Abstract
Main conclusion
This review provides a comprehensive overview of the CRISPR/Cas9 technique and the research areas of this gene editing tool in improving wheat quality.
Abstract
Wheat (Triticum aestivum L.), the basic nutrition for most of the human population, contributes 20% of the daily energy needed because of its, carbohydrate, essential amino acids, minerals, protein, and vitamin content. Wheat varieties that produce high yields and have enhanced nutritional quality will be required to fulfill future demands. Hexaploid wheat has A, B, and D genomes and includes three like but not identical copies of genes that influence important yield and quality. CRISPR/Cas9, which allows multiplex genome editing provides major opportunities in genome editing studies of plants, especially complicated genomes such as wheat. In this overview, we discuss the CRISPR/Cas9 technique, which is credited with bringing about a paradigm shift in genome editing studies. We also provide a summary of recent research utilizing CRISPR/Cas9 to investigate yield, quality, resistance to biotic/abiotic stress, and hybrid seed production. In addition, we provide a synopsis of the laboratory experience-based solution alternatives as well as the potential obstacles for wheat CRISPR studies. Although wheat’s extensive genome and complicated polyploid structure previously slowed wheat genetic engineering and breeding progress, effective CRISPR/Cas9 systems are now successfully used to boost wheat development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that by 2050, there will be 9.6 billion people in the world (Turhan et al. 2021). To keep the food demand of the booming population, the wheat yield must increase by 50% by 2034 (Zhang et al. 2019). According to a paper published by the World Health Organization (WHO) in 2020, almost 690 million people, 8.9% of the human population, do not have sufficient food to eat. Sustainable food and nutrition security demand sustainable and quality wheat production for the general health and well-being of the masses through responsible consumption and production, which are defined as priority sustainable improvement aims by the United Nations (UN) (Jarvis 2020).
When it comes to grains, wheat (Triticum aestivum L.) is one of the most extensively grown and consumed cereal crops in the world. Archaeological archives show that wheat was first grown in the foothills of Karacadag (Diyarbakır, Turkey), known as the Fertile Crescent region, around 10,000–12,000 BC (Karakas et al. 2022). Wheat is the world's most commerced and cultivated product, covering an area of 220 million hectares (mha), with a mean yield of 350 kg. da−1 (at 11% moisture content) and a total worldwide production of 773 million tons (USDA 2022). On a continent basis, 45.7% of global wheat production was realized in Asia, 33.5% in Europe, 15.5% in America, 3.3% in Africa, and 2% in Oceania (FAO 2022). More than fifty percent of the world's wheat production is used in human nutrition, and the rest is used in the animal feed and processing industry (Curtis et al. 2002).
Hexaploid bread wheat is thought to be a hybrid of tetraploid wheat (T. turgidum, ssp dicoccum, AABB, 2n = 4x = 28) and Aegilops tauschii (DD, 2n = 2x = 14) and has a genome size of roughly 17,000 Mbp (Awan et al. 2022; Kou et al. 2023). The wheat genome's this hexaploid structure makes it a valuable model for researching and optimizing genome editing technology (Kim et al. 2018). While people meet of their 19.0% of their calorie needs and 20.8% of their protein needs from bread and other wheat products in their daily diets, a significant part of the daily average nutritional elements required per person is also met from cereals and especially wheat bread (Arslan et al. 2021; Ozturk et al. 2022).
Crop development attempts to improve crop yield, quality, and biotic/abiotic stress tolerance (Erturk et al. 2015; Taspinar et al. 2017). For several decades, crop productivity has been significantly increased using modern agricultural technology (Liu et al. 2021). Today, biotechnologies, breeders, and scientists approach the issue in terms of improving the food quality rather than raising the production of wheat, which is extremely important for human nutrition, in proportion to population growth. Various breeding approaches, such as conventional crossing, molecular marker-assisted, radiation and chemically mediated mutation, and genetic engineering have been efficiently employed to enhance different crop properties (Chaudhary et al. 2019; Singha et al. 2022; Ramesh et al. 2020; Natalini et al. 2021; Puren et al. 2023). However, for polyploid crop breeding the traditional mutagenesis-based breeding procedures are lengthy and arduous (Parry et al. 2009).
