Abstract
Molecular genetic characterization using the ribosomal RNA (rDNA) gene accrues a wealth of knowledge regarding the true nature of species diversity of Kudoa Meglitsch, 1947 (Myxozoa: Myxosporea: Multivalvulida) and the biogeographical relationships of isolates from different host fish and sea areas. In the present study, we characterized morphologically and genetically three Kudoa spp. with four shell valves and polar capsules (SV/PC), forming pseudocysts in the myofiber of trunk muscles of Cheilodactylus zonatus or Acanthogobius hasta in the natural seawater around Japan. Myxospores from C. zonatus fished in the western Pacific Ocean off Kochi, Japan, were unequal quadrangular pyramids with one large and three smaller SV/PC, morphologically closest to Kudoa whippsi recorded in various pomacentrid and apogonid fish from the Australian Coral Sea. The 18S and 28S rDNA nucleotide sequences of the Japanese isolate were highly similar to some Australian K. whippsi isolates, but also displayed less similarity to other K. whippsi isolates from the same sea mainly due to instability of nucleotides at certain base positions and/or segments of different isolates. All the K. whippsi isolates including the present Japanese isolate, however, were distinct from Kudoa gunterae, K. whippsi’s closest kudoid species in morphology, molecular phylogeny, and biogeography. Our detection of K. whippsi from C. zonatus in the natural seawater around Japan is a new host and geographical record. Kudoid myxospores from A. hasta from the Sea of Ariake, a deep bay of the western part of Japan, exhibited two morphotypes, one resembling K. whippsi and the other Kudoa quadricornis with distinct posteriolateral SV projections. However, rDNA nucleotide sequencing revealed that these two Kudoa spp. were distinct from any known congeners; thus, Kudoa akihitoi n. sp. and Kudoa empressmichikoae n. sp. were erected. The morphological differentiation of K. akihitoi n. sp. from multiple Kudoa spp. with scalene stellate myxospores containing one large and three smaller SV/PC was difficult, whereas K. empressmichikoae n. sp. with spherical spore bodies extending small posteriolateral SV projections was distinct from known congeners with similar but elongated spore bodies and PC, i.e., K. quadricornis and Kudoa paraquadricornis, found in the trunk muscle of carangid fish from the Australian Coral Sea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kudoa Meglitsch, 1947 (Myxozoa: Myxosporea: Multivalvulida) is currently defined as myxosporeans with four or more shell valves and polar capsules (SV/PC) in equal numbers (Whipps et al. 2003a, 2004; Lom and Dyková 2006). Records of Kudoa spp., including multiple new species, have accrued at an accelerating pace in the last two decades, with currently more than 100 species (Moran et al. 1999; Lom and Dyková 2006; Sato 2011; Eiras et al. 2014). Myxospores of Kudoa spp. are relatively simple in morphology, being stellate, quadrate, or subspherical to ovoid in apical view with ellipsoidal or pyriform PC (Whipps et al. 2003a, 2004; Lom and Dyková 2006). Furthermore, surface ornamentations of SV are uncommon, and specialized techniques such as scanning electron microscopy are required to observe fine structures including apical projections of myxospores. Although information pertaining to geographical distribution, host specificity, and tissue tropism may aid species differentiation, it is still difficult to confidently identify a species based solely on myxospore morphology. Moreover, at present, the life cycle of Kudoa spp., i.e., alternate annelid hosts and actinospore stages, is poorly understood (Yokoyama et al. 2012; Eszterbauer et al. 2015). The recent application of 18S and 28S ribosomal RNA (rDNA) gene nucleotide sequencing has, however, dramatically improved the species identification of Kudoa spp. and other myxozoans. It has demonstrated the phenotypic plasticity of myxospores, low host specificity, and appreciable genetic variation of certain species, as well as higher phylogenetic relationships of species showing the same tissue tropism rather than morphological similarity of myxospores (Burger et al. 2007, 2008; Burger and Adlard 2010b, 2011; Abdel-Ghaffar et al. 2016).
Kudoa thyrsites (Gilchrist, 1924) causes post-mortem myoliquefaction and is distributed worldwide. It has been recorded from a variety of fish species and is responsible for precipitating great economic losses to global fisheries (Kent et al. 1994; Moran et al. 1999; Yokoyama et al. 2004; Whipps and Kent 2006; Kasai et al. 2016b). Myxospores of K. thyrsites are unequal quadrangular pyramids containing one large and three smaller SV/PC. In the last decade, with the benefit of molecular techniques, multiple Kudoa spp. with almost identical myxospore morphology to K. thyrsites have been differentiated and named. These include Kudoa minithyrsites Whipps et al., 2003; Kudoa lateolabracis Yokoyama et al., 2004; Kudoa megacapsula Yokoyama & Itoh, 2005; Kudoa whippsi Burger & Adlard, 2010; Kudoa gunterae Burger & Adlard, 2010; Kudoa cheilodipteri Heiniger et al., 2013; and Kudoa parathyrsites Kasai et al., 2016 (Whipps et al. 2003b; Yokoyama et al. 2004; Yokoyama and Itoh 2005; Burger and Adlard 2010a; Heiniger et al. 2013; Kasai et al. 2016b). Furthermore, K. thyrsites itself has been suggested to be a species complex with four major regional strains (Whipps and Kent 2006; Burger and Adlard 2010a, 2011).
