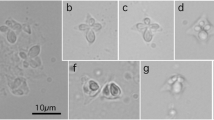
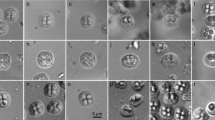
The trunk muscle of the northern bluefin tuna showed a normal gross appearance, whereas that of the yellowfin tuna exhibited striking post-harvest myoliquefaction. One gram of muscle removed from the northern bluefin tuna contained 7 × 107 spores (Fig. 1), with six equal-sized shell valves radially arranged in apical view (Fig. 2). In lateral view, the spores were pyramidal in shape without apical protrusions. This finding was confirmed by scanning electron microscopy (Fig. 3). Spores collected from jellied meat and intact meat of the yellowfin tuna showed the same morphology. All the isolates exhibited a morphology typical of K. neothunni: 9.5–11.4 (mean [n = 15], 10.6) μm in width; 7.3–8.6 (8.1) μm in suture width; 8.9–10.9 (9.7) μm in thickness; and 7.3–7.7 (7.6) μm in length. The polar capsules were equal in dimensions: 3.6–4.1 (3.8) μm in length and 1.8–2.3 (2.0) μm in width (Table 2). Coils in the polar capsules were not visible under a light microscope.
Although our specimens of K. neothunni had larger polar capsules in comparison to the original description by Arai and Matsumoto (1953), this species was differentiated from K. grammatorcyni by having larger spores, a more pointed shell valve edge in apical view, and a different lateral view (pyramidal versus ovoid; Table 2). K. neothunni and K. scomberomori had an obvious resemblance in spore morphology, but the former species had larger spores and polar capsules as well as a different lateral view (Table 2). K. thalassomi was differentiated from K. neothunni by having spores with lateral projections, whereas K. septempunctata was differentiated by its spore shape and demonstrating variations in polar capsule size (Table 2).
In the chub mackerel fillet, fished in the Sea of Japan, there were 20 white parasitic cysts delineated by a thin layer of fibrous tissue. The range of cyst sizes (mean ± SD; n = 7) was 0.30–0.75 (0.49 ± 0.15) mm by 0.20–0.40 (0.26 ± 0.07) mm. Spores were semiquadrate with four equal-sized shell valves in apical view (Figs. 4 and 5). In lateral view, the spores were ovoid in shape and apical projections on shell valves were not evident. Polar capsules were drop-like or ovoid. The morphometries of the spores were compared with other Kudoa spp. having similar morphology (Table 3).
Molecular phylogenetic analysis
The rDNA sequence of K. neothunni collected from the northern bluefin tuna was 6,226 bp in length: partial external transcribed spacer (ETS), 170 bp; 18S, 1,741 bp; internal transcribed spacer (ITS) 1 region, 705 bp; 5.8S, 158 bp; ITS2 region, 529 bp; and partial 28S, 2,923 bp (DDBJ/EMBL/GenBank accession no. AB693042). The rDNA sequence of K. neothunni collected from the yellowfin tuna was 5,609 bp in length: partial 18S, 1,720 bp; ITS1 region, 705 bp; 5.8S, 158 bp; ITS2 region, 588 bp; and partial 28S, 2,438 bp (DDBJ/EMBL/GenBank accession no. AB693049). The 18S and 5.8S rDNA sequences of these two isolates were essentially identical (99.9 % similarity across 1,720-bp length and 100 % similarity across 158-bp length, respectively). However, variations in the ITS1 (62.4 % similarity across 757-bp length), ITS2 (66.9 % similarity across 599-bp length), and 28S (99.0 % similarity across 2,245-bp length) rDNA sequences were observed between the two isolates.
In the ML phylogenetic trees based on the 18S and 28S rDNA sequences, K. neothunni positioned closest to K. grammatorcyni, forming a clade with Kudoa spp. with five or more SV/PC and tissue tropism to trunk muscles except for K. septempunctata and K. thalassomi (Figs. 6 and 7). The latter two Kudoa spp. formed a clade with kudoids with five or more SV/PC and characteristic tissue tropism to the brain. The similarities of the 18S and 28S rDNA sequences between K. neothunni and K. grammatorcyni were 99.4 % (10 base differences across 1,680-bp length) and 93.4 % (48 base differences across 726-bp length), respectively. The similarity of the 18S rDNA sequence between K. neothunni and K. scomberomori was 99.6 % (6 base differences across 1,680-bp length). Comparison of the 28S rDNA sequences was not possible due to a lack of deposited 28S rDNA sequences of K. scomberomori in the DDBJ/EMBL/GenBank databases.