Genetic modification is an important research instrument that allows the advance of products with valuable properties and advances the understanding of genes' functions (Lawrenson et al. 2015). Recombination and undirected mutagenesis are both random processes, making it difficult and time-consuming to further improve the current elite germplasm. Contrarily, the high precision of genome editing systems allows for unparalleled control over the mutation technique, permitting multiple advantageous traits to occur rapidly within a single generation (Scheben et al. 2017; Wolter et al. 2019). With technological advances, four nucleases, including meganucleases (MNs), zinc finger nucleases (ZFNs), TAL effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) (CRISPR/Cas9) are used for the development and implementation of genome editing systems (Guha et al. 2017; Hajiahmadi et al. 2019; González Castro et al. 2021). TALEN and ZFNs use protein–DNA interactions to identify DNA sequences, but these methods have definite drawbacks, made plasmid construction a challenge, and are also very expensive (Kumar et al. 2015; Soda et al. 2018). TALEN is less costly, easier to construct than ZFN technology, and more specific to the target sequence (Reegan et al. 2016). However, compared to ZFN and TALEN, the CRISPR system is currently considered the most effective, affordable, and flexible cost-effective genome editing technique (Liu et al. 2014; Chang et al. 2016).
The CRISPR/Cas9 system has accelerated the transition to precision breeding by allowing specific genetic manipulation on crops (He et al. 2018; Marwein et al. 2019). When compared to RNA interference (RNAi) and other genome-editing methods, CRISPR/Cas9 has proven more powerful due to its rapidity, heritability, and low off-target mutation rate. The main advantage of this strategy is that the Cas9 transgene may be eliminated in future generations through easy separation or crossing, which offers up a lot of possibilities for applying targeted mutagenesis for speedy crop breeding (Zhang et al. 2016; Zafar et al. 2020).
Another advantage of the CRISPR system over TALENs and ZFNs is the ease with which multiple sites can be targeted simultaneously using multiple sgRNAs (Zarei et al. 2019). Although CRISPR has been used very efficiently for targeted gene knockout in plants, the use of an individual gRNA limits its efficacy and biotechnological applications (Chen et al. 2019a, b). Therefore, recent studies have focused on using multiplexed strategies for multi-locus editing or transcriptional regulation rather than single-guided approaches (Ahmad et al. 2023). CRISPR/Cas9 made it possible to simultaneously modify many quantitative trait loci (QTL) with multiplex genome editing, accelerating the process of improving key crop traits QTL is a specific genetic locus that can result in variations in an organism's phenotypic characteristics, and it controls many yield and quality traits in wheat (Yimam et al. 2021). New research suggests that the range of phenotypic diversity could be increased by CRISPR/Cas9-induced cis-regulatory elements (CREs) mutations in genes affecting productivity traits (Rodríguez-Leal et al. 2017; Wang et al. 2021; Saeed et al. 2022). CRISPR/Cas9 is a valuable tool for knockout non-coding sequences, such as microRNA genes and other non-coding elements (e.g., cis-elements of promoters, enhancers, and transposons) in plants (Ding et al. 2016). For this, Cas9 and multiple gRNAs could be used to remove short microRNA fragments (Waheed et al. 2020). Furthermore, CRISPR/Cas9 is a potent device for the activation, transcription, and repression of protein-coding and non-protein-coding genes and can also be said to be an essential tool in plant biology by reversing methylation-induced gene silencing (Liu et al. 2017).
Beginning with a description of the CRISPR/Cas9 molecular platforms, this review concentrates on last advances in genome editing research in wheat. The review aims to assess the application possibilities of CRISPR/Cas9 in wheat. In addition, we list the research of CRISPR/Cas9 in wheat, including the genes, edited, the plasmids employed, the transformation procedure, and the frequency of the resulting changes. Furthermore, it is to bring to the attention of researchers the potential difficulties that may be encountered in wheat CRISPR studies and the laboratory experience-based solution alternatives for them.