During our recent survey of myxosporean infection in 354 individuals of 53 edible marine fish species in the natural seawater around Japan, two K. whippsi-like and one Kudoa quadricornis-like Kudoa spp., in addition to Kudoa trachuri Matsukane et al., 2011, in the white trevally Pseudocaranx dentex, were found in the myofiber of trunk muscles of Cheilodactylus zonatus from the western Pacific Ocean off Kochi, Japan, and Acanthogobius hasta from the Sea of Ariake, Japan. Since the two aforementioned Kudoa spp., K. whippsi and K. quadricornis Whipps et al., 2003, were originally recorded in Australian coral fish of Carangidae, Pomacentridae, and Apogonidae (Whipps et al. 2003a; Burger and Adlard 2010a; Heiniger et al. 2013), their phylogenetic relationships with the new Kudoa spp. collected from Japan’s seawater are of great benefit to the understanding of species diversity and/or phylogenetic relationships between isolates in distant sea areas.
Materials and methods
Fish samples and parasitological examination
Whole bodies of 354 individuals of 53 fish species, classified in 44 genera of 34 families, were purchased from local fish markets in Japan during the period June 2014 to February 2016 (Table 1). These fish were caught near the local fish markets in the Sea of Japan, East China Sea, Sea of Ariake, Inland Sea of Japan, and western Pacific Ocean. Following transportation of the samples on ice, fish were cut open and their gills and viscera removed and examined under a dissection microscope. Filleted fish meats were examined on the day of arrival or frozen until examination. Thin slices of muscle fillets were pressed between two glass plates and examined under a dissection microscope to detect the presence of myxosporean cysts or pseudocysts.
When myxosporean plasmodia were detected, muscle slices were placed in physiological saline and parasitized myofibers were carefully isolated with fine forceps. The release of myxospores from a pseudocyst in the myofiber or a cyst between myofibers was executed with fine forceps. Myxospores were observed using a microscope equipped with differential interference contrast imaging, photographed at a magnification of ×800, and then transformed into photographs with Adobe® Photoshop® ver. 11.0 (Adobe Systems, San Jose, California, USA). Photographs were then printed at a high magnification. Measurements were conducted on multiple printed photographs following the guidelines of Lom and Arthur (1989). All measurements are expressed in micrometer unless otherwise stated. Ranges with the means in parentheses are presented.
Following removal of a portion of the myxospores for DNA extraction, the parasite was fixed in 10% neutral-buffered formalin solution and 70% ethanol solution. Specimens collected in the present work were deposited in the Meguro Parasitological Museum, Tokyo, Japan, under collection nos. 21255–21257.
DNA extraction, PCR, and sequencing
Parasite DNA was extracted from a kudoid plasmodium using an Illustra™ tissue and cell genomicPrep Mini Spin Kit (GE Healthcare UK, Buckinghamshire, UK) according to the instructions of the manufacturer. Polymerase chain reaction (PCR) amplification of overlapping fragments of the rDNA was performed in a 20-μl volume containing a DNA polymerase, Blend Taq-Plus- (TOYOBO, Dojima Hama, Osaka, Japan), and primers as described previously (Li et al. 2013; Kasai et al. 2015). The PCR products were purified using a FastGene Gel/PCR Extraction Kit (NIPPON Genetics Co., Tokyo, Japan) and sequenced directly. When direct sequencing was not satisfactory, the purified PCR products were cloned into the plasmid vector pTA2 (TArget Clone™; TOYOBO) and transformed into Escherichia coli JM109 (TOYOBO) according to the instructions of the manufacturer. Following propagation, the plasmid DNA was extracted using a FastGene Plasmid Mini Kit (NIPPON Genetics Co.) and inserts from multiple independent clones, at least three, were sequenced using universal M13 forward and reverse primers. The nucleotide sequences obtained in the present study are available from the DDBJ/EMBL/GenBank databases under the accession nos. LC190919–LC190927.
Phylogenetic analysis
For phylogenetic analysis, the newly obtained rDNA nucleotide sequences of Kudoa spp. in the present study and related Kudoa sequences retrieved from the DDBJ/EMBL/GenBank databases were aligned using the CLUSTAL W multiple alignment program (Thompson et al. 1994), with subsequent manual adjustment. The accession numbers of the sequences analyzed in the present study are given in the figures showing phylogenetic trees. Regions judged to be poorly aligned and characters with a gap in any sequence were excluded from subsequent analyses; 1399 characters, of which 238 were variable, and 460 characters, of which 152 were variable, remained for subsequent analysis for the 18S and 28S rDNAs, respectively. Maximum likelihood (ML) analysis was performed with the program PhyML as described previously (Matsukane et al. 2010; Li et al. 2013). Kudoa carcharhini and Kudoa hemiscylli, two Kudoa spp. of elasmobranchs in the natural seawater around Australia, were used as an outgroup for the construction of ML phylogenetic trees. They were used for this purpose because they are positioned near the root of a great majority of Kudoa spp. in phylogenetic trees based on the rDNA (Gleeson et al. 2010).