The kudoid species isolated from the chub mackerel formed a clade with K. thunni and “K. crumena” in both ML phylogenetic trees (Figs. 6 and 7). The similarities of the 18S and 28S rDNA sequences between this kudoid isolate and K. thunni were 98.5 % (27 base differences across 1,765-bp length) and 96.3 % (30 base differences across 804-bp length), respectively. Similarly, those between the present isolate and “K. crumena” were 98.8 % (20 base differences across 1,631-bp length) and 96.6 % (28 base differences across 815-bp length), respectively. The similarities of the 18S and 28S rDNA sequences between the present isolate and K. trachuri were 96.2 % (across 1,780-bp length) and 90.4 % (across 771-bp length), respectively. This morphologically and genetically characterized kudoid is described as a new species.
Description
Kudoa scomberi n. sp. (Myxosporea: Multivalvulida) (Figs. 4 and 5; Table 3).
Plasmodia were delineated by a thin layer of fibrous tissue in the trunk muscle, seen as minute white cysts measuring 0.30–0.75 (average 0.49) mm by 0.20–0.40 (average 0.26) mm (n = 7).
Spores semiquadrate in shape in apical view, with four shell valves and polar capsules of equal sizes. Shell valves without ornamentation, at least by light microscopy assessment. Polar capsules drop-like, located at the anterior portion of shell valves. In apical view, polar capsules accounted for approximately 50 % or less of the total radius of shell valves. In lateral view, spores ovoid with somewhat flattened bottom. Coils of polar filament were not visible in wet preparations. Spores (n = 30; average and range in parentheses; measurements in μm): width 9.2 (8.2–10.5), thickness 8.1 (7.0–8.8), length 6.4 (6.1–6.8), polar capsule length 2.9 (2.5–3.4), and polar capsule width 1.6 (1.3–2.0).
Partial nucleotide sequences of the 18S, ITS1, and 5.8S rDNA (2,232 bp in length) and partial 5.8S, ITS2, and 28S rDNA (1,052 bp in length) have been deposited in DDBJ/EMBL/GenBank (accession nos. AB693044 and AB693045).
Taxonomic summary
Type host: S. japonicus Houttuyn, 1782, chub mackerel (Actinopterygii: Perciformes: Scombridae).
-
Site of infection: Cysts in somatic muscles.
-
Type locality: Sea of Japan, off Nagasaki, Nagasaki Prefecture, Japan.
-
Specimen deposited: Hapantotype NSMT-Pr323, National Museum of Nature and Science, Tokyo, Japan.
-
Etymology: The specific name comes from its host, S. japonicus.
-
Prevalence: Unknown.
Remarks
K. caudata has been reported from the same host species (S. japonicus), but fished in the southeastern Pacific Ocean off Peru (Kovaleva and Gaevskaya 1983). Although the general appearances of K. caudata and K. scomberi n. sp. spores resemble one another, K. caudata is differentiated from the new species by possession of a hair-like filamentous projection on the lateral surface of each shell valve. Similar fine but shorter projections on the lateral surface of each shell valve have been documented in K. aequidens from Aequidens plagiozonatus in the Amazonian estuarine region and K. camarguensis from Pomatoschistus minutus in the Mediterranean Sea (Pampoulie et al. 1999; Casal et al. 2008). Since its original description, K. caudata has not been recorded again. The rDNA sequence of this kudoid is currently unavailable, so its genetic relationship with the new species is unknown. K. scomberi n. sp. formed a clade with Kudoa spp. with semiquadrate spores such as K. thunni, “K. crumena,” and K. trachuri in both of the phylogenetic trees based on 18S and 28S rDNA sequences. In addition to a substantially lower similarity of the 18S and 28S rDNA sequences (98.8 % or lower, and 96.6 % or lower, respectively), the new species is morphologically differentiated from K. crumena on the basis of a different lateral view of spores (ovoid versus pouch-shaped with truncated apex), smaller spore length, and smaller polar capsule size. Furthermore, it is morphologically differentiated from K. trachuri by a larger spore size (Table 3). The morphological differentiation of K. scomberi n. sp. from K. thunni based solely on light microscopy assessment is difficult. In addition to a lower similarity of the 18S and 28S rDNA sequences (98.5 % and 96.3 %, respectively), the dimensions of cysts in the trunk muscle formed by K. thunni are clearly larger than those of the new species (Matsukane et al. 2011): 1.5 (1.3–2.0) mm by 1.2 (1.1–1.4) mm (n = 14) vs. 0.49 (0.30–0.75) mm by 0.26 (0.20–0.40) mm (n = 7), respectively.