CRISPR/Cas system
History of CRISPR/Cas system
CRISPR is the key element of prokaryotes (such as archaea and bacteria) RNA-mediated adaptive immunity which was first found in Escherichia coli in the 1980s and then reported in 1987 by Ishino et al. (1987). At the time, it took many months to sequence these challenging-to-work DNA fragments, and discoverers didn't know where they came from or what role they played in the bacterial cell (Gostimskaya et al. 2022). However, the first surprising experimental information about how the CRISPR system works came when it was discovered that Streptococcus thermophilus could become resistant to a bacteriophage by putting a piece of an infectious virus’s genome fragment into the CRISPR locus (Doudna and Charpentier 2014; Samso et al. 2015). Due to this discovery, the authors were awarded one of the first patents in the field, and the start of bacterial cultures “vaccination” using CRISPR systems by Danisco in 2005 (Isaacson 2021). In 2012, it was found that the bacterium Streptococcus pyogenes (Sp) could be used for gene engineering in eukaryotes. In 2013, researchers reported the first effective use of successful application CRISPR/Cas9 editing for both transient expression and recovery of stable transgenic lines in plants containing Arabidopsis thaliana, Nicotiana benthamiana, and Sorghum bicolor (Jiang et al. 2013a, b; Li et al. 2013; Nekrasov et al. 2013).
Biology of CRISPR/Cas system
Many bacteria and most archaea have CRISPR locus and Cas genes, which are part of adaptive immune systems that are guided by RNA and protect against bacteriophage infection and plasmid transfer (Sapranauskas et al. 2011; Wang et al. 2016; Jiang and Doudna 2017). Generally, CRISPR/Cas-mediated systems work in three steps to develop a comprehensive immune response to the intrusive foreign DNA (Gupta et al. 2019). First, at the acquisition or immunization stage, foreign DNA fragments of intrusive plasmids or phages (the protospacers) are introduced into the host CRISPR locus (spacers) between CRISPR RNAs (crRNA) repeat to establish the genetic memory of the cell (Nussenzweig and Marraffini 2020). To cleave foreign RNA or DNA, organisms containing CRISPR obtain DNA fragments from infestive bacteriophages and plasmids, which they then transcribe into crRNAs (Wang et al. 2016). Thus, the host can protect itself from a recurrence of the same invader thanks to the genetic record of the infection (Barrangou et al. 2007; Makarova et al. 2006). Afterward, crRNA is expressed and processed, and Cas proteins are produced. RNA polymerase creates RNA molecules from the CRISPR site's spacer regions; these molecules are known as pre-CRISPR RNAs (pre-crRNAs) (Li et al. 2019a, b; Wang et al. 2022a, b, c). Trans-activating crRNA (tracrRNA) is transcribed upstream of the CRISPR locus and serves two crucial roles: first, stimulating the formation of mature RNase III crRNA, and second, launching crRNA-directed DNA cleavage (Jinek et al. 2012; Nussenzweig and Marraffini 2020). The tracrRNA: crRNA complex then binds to Cas9 to create an active ribonucleoprotein (RNP) complex. In the third phase, crRNA directs Cas proteins to the appropriate target, where they cause a double-strand break (DSB) in the invading foreign genome and thereby shield the host cells from infection (Wang et al. 2022a, b, c). In the event of a second infection, the crRNA spacer hybridizes with a foreign target sequence (protospacer), which in turn activates the sequence-specific cleavage of invasive DNA or RNA by Cas nucleases (Marraffini and Sontheimer 2008; Garneau et al. 2010).
Classification of CRISPR/Cas system
Based on the sequence and structure of the Cas proteins, CRISPR/Cas systems are separated into two classes I (heteromeric multiprotein effector modules) and classes II (single proteins effector modules), 6 types (Class I; type I, III, and IV and Class II; type II, V, and VI), 18 subtypes and multiple variants (Makarova et al. 2020; Ilhan et al. 2021). While there is an enlargement in the classification because of newly investigated systems with recent studies, it is estimated that the current classification is almost complete at the "type" level (Russel et al. 2020). The classification of the CRISPR system is summarized in Table 1.