Results
Incidence of kudoid infection
A survey of 354 samples of edible marine fish (53 species of 44 genera in 34 families) in the natural seawater around Japan (Table 1) revealed myxosporean infection in trunk muscles of Pseudocaranx dentex fished in the East China Sea off Kagoshima, Japan (body standard length, 23–26 cm; and body weight, 315–403 g), C. zonatus fished in the western Pacific Ocean off Kochi, Japan (body standard length, 30–33 cm; body weight, 609–733 g), and A. hasta in the Sea of Ariake, Japan (body standard length, 26–31 cm; body weight, 160–203 g). Plasmodia of K. trachuri from two of six P. dentex individuals examined were found in fibrous cysts between the myofibers, and 35 and nine cysts were dispersed in the trunk muscles of each of the infected fish individuals. Kudoid cysts in the former individual contained 15 intact, well-grown plasmodia and 20 degenerated ones. The morphology and 18S rDNA nucleotide sequence of myxospores isolated from intact cysts coincided well with K. trachuri from Japanese jack mackerel Trachurus japonicus (Matsukane et al. 2011; Kasai et al. 2015). Therefore, this kudoid was not further investigated in the present study.
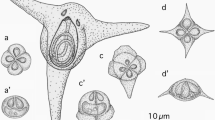
K. whippsi-like species from C. zonatus had unequal quadrangular pyramid-shaped myxospores (Fig. 1), forming ten pseudocysts in the myofibers. The infection was found in one of six fish individuals examined. In six A. hasta individuals, 51 to 106 (average 67) pseudocysts occurred in the myofibers, and two morphotypes of Kudoa myxospores were found: K. whippsi-like spores (Fig. 2) in two individuals and a K. quadricornis-like species with spherical spore bodies bearing small posteriolateral SV projections (Fig. 3) in all six fish individuals examined. Pseudocysts of the two Kudoa spp. from A. hasta could not be differentiated by their shape or dimensions under a dissection microscope. Stylized diagrams of each kudoid species are shown in Fig. 4. No inflammation was noted around the myofibers containing myxosporean plasmodia. Phylogenetic trees based on the 18S rDNA and 28S rDNA nucleotide sequences indicated that the Kudoa isolate from C. zonatus had a close affinity with certain isolates of K. whippsi recorded in the Australian Coral Sea, whereas the two Kudoa spp. from A. hasta showed no notable genetic affinities with known Kudoa spp. (Figs. 5 and 6). Consequently, for the two latter Kudoa spp. from A. hasta, new myxosporean species, Kudoa akihitoi n. sp. and Kudoa empressmichikoae n. sp., are erected in the present study.
ML phylogenetic tree based on the 18S rDNA sequence. The species name of the isolates collected in the present study (with gray background) is followed by the name of the isolate, fish host, country of collection, and DDBJ/EMBL/GenBank accession number. The name of the other species is followed by the country of collection and DDBJ/EMBL/GenBank accession number. Abbreviations of country names: AU Australia, CA Canada, CN People’s Republic of China, ES Spain, IL Israel, IS Iceland, JP Japan, KR Korea, PH Philippines, UA Ukraine, US USA, ZA South Africa
ML phylogenetic tree based on the 28S rDNA sequence (see Fig. 5 legend for details)
K. whippsi (Myxosporea: Multivalvulida) from C. zonatus (Figs. 1 and 4a, a’; Table 2)
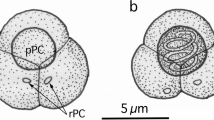
Elongated plasmodia with tapering ends, 1.13–3.91 mm (2.15) by 0.19–0.30 mm (0.24) (n = 10), forming pseudocysts, were found in the myofiber of trunk muscles. Plasmodia were polysporic with synchronized spore development. Myxospores from pseudocysts were scalene stellate with four unequal SV/PC in apical view. When the average length of the largest PC was assumed to be 10, the ratio of the four PC was 10:6.3–7.9 (7.0):6.1–7.6 (6.7):5.7–7.0 (6.2) (n = 20). General spore morphology was closest to K. whippsi. PC were drop-like, occupying most parts of spores. In lateral view, spores were scalene pyramidal, extending sharp SV corners posteriolaterally. Coils of polar filament were not seen in wet preparations. Measurements of myxospores are shown in Table 2. Although myxospore morphometrics of the present specimens were similar to most or some of the values of the eight species listed in Table 2, the closest ones were K. whippsi and K. cheilodipteri recorded from various Australian coral fish of Carangidae and Pomacentridae (Burger and Adlard 2010a; Heiniger et al. 2013).
Four nucleotide sequences of the serial 18S to 28S rDNA, 5417–5431 bp in length, were obtained by extensive DNA clonings from a single plasmodium. These sequences contained 1727-bp-long partial 18S rDNA, 518-bp-long internal transcribed spacer 1 (ITS1), 158-bp-long 5.8S rDNA, 434-bp-long internal transcribed spacer 2 (ITS2), and 2580–2594-bp-long partial 28S rDNA (DDBJ/EMBL/GenBank accession nos. LC190919–LC190922), showing intraindividual nucleotide changes, i.e., nucleotide substitutions and indels (insertion/deletion), at certain base positions and segments in the 28S rDNA nucleotide sequences. The remaining parts (18S rDNA, ITS1, 5.8S rDNA, and ITS2) of the four sequences, 2837 bp in length, were absolutely identical. As shown in Figs. 5 and 6, the Japanese K. whippsi isolate from C. zonatus formed a clade with Australian K. whippsi isolates and K. gunterae isolates from various pomacentrid and apogonid fish in both phylogenetic trees based on either the 18S rDNA or 28S rDNA, and was distant from K. cheilodipteri and other kudoids. When their 18S and 28S rDNA nucleotide sequences were aligned and precisely compared, K. gunterae isolates were clearly different from all the K. whippsi isolates at several base positions and certain segments of the sequences (data not shown). The Australian and Japanese isolates of K. whippsi showed nucleotide changes at certain base positions and segments (Tables 3 and 4), and combinations of these nucleotide changes were variable by isolate. Certain Australian K. whippsi isolates (e.g., NR isolate, followed by LI1 or KwAb2 isolates) showed partial but higher commonality of the rDNA nucleotide sequences with the Japanese isolate rather than the other Australian K. whippsi isolates. The present isolation of K. whippsi from spottedtail morwong C. zonatus fished in the western Pacific Ocean off Kochi, Japan, is a new host and new geographical record.