As reported previously (Matsukane et al. 2011), K. crumena has been recorded from Spanish mackerel (Scomberomorus maculatus) in southern Florida, USA (Iversen and Van Meter 1967), but also from yellowfin tuna (T. albacares) collected in North Carolina, USA (Moran et al. 1999). The 18S and 28S rDNA sequences deposited in DDBJ/EMBL/GenBank for K. crumena (accession nos. AF378347 and FJ417057, respectively) were based on an isolate from T. albacares in North Carolina, USA, and another isolate from southern bluefin tuna (T. maccoyii) in Tasmania, Australia, respectively, and not from the type host for K. crumena, Scomberomorus maculatus (Kent et al. 2001; Bartosová et al. 2009). The closeness of the relationships of the 18S and 28S rDNA sequences of K. thunni with the deposited sequences of “K. crumena” from Thunnus spp. requires further confirmation in order to establish whether K. crumena described by Iversen and Van Meter (1967) and “K. crumena” from Thunnus spp. are indeed identical.
Discussion
K. neothunni has been recorded not only from the jellied meat of yellowfin tuna but also from the jellied meat of bigeye tuna (T. obesus) fished in the Pacific Ocean near Taiwan and northern bluefin tuna in the Pacific Ocean near northern Japan (Arai and Matsumoto 1953; Konagaya 1982, 1984). In the present study, we have characterized morphologically and genetically two K. neothunni isolates from different tuna species (T. albacares and T. thynnus) with/without post-harvest myoliquefaction. In addition to morphological similarity, the 18S rDNA sequences of these two isolates were essentially identical; only two base substitutions were detected across the 1,720-bp length of the 18S rDNA sequence. There are only two base substitutions across the comparable 1,679-bp length of 18S rDNA sequence of K. alliaria infecting Argentinian hoki (Macruronus magellanicus) without myoliquefaction and K. rosenbuschi recovered from Argentinean hake (Merluccius hubbsi) with post-harvest myoliquefaction. Furthermore, these two species show an identical morphology and geographical distribution (Abollo et al. 2005; Whipps and Diggles 2006). This illustrates the use of a single pathophysiological character (post-mortem tissue degradation) as a major criterion for species discrimination. When we apply this taxonomic code, it appears likely that the two K. neothunni isolates found in tunas with/without post-harvest myoliquefaction are distinct species. In this regard, however, we should take into account the fact that the degree of post-harvest myoliquefaction, i.e. enzymatic digestion of the host muscle, can be affected by several factors such as time of evaluation post-mortem, temperature during storage, intensity of the infection, developmental stage of plasmodia, host response to infected myofibers, muscle pH associated with the inherent flesh quality, etc. (Konagaya 1984; Dawson-Coates et al. 2003; Funk et al. 2007, 2008; Zhou and Li-Chan 2009). Even if a close association between particular Kudoa spp. and post-harvest myoliquefaction is regarded as a general tendency, the condition of fish meats at a particular time point could be highly variable, and for an individual case of infection it is inadvisable to use this character as a major criterion for species differentiation.