The Class I systems possess multisubunit crRNA effector complexes and encompass the most common and varied type I and III, which is more common in archaea (nearly all systems) than in bacteria (75%), along with the extremely rare type IV, which consists of primitive CRISPR/Cas loci missing the adaptation module (Makarova et al. 2015; Koonin et al. 2017). Type I includes 8 different Cas operons; type III includes 8 Cas operons and Csm/Cmr complexes and type IV includes 2 Cas operons and 4 DinG/Csf proteins (Koonin et al. 2017). All type I system includes the signature Cas3 protein, which uses the DNase domain and helicase to cleavage the target (Sinkunas et al. 2011). The Cas10 signature gene is found in all Type III systems and codes a multidomain protein, the biggest subunit of Type III crRNA-effector complexes. Type III systems (Type III-A and III-B) are distinguishable because they have distinct genes that code for small subunits, called Csm2 (Type III-A) and Cmr5 (Type III-B) (Makarova et al. 2015). Type IV belongs to a putative class 1 system and is less recognized as per types I and III. In type IV systems which are generally localized on plasmids, the effector molecular is significantly much smaller (although still composed of multiprotein), and it does not have any domains that are able to cleave targets or insert spacers (cas1 and cas2) (Pinilla-Redondo et al. 2020).
Class II systems are present almost only in bacteria and consist of an RNP complex including a Cas protein and a crRNA (Gasiunas et al. 2012; Scholz et al. 2013; Shmakov et al. 2017). Because of its simplicity and flexibility in practical applications, class II systems have mostly been used to improve CRISPR/Cas-mediated gene editing technologies. Class II is further subdivided into type II, V, and VI systems. Type II and V CRISPR/Cas9 systems contain, respectively, signature Cas9 or Cas12 effectors with DNA interference activity, while type VI systems have Cas13 effectors with RNA interference activity (Zhang et al. 2021a, b, c). In type V systems, effectors such as C2c1, C2c3, and Cpf1 work with a single RuvC nuclease domain, but not the HNH domain (Abudayyeh et al. 2016; Tamulaitis et al. 2017; Perez Rojo et al. 2018).
Type VI depends on the Cas13 protein and two higher eukaryotes and prokaryotes nucleotide-binding (HEPN) nuclease domains (Schindele et al. 2018; O'Connell 2019). Only with Cas13, does collaboration activity after recognition happens, and later, there is non-specific destruction of surrounding transcripts. There is potential for this phenomenon to be developed as an application for RNA targeting because it happens in bacteria but not in animal and plant cells (Gootenberg et al. 2018).
Components of CRISPR/Cas9 system
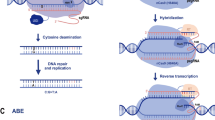
The type II system based on Cas9 obtained from Streptococcus pyogenes (SpCas9) is the most researched and applied among these various CRISPR/Cas systems since its ease of use, adaptability, efficiency, and specificity (Zhang et al. 2021a, b, c). These systems, contain the commonly acknowledged Cas9 effector protein, including RuvC (I, II, and III subdomains) and HNH (His-Asn-His) nuclease domains (Tamulaitis et al. 2017; Janik et al. 2020). Moreover, the Cas9 nuclease comprises two lobes: a nuclease lobe (NUC) and a target recognition lobe (REC). The NUC lobe includes the HNH, the RuvC, and the protospacer adjacent motifs (PAM)-interacting domains. Target DNA strands that are complementary to the short guide RNA (sgRNA) are cut by the HNH nuclease domain, while other target DNA strands are cut by the RuvC nuclease domain (Chen et al. 2019a, b). The recognition site, the REC lobe, recognizes the specific 10–12 nt “seed site” of the sgRNA and has an important role in directing Cas9 to initiate cleavage at the target site (Uniyal et al. 2019).
Cas9 creates an RNP complex with crRNA and a tracrRNA, then this complex cleaves DNA in a crRNA-guided manner (Zhang 2019). sgRNA is another vital element of CRISPR/Cas9. The sgRNA, which is formed when a tracrRNA and a crRNA hybridize, guides the gene-targeting process in CRISPR/Cas (Mali et al. 2013). tracrRNA has three stem-loop hairpin structures that are complementary to the repeat sequence (Chylinski et al. 2013; Nishimasu et al. 2015). The guide sequence in sgRNA is 20 bp long and completely matches the target site's sequence in all genomes. When selecting the target site, care should be taken that the "NGG" trinucleotide motif, known as PAM, is right next to the protospacer target at its 3' end. The Cas9 cleavage site is found within the protospacer, approximately 3–4 bp upstream of the 5'-terminal end of the PAM (Zarei et al. 2019). Cas9 cannot cleave the target sequence in the lack of PAM (Jiang et al. 2013a, b). CRISPR/Cas9 can be derived from various species, each of which has its unique PAM sequence. For instance, the PAM sequence in Staphylococcus aureus, Campylobacter jejuni, Neisseria meningitidis, Pasteurella pneumotropica, and Francisella novicida are 5'-NNGRRN-3', 5'-NNNNACA-3', 5'-NNNNGATT-3', 5'-GNNNCNNA -3' and 5'-NG -3', respectively (Kim et al. 2017; Fedorova et al. 2020; Hashemi 2020; Caruso et al. 2022; Stevanovic et al. 2022). Also, the sgRNA has a "scaffold" sequence, which is essential for Cas9 to attach to it (Anders et al. 2014).