Description
K. akihitoi n. sp. (Myxosporea: Multivalvulida) (Figs. 2 and 4b, b’; Table 2)
Elongated plasmodia with tapering ends, 0.79–5.40 (1.98) mm by 0.12–0.44 (0.26) mm (n = 16), forming pseudocysts, in the myofiber of trunk muscles. Polysporic and synchronized spore development. Myxospores scalene stellate with four unequal SV/PC in apical view, without SV ornamentation. In lateral view, myxospores scalene pyramidal, PC drop-like, occupying most parts of myxospores. When the average length of the largest PC was assumed to be 10, the ratio of the four PC was 10 : 7.2–9.3 (8.4) : 6.2–9.2 (7.8) : 5.9–8.2 (7.0) (n = 20). Coils of polar filament not seen in wet preparations. The spores having dimensions of: width 9.1–12.5 (10.6); thickness 6.8–9.4 (8.1); sutural thickness 5.3–6.7 (6.1); length 5.3–7.0 (6.4); largest PC 4.2–5.1 (4.8) by 2.2–2.8 (2.5); and three smaller PC 2.8–4.6 (3.7) by 1.5–2.3 (1.9).
Two serial nucleotide sequences of the 18S to 28S rDNA, 5950 and 5959 bp in length, were obtained from a single plasmodium. These sequences contained 1717-bp-long partial 18S rDNA, 594- and 609-bp-long ITS1, 158-bp-long 5.8S rDNA, 576- and 570-bp-long ITS2, and 2905-bp-long partial 28S rDNA (DDBJ/EMBL/GenBank accession nos. LC190923 and LC190924). The two nucleotide sequences were absolutely identical regarding their 18S, 5.8S, and 28S rDNAs, whereas their ITS1 and ITS2 regions showed 93.8 and 94.9% identities, respectively, partly ascribed to different numbers of repeats of a few nucleotide units such as “TG,” “GT,” or “TGAAA.”
Taxonomic summary
Host: Acanthogobius hasta (Temminck & Schlegel, 1845) (Actinopterygii: Perciformes: Gobiidae).
Locality: The Sea of Ariake, a deep bay surrounded by Fukuoka, Saga, Nagasaki, and Kumamoto Prefectures on Kyushu Island, Japan.
Site of infection: Pseudocysts in somatic muscles.
Materials deposited: Hapantotype no. 21256, Meguro Parasitological Museum, Tokyo, Japan.
Prevalence: Two of six fish individuals were collected in the same sea area. These two fish individuals were coinfected with another species described in the following, K. empressmichikoae n. sp., with 74 and 106 pseudocysts detected in them, whereas the four other fish individuals with only K. empressmichikoae n. sp. infection were loaded with 51 to 70 (average 57) pseudocysts. Due to the similarity of plasmodia in morphology and dimensions, the exact numbers of K. akihitoi n. sp. plasmodia in infected A. hasta individuals were unable to be determined.
Etymology: The species is named in honor of Akihito, the reigning Emperor of Japan, who has a great interest in science and ichthyological research, particularly the taxonomy of the family Gobiidae, and has previously published in the field (Akihito 1992; Akihito et al. 2000).
Remarks
As shown in Table 2, there are multiple K. whippsi-like kudoids with unequal quadrangular pyramid-shaped myxospores containing one large and three smaller SV/PC. It is rather difficult to differentiate one from the other based solely on myxospore morphology. Furthermore, K. whippsi, K. gunterae, and K. cheilodipteri are found in the same fish hosts in the same coastal sea around Australia (Burger and Adlard 2010a; Heiniger et al. 2013). From several standpoints, the morphometrics of the present new species were closest to those of K. whippsi and K. cheilodipteri. However, molecular genetic analyses using the currently available 18S and 28S rDNA nucleotide sequences clearly separated K. akihitoi n. sp. from known Kudoa spp. with unequal quadrangular pyramid-shaped myxospores (listed in Table 2), albeit they demonstrated intraspecific nucleotide variations to some extent. The present new species showed 96.6–98.2% nucleotide identities of the 18S rDNA and 91.4–95.6% nucleotide identities of the 28S rDNA with the aforementioned Kudoa spp., which were satisfactory differences for species differentiation. The low affinities of K. akihitoi n. sp. with K. whippsi and other related species were reflected in isolation of the present new species in both ML phylogenetic trees based on the 18S and 28S rDNAs (Figs. 5 and 6).