In contrast to their 18S rDNA sequence, the two K. neothunni isolates collected in this study had significant differences in the nucleotide sequences of their ITS1 (only 62.4 % similarity across 757-bp length), ITS2 (only 66.9 % similarity across 599-bp length), and 28S (22 base changes across 2,245-bp length) rDNA regions. Collection of more isolates from different origins and genetic characterization of their ITS region and/or 28S rDNA may disclose cryptic species or different lineages of K. neothunni, and could contribute to understanding the significance(s) of such ITS variation in the species. When conducting such research, kudoid specimens should be collected from infected fish with post-harvest myoliquefaction and from infected fish with an intact appearance, although it is easier to collect samples from the former. In a work on cosmopolitan K. thyrsites, assumed to be a species complex by many researchers, the 18S rDNA sequences obtained from hosts in different regions (seas around Canada, USA, Chile, UK, South Africa, Australia, and Japan) showed little sequence divergence (0.2–1.0 %). Greater variation in the 28S rDNA sequences (3.0–9.6 % variation across 700-bp length) and ITS1 regions (maximum difference of 65.6 % for 393–493-bp length) was found (Whipps and Kent 2006). Greater base variation in the 28S rDNA sequence than in the 18S rDNA sequence has been shown for several Kudoa spp. (Burger and Adlard 2011). Genetic comparison of 18 K. thalassomi isolates from 18 fish species of six different families on the Great Barrier Reef, Queensland, Australia revealed a minimum 99.7 % identity for 18S rDNA (range of 0–10 nucleotide differences across 1,539-bp length) and 98.5 % identity for 28S rDNA (range of 0–10 nucleotide differences across 814-bp length) regardless of host origin (Burger and Adlard 2011). The possible usefulness of molecular markers other than the 18S rDNA sequence awaits evaluation for Kudoa spp. including K. neothunni.
Recently, Burger and Adlard (2010b) identified a Kudoa isolate having five SV/PC in the brain of sand whitings around Australia as K. yasunagai—originally described as having seven SV/PC in the brain of olive flounders (P. olivaceus) and bluefin tunas—based on 100 % identity of the 18S rDNA. This morphological variation of a single species appears to be distinct from the previously reported plasticity of numbers of SV/PC in a single plasmodium of K. chaetodoni (Burger et al. 2007), K. crumena (Iversen and Van Meter 1967), K. iwatai (Egusa and Shiomitsui 1983; Diamant et al. 2005), K. lethrini (Burger et al. 2007), K. monodactyli (Gunter et al. 2006), K. neurophila (Grossel et al. 2003), K. permulticapsula (Whipps et al. 2003), K. septempunctata (Matsukane et al. 2010), K. thalassomi (Burger and Adlard 2011), and K. yasunagai (Hsieh and Chen 1984). The same researchers (Burger et al. 2007), however, differentiated brain-parasitizing K. chaetodoni from K. yasunagai based on notable differences in spore sizes and the number of polar capsules (8/9), although these two species have 0.19 % difference (3 bases) in the 18S rDNA and 0.6–1.3 % difference (4–8 bases) in the partial 28S rDNA. Similarly, Burger and Adlard (2010a) found dimensional differences, but not in shape, between spores of K. paraquadricornis and K. quadricornis, although these two species have only one nucleotide difference in the 18S rDNA of 1,683-bp length (99.94 % identity) and 27 base differences in the 28S rDNA of 715-bp length (96.22 % identity). Likewise, K. prunusi, recorded from the brain of aquacultured northern bluefin tunas, shows a close resemblance in morphology with K. yasunagai ex S. ciliata, i.e., having five SV/PC, and has a 0.3 % (5 bases) difference in the 18S rDNA and a 1.7 % (11 bases) difference in the 28S rDNA from those of K. yasunagai (Meng et al. 2011). As mentioned above, collection of more Kudoa isolates from different origins and geographical areas and subsequent genetic characterization of them together with intensive morphological observation may help us to determine whether the observed divergence is ascribed to intraspecific variation or critical difference separating species of the myxosporean parasites.
Although a close relationship of K. scomberi n. sp. to K. thunni appears apparent in both ML phylogenetic trees based on the 18S and 28S rDNA sequences (Figs. 6 and 7), this is ascribed not to conspecificity of these two species, but to extensive trimming of certain variable areas containing gaps for construction of the molecular phylogenetic tree. Since kudoid species with quadrate or semiquadrate spores with four SV/PC resembling each other are abundant, it is critical to perform genetic analyses not only for 18S rDNA but also for 28S rDNA for their species identification.