DNA repair pathways in CRISPR/Cas9 system
When the gRNA and the target sequence are homologous, the HNH and the RuvC catalytic domains of Cas9 cause a DSB in the DNA (Loureiro et al. 2019). DSBs generally be repaired through two major endogenous DNA repair: NHEJ and HDR pathways (Yeh et al. 2019; Zhang et al. 2021a, b, c; Sun et al. 2022). Furthermore, microhomology-mediated end joining (MMEJ) and single-stranded annealing (SSA) are the other repair pathways, both of which are more error-prone (Xue and Greene 2021).
NHEJ is an active, dominant, and error-prone repair mechanism (Chavez et al. 2022). Repairing a DSB with NHEJ typically causes 1–10 bp small insertions or deletions of nucleotides (indels) at the cleavage site (Waters et al. 2014; Ryu et al. 2019). These indels usually reason a frameshift and, eventually, knockouts of the corresponding gene and loss of protein function. Therefore, NHEJ can be used in place of RNAi and other methods of gene silencing (Unniyampurath et al. 2016). HDR differs from NHEJ in that a DNA template (undamaged sister chromatid) including the sequence to be conveyed to the cell alongside Cas9 and the gRNA is necessary (Shrivastav et al. 2008). HDR stimulates specific gene modification or foreign DNA knock-in (precise insertion) when a DNA template is present (Sun et al. 2016).
The occurrence of HDR in nature is low, and eukaryotic cells preferentially use NHEJ instead of HDR through various mechanisms (Pickar-Oliver and Gersbach 2019). One of the reasons for this is since NHEJ is active throughout the cell cycle except in mitosis, while HDR is limited to the S and G2 phases (Ma et al. 2018). Even, in plants, the rate of gene knockouts arising from DSB repair via the NHEJ mechanism ranges from 30 to 70% and can even reach 100% in some circumstances (Rozov et al. 2019). Accordingly, many scientists have tried strategies to develop the HDR repair mechanism process and/or suppress the NHEJ pathway by targeting essential components for more effective HDR-mediated precise genome editing (Riesenberg and Maricic 2018; Jayavaradhan et al. 2019; Li et al. 2019a, b).
CRISPR/Cas9 geneediting system in wheat
Today, functional genomics and molecular breeding studies in wheat have gained momentum as the CRISPR/Cas9 technique is used to target more than one homoallele simultaneously (Li et al. 2021a, b). Forward genetic methods like mapping-based cloning of quantitative trait loci (QTLs) and insertion mutagenesis (T-DNA or transposon tag) have helped identified many genes and alleles (Appels et al. 2018; Bettgenhaeuser and Krattinger 2019; Pereira et al. 2020; Sahu et al. 2020). These genes have been used to improve wheat yield with reverse genetic methods such as CRISPR/Cas9, ZFN, TALENs, meganucleases, RNAi, overexpression, and target-induced local lesions in the genome (TILLING) (Aglawe et al. 2018; Savadi et al. 2018; Nerkar et al. 2022). The development of gene editing technologies and understanding of gene functions in wheat have significantly increased studies on modifications in genes that regulate crucial traits such as yield, quality, biotic/abiotic stress tolerance, and hybrid seed production (Lv et al. 2020; Usman et al. 2021; Liu et al. 2022). The investigations on the CRISPR/Cas9 methodology in wheat are summarized in Table 2, which also includes information on the cultivar or genotype, targeted gene(s), gene function, genome editing approach, CRISPR vector, sgRNA promoter, and delivery techniques.