K. empressmichikoae n. sp. (Myxosporea: Multivalvulida) (Figs. 3 and 4c, c’; Table 5)
Elongated plasmodia with tapering ends, 0.79–5.40 (1.98) mm by 0.12–0.44 (0.26) mm (n = 16), forming pseudocysts, in the myofiber of trunk muscles. Polysporic and synchronized spore development. Myxospores spheroidal with four almost equal SV/PC and small posteriolateral SV projections. Apical digitate projections evident, without other SV ornamentation; PC drop-like, slightly variable in size. Coils of polar filament not seen in wet preparations. The spores having dimensions of: width 9.2–11.8 (10.5); thickness 6.2–9.1 (7.5); surural thickness 4.8–7.0 (5.7); length 5.1–6.1 (5.6); PC length 1.7–2.7 (2.1); PC width 1.2–1.8 (1.5); posteriolateral SV projection length 2.2–3.4 (2.9); and posteriolateral SV projection width 1.8–2.9 (2.2).
Three serial nucleotide sequences of the 18S to 28S rDNA, 6002–6016 bp in length, were obtained from three different plasmodia isolated from fish individual no. 1, no. 3, and no. 4. These sequences contained 1718-bp-long partial 18S rDNA, 651–665-bp-long ITS1, 158-bp-long 5.8S rDNA, 566-bp-long ITS2, and 2909-bp-long partial 28S rDNA (DDBJ/EMBL/GenBank accession nos. LC190925–LC190927). The 18S, 5.8S, ITS2, and 28S rDNA nucleotide sequences of these three isolates were highly similar (99.4–100% identities), whereas those of the ITS1 region showed 83.3–94.2% identities, partly ascribed to different numbers of repeats of a few nucleotide units such as “TTG,” GT, or “AGTG.”
Taxonomic summary
Host: Acanthogobius hasta (Temminck & Schlegel, 1845) (Actinopterygii: Perciformes: Gobiidae).
Locality: The Sea of Ariake, a deep bay surrounded by Fukuoka, Saga, Nagasaki, and Kumamoto Prefectures on Kyushu Island, Japan.
Site of infection: Pseudocysts in somatic muscles.
Materials deposited: Hapantotype no. 21257, Meguro Parasitological Museum, Tokyo, Japan.
Prevalence: All six fish individuals were examined. As stated earlier, all six A. hasta individuals examined had 51–106 (average 67) pseudocysts, and two of them were coinfected with undetermined numbers of K. akihitoi n. sp. plasmodia. The four fish individuals solely infected with K. empressmichikoae n. sp. had 51–70 (average 57) pseudocysts in their trunk muscles.
Etymology: The species is named in honor of Empress Michiko, the wife of Japan’s Emperor Akihito, who unfailingly supports his role of monarch and its associated duties.
Remarks
The present new species, K. empressmichikoae n. sp., was highly prevalent in A. hasta in the Sea of Ariake and sometimes coinfected the trunk muscles with K. akihitoi n. sp. This species is the third Kudoa sp. to have unique myxospore morphology with distinct posteriolateral SV projections and trunk muscle tropism, similar to K. quadricornis and Kudoa paraquadricornis recorded in carangid fish (Perciformes) such as Carangoides fulvoguttatus, Carangoides plagiotaenia, and Caranx ignobilis from the Australian Coral Sea (Whipps et al. 2003a; Burger and Adlard 2010a). Both of these known species have a pyriform spore body and elongated club-like PC, whereas the present new species has a spherical spore body and drop-like PC. Therefore, morphological differentiation of K. empressmichikoae n. sp. from the two other Kudoa spp. is feasible. Phylogenetically, the present new species showed the highest affinity with K. parathyrsites (99.4% identity over 1560-bp-long 18S rDNA; and 95.7% over 553-bp-long 28S rDNA), followed by some other K. thyrsites-like species such as K. megacapsula (98.7% over 1565-bp-long 18S rDNA) and K. minithyrsites (98.2% over 1684-bp-long 18S rDNA), indicating its uniqueness as a lineage of kudoids. K. parathyrsites, phylogenetically closest to the present new species, was recorded from the myofiber of trunk muscles of a black scraper (Thamnaconus modestus) in the Inland Sea of Japan (Seto-naikai) and had K. whippsi- or K. cheilodipteri-like myxospores (Kasai et al. 2016b).
Discussion
Nucleotide sequencing of the 18S and 28S rDNAs has provided us with great insight into the phylogenetic positions of Kudoa isolates from diverse fish resources. This molecular technology has affirmed the conspecificity of different morphotypes of myxospores, e.g., Kudoa yasunagai with five or seven SV/PC, Kudoa chaetodoni with eight or nine SV/PC, Kudoa thalassomi with six or seven SV/PC, and Kudoa septempunctata with six or seven SV/PC (Burger et al. 2007; Burger and Adlard 2010b, 2011; Kasai et al. 2016b). However, until recently, the number of SV/PC was believed to be the clearest morphological marker to separate the species or genera of Multivalvulida, with Kudoa, Pentacapsula Naidenova & Zaika, 1970, Hexacapsula Arai & Matsumoto, 1953, and Septemcapsula Hsieh & Chen, 1984, being settled in the classical systematics of Multivalvulida (Whipps et al. 2003a, 2004; Lom and Dyková 2006). Furthermore, the morphometrical variation of the small-sized myxospores of Kudoa spp. presents another problem requiring clarification for species differentiation (Matsukane et al. 2011). Fortunately, rDNA nucleotide sequencing has dramatically improved our recognition of the species and systematics of Kudoa. Therefore, incorporation of genetic analysis in addition to morphological characterization of kudoid myxospores is critically important to facilitate their unambiguous specific identification, as is the case with other myxosporeans (Burger and Adlard 2011; Morsy et al. 2012; Abdel-Ghaffar et al. 2012, 2016).