Recently, a subtle but significant public health impact of K. septempunctata has been indicated by Japanese researchers (Konishi 2011; Ohnishi 2011; Grabner et al. 2012; Harada et al. 2012; Kawai et al. 2012). Indeed, two to three dozen outbreaks of symptomatic patients per year have been recorded in the last few years. This food poisoning, with a moderate prognosis, is manifested by vomiting and diarrhea within 12 hours after consumption of fresh flounder slices in the raw state as sashimi or sushi. The Ministry of Health, Labour and Welfare of Japan has issued an official notice to regard K. septempunctata as a potential cause of food poisoning following consumption of fresh fish foods. Since this food poisoning is dose- and spore viability-dependent, it is advisable to avoid consumption of fresh fish with kudoid infection in the raw state. With kudoid species that form pseudocysts in the trunk muscle, like K. septempunctata in the flounder and K. neothunni in the tuna, it is difficult to perceive their infection with the naked eye, even heavy ones as shown in Fig. 1, resulting in the oral intake of kudoid spores in bulk. In Spain, Martínez de Velasco et al. (2008) noticed a potential public health impact of Kudoa spp. as a food-borne allergen for humans with allergic gastrointestinal symptoms. Feasible and reliable techniques to identify Kudoa spp. defined by taxonomical experts would facilitate public health workers to approach and control the cause of diseases, and specify the fish species that put our health at risk.
References
Abollo E, Novoa B, Figueras A (2005) SSU rDNA analysis of Kudoa rosenbuschi (Myxosporea) from the Argentinean hake Merluccius hubbsi. Dis Aquat Org 64:135–139
Adlard RD, Bryant MS, Whipps CM, Kent ML (2005) Multivalvulid myxozoans from eastern Australia: three new species of Kudoa from scombrid and labrid fishes of the great barrier reef, Queensland, Australia. J Parasitol 91:1138–1142
Anisimova M, Gascuel O (2006) Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol 55:539–552
Arai Y, Matsumoto K (1953) On a new sporozoa, Hexacapsula neothunni gen. et sp. nov., from the muscle of yellowfin tuna, Neothunnus macropterus. Bull Jpn Soc Sci Fish 18:293–299
Bartosová P, Fiala I, Hypsa V (2009) Concatenated SSU and LSU rDNA data confirm the main evolutionary trends within myxosporeans (Myxozoa: Myxosporea) and provide an effective tool for their molecular phylogenetics. Mol Phylogenet Evol 53:81–93
Burger MAA, Adlard RD (2010a) Four new species of Kudoa Meglitsch, 1947 (Myxosporea: Multivalvulida) from Australia with recommendations for species descriptions in the Kudoidae. Parasitology 137:793–814
Burger MAA, Adlard RD (2010b) Phenotypic variation in a significant spore character in Kudoa (Myxosporea: Multivalvulida) species infecting brain tissue. Parasitology 137:1759–1772
Burger MAA, Adlard RD (2011) Low host specificity in the Kudoidae (Myxosporea: Multivalvulida) including seventeen new host records for Kudoa thalassomi. Folia Parasitol 58:1–16
Burger MAA, Cribb TH, Adlard RD (2007) Patterns of relatedness in the Kudoidae with descriptions of Kudoa chaetodoni n. sp. and K. lethrini n. sp. (Myxosporea: Multivalvulida). Parasitology 134:669–681
Casal G, Matos E, Matos P, Azevedo C (2008) Ultrastructural description of a new myxosporean parasite Kudoa aequidens sp. n. (Myxozpa, Myxosporea), found in the sub-opercular musculature of Aequidens plagiozonatus (Teleostei) from the Amazon river. Acta Protozool 47:135–141
Dawson-Coates JA, Chase JC, Funk V, Booy MH, Haines LR, Falkenberg CL, Whitaker DJ, Olafson RW, Pearson TW (2003) The relationship between flesh quality and numbers of Kudoa thyrsites plasmodia and spores in farmed Atlantic salmon, Salmo salar L. J Fish Dis 26:451–459
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie J-M, Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36:465–469
Diamant A, Ucko M, Paperna I, Colorni A, Lipshitz A (2005) Kudoa iwatai (Myxosporea: Multivalvulida) in wild and cultured fish in the red sea: Redescription and molecular phylogeny. J Parasitol 91:1175–1189
Egusa S, Shiomitsu T (1983) Two new species of the genus Kudoa (Myxosporea: Multivalvulida) from marine cultured fishes in Japan. Fish Pathol 18:163–171 (in Japanese with English summary)
Funk VA, Raap M, Sojonky K, Jones S, Robinson J, Falkenberg C, Miller KM (2007) Development and validation of an RNA- and DNA-based quantitative PCR assay for deterimination of Kudoa thyrsites infection levels in Atlantic salmon Salmo salar. Dis Aquat Org 75:239–249
Funk VA, Olafson RW, Raap M, Smith D, Aitken L, Haddow JD, Wang D, Dawson-Coates JA, Burke RD, Miller KM (2008) Identification, characterization and deduced amino acid sequence of the dominant protease from Kudoa paniformis and K. thyrsites; a unique cytoplasmic cysterine protease. Comp Biochem Physiol B 149:477–489
Grabner DS, Yokoyama H, Shirakashi S, Kinami R (2012) Diagnostic PCR assays to detect and differentiate Kudoa septempunctata, K. thyrsites and K. lateolabracis (Myxozoa, Multivalvulida) in muscle tissue of olive flounder (Paralichthys olivaceus). Aquaculture 338–341:36–40
Grossel GW, Dykova I, Handlinger J, Munday BL (2003) Pentacapsula neurophila sp. n. (Multivalvulida) from the central nervous system of striped trumbeter, Latris lineata (Forster). J Fish Dis 26:315–320
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Gunter NL, Whipps CM, Cribb TH, Adlard RD (2006) Characterisation of Kudoa monodactyli n. sp. (Myxozoa: Multivalvulida) from Monodactylus argenterus (Teleostei: Monodactylidae) from Moreton Bay, Queensland, Australia. J Eukaryot Microbiol 53:374–378
Harada T, Kawai T, Sato H, Yokoyama H, Kumeda Y (2012) Development of a quantitative polymerase chain reaction assay for detection of Kudoa septempunctata in olive flounder (Paralichthys olivaceus). Int J Food Microbiol 155:161–167
Hsieh S, Chen C (1984) Septemcapsula yasunagai gen. et sp. nov., representative of a ne family of the class Myxosporea. Acta Zootax Sinica 9:225–227 (in Chinese with English summary)
Iversen ES, Van Meter NN (1967) A new myxosporidian (Sporozoa) infecting the Spanish mackerel. Bull Mar Sci 17:268–273
Kawai T, Sekizuka T, Yahata Y, Kuroda M, Kumeda Y, Iijima Y, Kamata Y, Sugita-Konishi Y, Ohnishi T (2012) Identification of Kudoa septempunctata as the causative agent of novel food poisoning outbreaks in Japan by consumption of Paralichthys olivaceus in raw fish. Clin Infect Dis 54:1046–1052
Kent ML, Andree KB, Bartholomew JL, El-Matbouli M, Desser SS, Devlin RH, Feist SW, Hedrick RP, Hoffmann RW, Khattra J, Hallett SL, Lester RJG, Longshaw M, Palenzuela O, Siddall ME, Xiao C (2001) Recent advances in our knowledge of the Myxozoa. J Eukaryot Microbiol 48:395–413
Konagaya S (1982) Histological observation of jellied yellowfin tuna meat. Bull Tokai Reg Fish Res Lab 106:55–75 (in Japanese with English summary)
Konagaya S (1984) Studies on the jellied meat of fish, with special reference to that of yellowfin tuna. Bull Tokai Reg Fish Res Lab 114:1–101 (in Japanese with English summary)
Konishi Y (2011) Overview of the study on new food-borne diseases associated with raw fish and flesh. Food Sanit Res 740:7–12 (in Japanese)
Kovaleva AA, Gaevskaya AV (1983) [First data on fish myxosporidia of the southeastern Pacific open waters.] Vestnik Zoologii 17:6-11. (In Russian)
Lom J, Arthur JR (1989) A guideline for the preparation of species descriptions in Myxosporea. J Fish Dis 12:151–156
Lom J, Noble ER (1984) Revised classification of the Myxosporea Bütschli, 1881. Folia Parasitol 31:193–205