Nevertheless, appreciable variations in the nucleotide sequences of the rDNA, a gene believed to be relatively consistent and not rapidly evolving in a species, have been reported for some Kudoa spp. such as K. thyrsites, K. thalassomi, K. whippsi, K. gunterae, Kudoa iwatai, Kudoa amamiensis, and K. hemiscylli (Diamant et al. 2005; Whipps and Kent 2006; Burger et al. 2008; Burger and Adlard 2010a, b, 2011; Gleeson et al. 2010; Matsukane et al. 2011; Heiniger et al. 2013). The 28S rDNA nucleotide sequencing of K. whippsi from C. zonatus in Japan required DNA cloning instead of direct sequencing due to intraindividual nucleotide changes, particularly indels, in several rDNA regions, i.e., the areas between 665 and 679 base positions (5 to 15 nucleotides by 4 clones), 955 and 965 base positions (11 to 15 nucleotides), 1284 (1 to 7 nucleotides), and 1881 and 1891 (11 to 19 nucleotides) of Japanese K. whippsi TKN isolate clone A (DDBJ/EMBL/GenBank accession no. LC190919). Similarly, interindividual nucleotide variations of multiple Australian isolates of K. whippsi are evident in Tables 3 and 4, which do not reflect the fish host or geographical distribution (Heiniger et al. 2013). Similar individual and segmental nucleotide variations of the 28S rDNA nucleotide sequence have also been observed for K. thyrsites isolates of different origins (Whipps and Kent 2006), partially reflected in the phylogenetic tree presented in Fig. 6. If, in the future, the rDNAs of multiple isolates are sequenced instead of only one isolate/a few isolates as is currently the case, similar genetic complexity of a species could be observed for more Kudoa spp. As indicated by Whipps and Kent (2006), parasite gene flow in a region appears to be critical to determine the level of genetic variation of a given Kudoa species. In other words, noticeable genetic variation could be seen when parasite gene flow is not satisfactory due to a limited time since speciation–dispersal–colonization or natural obstacles for fluent gene flow between individuals of a species. This should be taken into consideration henceforth when conducting the specific identification of Kudoa spp. using molecular genetic characterization.
The speciation of Kudoa spp. with unequal quadrangular pyramidal myxospores has progressed well, with multiple species being described: K. thyrsites, K. minithyrsites, K. whippsi, K. gunterae, K. lateolabracis, K. megacapsula, K. cheilodipteri, and K. parathyrsites. The present study adds one more species, K. akihitoi n. sp. from A. hasta in the Sea of Ariake, Japan. Therefore, just around Japan, at least six species of this morphotype group, i.e., K. thyrsites, K. lateolabracis, K. megacapsula, K. parathyrsites, K. whippsi, and K. akihitoi n. sp., are currently found (Yokoyama et al. 2004; Yokoyama and Itoh 2005; Kasai et al. 2016b; the present study). As seen in Figs. 5 and 6, almost all members of K. thyrsites and relatives with a similar myxospore morphology form a well-supported clade in the phylogenetic trees based on the rDNA. Intriguingly, K. empressmichikoae n. sp. with distinct morphological characters, i.e., spherical spore body with posteriolateral SV projections like K. quadricornis and K. paraquadricornis, is positioned in the same clade as K. thyrsites and its relatives. Burger et al. (2007) evaluated the 18S and 28S rDNA phylogenetic trees to reflect the tissue tropism of each kudoid species rather than its myxospore morphotype. Since A. hasta is a fish that lives in the sand and mud bottoms of deep bays in Japan (Sea of Ariake and Sea of Yatsushiro, both located on Kyushu Island), Korea, and Taiwan (Masuda et al. 1984; Shao et al. 1993), it would be of great interest to ascertain whether the two Kudoa spp. recorded here as new species are also distributed in Korea and Taiwan and to determine how much genetic diversity is displayed by these two species distributed in clearly isolated sea areas.
The present study and earlier studies (Adlard et al. 2005; Burger et al. 2008; Burger and Adlard 2010b; Shirakashi et al. 2014) have expanded the geographical distribution of some Kudoa spp. such as K. amamiensis, K. thalassomi, and K. whippsi, which are believed to have local distribution in the natural seawater around Japan or Australia. The detection of kudoid infection is often difficult, as demonstrated here in which only 9 out of 354 fish individuals examined had kudoids (2.5%; Table 1) and in other surveys (Egusa and Nakajima 1980; Burger et al. 2008; Matsukane et al. 2010; Kasai et al. 2016b). Rarely is the prevalence of kudoid infection satisfactorily high (Gleeson et al. 2010; Shirakashi et al. 2014; Kasai et al. 2016a), partly dependent on batch differences and not natural prevalence, because we examined only a limited number of fish. Full genetic characterization of kudoid species obtained by chance could provide us with more insight into the genetic variation of a known species on the distributional borders or genetic changes after speciation and geographical dispersal. At the same time, it is highly likely that more kudoid species remain to be discovered like K. akihitoi n. sp. and K. empressmichikoae n. sp. in local fish with limited distribution, since the majority of research on kudoid infection is conducted by experts in a limited number of countries that do not cover all areas of our planet.