Martínez de Velasco G, Rodero M, Cuéllar C, Chivato T, Mateos JM, Laguna R (2008) Skin prick test of Kudoa sp. antigens in patients with gastrointestinal and/or allergic symptoms related to fish ingestion. Parasitol Res 103:713–715
Matsukane Y, Sato H, Tanaka S, Kamata Y, Sugita-Konishi Y (2010) Kudoa septempunctata n. sp. (Myxosporea: Multivalvulida) from an aquacultured olive flounder (Paralichthys olivaceus) imported from Korea. Parasitol Res 107:865–872
Matsukane Y, Sato H, Tanaka S, Kamata Y, Sugita-Konishi Y (2011) Kudoa iwatai and two novel Kudoa spp., K. trachuri n. sp. and K. thunni n. sp. (Myxosporea: Multivalvulida), from daily consumed marine fish in western Japan. Parasitol Res 108:913–926
Meng F, Yokoyama H, Shirakashi S, Grabner D, Ogawa K, Ishimaru K, Sawada Y, Murata O (2011) Kudoa prunusi n. sp. (Myxozoa: Multivalvulida) from the brain of Pacific bluefin tuna Thunnus orientalis (Temminck & Schlegel, 1844) cultured in Japan. Parasitol Int 60:90–96
Moran JDW, Whitaker DJ, Kent ML (1999) A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture 172:163–196
Naidenova NN, Zaika VE (1970) Three new genera of myxosporean parasites of fish from the Indian Ocean. Zool Zh 49:451–454 (in Russian with English summary)
Ohnishi T (2011) Food poisoning caused by Kudoa septempunctata. Food Sanit Res 740:13–20 (in Japanese)
Pampoulie C, Marques A, Rosecchi E, Crivelli AJ, Bouchereau J-L (1999) A new myxosporean parasite, Kudoa camarguensis n. sp., recorded on two goby species (Teleostei: Pisces) in the Rhône Delta (Mediterranean Sea, France). J Eukaryot Microbiol 46:304–310
Sato H (2011) Biology of the Myxozoa, a newly recognized parasitic pathogen causing food poisoning. Yamaguchi J Vet Med 38:1–26 (in Japanese with English summary)
Smythe AB, Sanderson MJ, Nadler SA (2006) Nematode small subunit phylogeny correlates with alignment parameters. Syst Biol 55:972–992
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Whipps CM, Diggles BK (2006) Kudoa alliaria in flesh of Argentinian hoki Macruronus magellanicus (Gadiformes; Merlucciidae). Dis Aquat Organ 69:259–263
Whipps CM, Kent ML (2006) Phylogeography of the cosmopolitan marine parasite Kudoa thyrsites (Myxozoa: Myxosporea). J Eukaryot Microbiol 53:364–373
Whipps CM, Adlard RD, Bryant MS, Kent ML (2003) Two unusual myxozoans, Kudoa quadricornis n. sp. (Multivalvulida) from the muscle of goldspotted trevally (Carangoides fulvoguttatus) and Kudoa permulticapsula n. sp. (Myltivalvulida) from the muscle of Spanish mackerel (Scomberomorus commerson) from the Great Barrier Reef, Australia. J Parasitol 89:168–173
Whipps CM, Grossel G, Adlard RD, Yokoyama H, Bryant MS, Munday BL, Kent ML (2004) Phylogeny of the Multivalvulidae (Myxozoa: Myxosporea) based on comparative ribosomal DNA sequence analysis. J Parasitol 90:618–622
Yokoyama H (2003) A review: gaps in our knowledge on myxozoan parasites of fishes. Fish Pathol 38:125–136
Yokoyama H, Whipps CM, Kent ML, Mizuno K, Kawakami H (2004) Kudoa thyrsites from Japanese flounder and Kudoa lateolabracis n. sp. from Chinese sea bass: causative myxozoans of post-mortem myoliquefaction. Fish Pathol 39:79–85
Zhou LS, Li-Chan ECY (2009) Effect of Kudoa spores, endogenous protease activity and frozen storage on cooked texture of minced Pacific hake (Merluccius productus). Food Chem 11:1076–1082
Acknowledgments
This study was supported in part by a Grant-in-Aid (H23-shokuhin-ippan-007) from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, YC., Sato, H., Tanaka, S. et al. Characterization of the ribosomal RNA gene of Kudoa neothunni (Myxosporea: Multivalvulida) in tunas (Thunnus spp.) and Kudoa scomberi n. sp. in a chub mackerel (Scomber japonicus). Parasitol Res 112, 1991–2003 (2013). https://doi.org/10.1007/s00436-013-3357-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3357-8