References
Abdel-Ghaffar F, Morsy K, Mehlhorn H, Bashtar AR, Shazly MA, Saad AH, Abdel-Gaber R (2012) First report of Kudoa species (Myxozoa: Kudoidae) infecting the spotted coral grouper Plectropomus maculates from the Red Sea. A light and ultrastructural study Parasitol Res 111:1579–1585
Abdel-Ghaffar F, Abdel-Gaber R, Maher S, Al Quraishy S, Mehlhorn H (2016) Morphological re-description and molecular characterization of Kudoa pagrusi (Myxosporea: Multivalvulida) infecting the heart muscles of the common sea bream fish Pagrus pagrus (Perciformes: Sparidae) from the Red Sea. Egypt. Parasitol Res. 115:3175–3184
Adlard RD, Bryant MS, Whipps CM, Kent ML (2005) Multivalvulid myxozoans from eastern Australia: three new species of Kudoa from scombrid and labrid fishes of the Great Barrier Reef, Queensland, Australia. J Parasitol 91:1138–1142
Akihito (1992) Early cultivators of science in Japan. Science 258:578–580
Akihito SK, Ikeda Y, Iwata A (2000) Gobioidei. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species, second edn. Tokai University Press, Tokyo, pp. 1139–1330 1606–1628 (in Japanese)
Burger MAA, Adlard RD (2010a) Four new species of Kudoa Meglitsch, 1947 (Myxosporea: Multivalvulida) from Australia with recommendations for species descriptions in the Kudoidae. Parasitology 137:793–814
Burger MAA, Adlard RD (2010b) Phenotypic variation in a significant spore character in Kudoa (Myxosporea: Multivalvulida) species infecting brain tissue. Parasitology 137:1759–1772
Burger MAA, Adlard RD (2011) Low host specificity in the Kudoidae (Myxosporea: Multivalvulida) including seventeen new host records for Kudoa thalassomi. Folia Parasitol 58:1–16
Burger MAA, Cribb TH, Adlard RD (2007) Patterns of relatedness in the Kudoidae with descriptions of Kudoa chaetodoni n. sp. and K. lethrini n. sp. (Myxosporea: Multivalvulida). Parasitology 134:669–681
Burger MA, Brames AC, Adlard RD (2008) Wildlife as reservoirs for parasites infecting commercial species: host specificity and redescription of Kudoa amamiensis from teleost fish in Australia. J Fish Dis 31:835–844
Diamant A, Ucko M, Paperna I, Colorni A, Lipshitz A (2005) Kudoa iwatai (Myxosporea: Multivalvulida) in wild and cultured fish in the red sea: redescription and molecular phylogeny. J Parasitol 91:1175–1189
Egusa S, Nakajima K (1980) Kudoa amamiensis n. sp. (Myxosporea: Multivalvulida) found in cultured yellowtails and wild damselfishes from Amami-Ohshima and Okinawa, Japan. Bull Jpn Soc Sci Fish 46:1193–1198
Eiras JC, Saravia A, Cruz C (2014) Synopsis of the species of Kudoa Meglitsch, 1947 (Myxozoa: Myxosporea: Multivalvulida). Syst Parasitol 87:153–180
Eszterbauer E, Atkinson S, Diamant A, Morris D, El-Matbouli M, Hartikainen H (2015) Myxozoan life cycles: practical approaches and insights. In: Okamura B, Gruhl A, Bartholomew JL (eds) Myxozoan evolution, ecology and development. Springer International Publishing, Switzerland, pp. 175–198
Gleeson RJ, Bennett MB, Adlard RD (2010) First taxonomic description of multivalvulidan myxosporean parasites from elasmobranchs: Kudoa hemiscylli n. sp. and Kudoa carcharhini n. sp. (Myxosporea: Multivalvulidae). Parasitology 137:1885–1898
Heiniger H, Cribb TH, Adlard RD (2013) Intra-specific variation of Kudoa spp. (Myxosporea: Multivalvulida) from apogonid fishes (Perciformes), including the description of two new species, K. cheilodipteri n. sp. and K. cookii n. sp., from Australian waters. Syst Parasitol 84:193–215
Kasai A, Li Y-C, Setsuda A, Mafie E, Sato H (2015) Genetic characterization of Kudoa iwatai and Kudoa trachuri in commercial marine fish (Platycephalus sp. and Trachurus japonicus) for human consumption. Jpn J Vet Parasitol 14:22–30
Kasai A, Li Y-C, Setsuda A, Mafie E, Sato H (2016a) Morphological and molecular genetic characterization of two Kudoa spp., K. musculoliquefaciens and K. pleurogrammi n. sp. (Myxosporea: Multivalvulida), causing myoliquefaction of commercial marine fish. Parasitol Res 115:1883–1892
Kasai A, Li Y-C, Mafie E, Sato H (2016b) New host records of monacanthid fish for three Kudoa spp. (K. septempunctata, K. thyrsites, and K. shiomitsui) prevalent in the olive flounder (Paralichthys olivaceus), with the description of K. parathyrsites n. sp. from a black scraper (Thamnaconus modestus). Parasitol Res 115:2741–2755
Kent ML, Margolis L, Whitaker DJ, Hoskins GE, McDonald TE (1994) Review of Myxosporea of importance in salmonid fisheries and aquaculture in British Columbia. Folia Parasitol 41:27–37
Li Y-C, Sato H, Tanaka S, Ohnishi T, Kamata Y, Sugita-Konishi Y (2013) Characterization of the ribosomal RNA gene of Kudoa neothunni (Myxosporea: Multivalvulida) in tunas (Thunnus spp.) and Kudoa scomberi n. sp. in a chub mackerel (Scomber japonicus). Parasitol Res 112:1991–2003
Lom J, Arthur JR (1989) A guideline for the preparation of species descriptions in Myxosporea. J Fish Dis 12:151–156
Lom J, Dyková I (2006) Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol 53:1–36
Masuda H, Amaoka K, Araga C, Uyeno T, Yoshino T (1984) The fishes of the Japanese archipelago, vol 1. Tokai University Press, Tokyo
Matsukane Y, Sato H, Tanaka S, Kamata Y, Sugita-Konishi Y (2010) Kudoa septempunctata n. sp. (Myxosporea: Multivalvulida) from an aquacultured olive flounder (Paralichthys olivaceus) imported from Korea. Parasitol Res 107:865–872
Matsukane Y, Sato H, Tanaka S, Kamata Y, Sugita-Konishi Y (2011) Kudoa iwatai and two novel Kudoa spp., K. trachuri n. sp. and K. thunni n. sp. (Myxosporea: Multivalvulida), from daily consumed marine fish in Japan. Parasitol Res 108:913–926
Moran JDW, Whitaker DJ, Kent ML (1999) A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture 172:163–196
Morsy K, Abdel-Ghaffar F, Bashtar AR, Mehlhorn H, Al Quraishy S, Abdel-Gaber R (2012) Morphological and small subunit ribosomal DNA sequence of Henneguya suprabranchiae (Myxozoa), a parasite of the catfish Clarias gariepinus (Clariidae) from the River Nile. Egypt Parasitol Res 111:1423–1435
Sato H (2011) Biology of the Myxozoa, a newly recognized parasitic pathogen causing food poisoning. Yamaguchi J Vet Med 38:1–26 (in Japanese with English summary)
Shao K-T, Chen J-P, Kao P-H, Wu C-Y (1993) Fish fauna and their geographical distribution along the western coast o Taiwan. Acta Zool Taiwanica 4:113–140
Shirakashi S, Yamane K, Ishitani H, Yanagida T, Yokoyama H (2014) First report of Kudoa species in the somatic muscle of the Japanese parrotfish Calotomus japonicus (Scaridae) and a description of Kudoa igami n. sp. (Myxozoa: Multivalvulida). Parasitol Res 113:2515–2524
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Whipps CM, Kent ML (2006) Phylogeography of the cosmopolitan marine parasite Kudoa thyrsites (Myxozoa: Myxosporea). J Eukaryot Microbiol 53:364–373
Whipps CM, Adlard RD, Bryant MS, Kent ML (2003a) Two unusual myxozoans, Kudoa quadricornis n. sp. (Multivalvulida) from the muscle of goldspotted trevally (Carangoides fulvoguttatus) and Kudoa permulticapsula n. sp. (Myltivalvulida) from the muscle of Spanish mackerel (Scomberomorus commerson) from the Great Barrier Reef, Australia. J Parasitol 89:168–173
Whipps CM, Adlard RD, Bryant MS, Lester RJG, Findlay V, Kent ML (2003b) First report of three Kudoa species from eastern Australia: Kudoa thyrsites from mahi mahi (Coryphaena hippurus), Kudoa amamiensis and Kudoa minithyrsites n. sp. from sweeper (Pempheris ypsilychnus). J Eukaryot Microbiol 50:215–219
Whipps CM, Grossel G, Adlard RD, Yokoyama H, Bryant MS, Munday BL, Kent ML (2004) Phylogeny of the multivalvulidae (Myxozoa: Myxosporea) based on comparative ribosomal DNA sequence analysis. J Parasitol 90:618–622
Yokoyama H, Itoh N (2005) Two multivalvulid myxozoans causing postmortem myoliquefaction: Kudoa megacapsula n. sp. from red barracuda (Sphyraena pinguis) and Kudoa thyrsites from splendid alfonso (Beryx splendens). J Parasitol 91:1132–1137
Yokoyama H, Whipps CM, Kent M, Mizuno K, Kawakami H (2004) Kudoa thyrsites from Japanese flounder and Kudoa lateolabracis n. sp. from Chinese sea bass: causative myxozoans of post-mortem myoliquefaction. Fish Parasitol 39:79–85
Yokoyama H, Grabner D, Shirakashi S (2012) Transmission biology of the Myxozoa. In: Carvalho ED, David GS, Silva RJ (eds) Health and environment in Aquaculure. InTech Europe, Croatia, pp. 1–42
Acknowledgements
This study was supported in part by Grant-in-Aid for Scientific Research 2015 from The Towa Foundation for Food Science and Research (HS), Grant-in-Aid for International Collaboration Research in Asia 2016 from the Heiwa Nakajima Foundation (HS), and JSPS KAKENHI Grant Number 15K07722.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasai, A., Setsuda, A. & Sato, H. Morphological and genetic characterization of Kudoa whippsi (Myxosporea: Multivalvulida) from Cheilodactylus zonatus in the western Pacific Ocean off Japan, and two new Kudoa spp. (K. akihitoi n. sp. and K. empressmichikoae n. sp.) from Acanthogobius hasta in the Sea of Ariake, Japan. Parasitol Res 116, 647–659 (2017). https://doi.org/10.1007/s00436-016-5329-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5329-2