Improving wheat yields
An enormous rise in wheat production is needed to feed the rising human population. Wheat's global genetic improvements of less than 1% per year are woefully inadequate to meet the expected future demand (Ober et al. 2021)). (Kumar et al. 2019)). This fact has prompted researchers to explore and breed new types of wheat. In late years, scientists have used the CRISPR/Cas9 technique for ameliorating wheat production and quality, which is a much faster and more precise manner to create more allelic variations and silence several regulatory genes (Zhang et al. 2021a, b, c)). Achieving high yields to increase production has been the top priority in wheat breeding. Specific characteristics linked to grain yield include tiller number per panicle, grain number and size per panicle, grain weight, and grain size (Liu et al. 2022)). Gupta et al. (2022) modified the microRNA-156 recognition elements (MRE) of TaSPL13 via CRISPR/Cas9 in hexaploid wheat and observed increased grain size and number in TaSPL13 mutants. The yield of bread wheat is highly dependent on the architecture of its inflorescence, which determines the number of grains per spike (Gao et al. 2019). Wang et al. (2022a, b, c) described DUO-B1 as a transcription factor encoding an APETALA2/ethylene response factor (AP2/ERF) that modulates spike blooming architecture in wheat. Gene editing in DUO-B1 created using CRISPR/Cas9 resulted in mild supernumerary spikelets, a rise in grain number per spike, and a considerable yield under field circumstances, without affecting other essential agronomical characteristics. It is known that DUO-B1 is not used in plant breeding, and it is predicted that wheat yield can be increased further with CRISPR/Cas9 studies to be carried out. A mutation in FT-D1 (flowering time gene) led to a rise in total spikelet number and heading date when compared to the wild type. In addition, one simple, easy-to-use, and strong marker designed corresponding to the polymorphic location of FTD1 showed that this specific G indel had been favorably selected for adaptation to varying environmental conditions (Chen et al. 2022a, b). By modifying TaGW7 homologs in the wheat B and D genomes using the CRISPR/Cas9 system, researchers were able to improve both grain size and weight (Wang et al. 2019a, b). The growth of the endosperm and the filling of the grain are the primary factors that determine the grain yield and nutritional quality. GRAIN WIDTH AND WEIGHT2 (GW2) gene encoding by the RING-type E3 ubiquitin ligase is arising as a major genetic driver of grain weight in cereal (Achary and Reddy 2021). To be silenced the TaGW2 lipoxygenase gene using CRISPR/Cas9 increased grain weight as well as protein content in wheat (Zhang et al. 2018). The CRISPR/Cas9 technology was applied to knockout the TaLOX2 gene in wheat, which changed grain size and weight and improved wheat’s ability to be stored (Zhang et al. 2016). Regardless of cultivar background, thousand-grain weight was dramatically raised when CRISPR/Cas9 was used to simultaneously target all three TaGASR7 homeolog (Zhang et al. 2016).
Seed dormancy and germination are the most significant agronomic and trading feature of cereal crops (Cheng et al. 2022). The most important among dormancy genes is the quantitative trait locus for seed dormancy 1 (Qsd1), which was identified in barley. Abe et al. (2019) performed mutations in TaQsd1 genes in subgenomes A, B, and D with CRISPR/Cas9 in wheat, and the mutant plants indicated an importantly longer seed dormancy period than the wild type. Knockout of the TaCKX2-D1 (cytokinin oxidase/dehydrogenase) gene with the CRISPR/Cas9 in wheat increased grain yield (Zhang et al. 2019).
Improvement of wheat grain quality
Agriculture needs to meet increasing food demands by bio-enriching high nutritional value crops through several food technological procedures to struggle with malnourishment and feed the growing global population (Rao and Wang 2021). Therefore, CRISPR/Cas offers a significant amount of promise for ensuring food security and feeding the expanding population of the globe through environmentally responsible agricultural practices. CRISPR/Cas9 for quality traits amelioration requires some specific characteristics; physical characteristics (e.g., size, color, texture, and fragrance), nutrients (e.g., lipids, minerals, proteins, and starch), and bioactive compounds (e.g., carotenoids, flavonoids, lycopene, and γ-aminobutyric acid) (Liu et al. 2021; Achary and Reddy 2021; Vats et al. 2022). Phytic acid (PA) has a high density of negative charges owing to its six phosphate groups, which are partly ionized at physiological pH and balanced by cations, mainly sodium ions (Nassar et al. 2021). Therefore, it can form insoluble complexes with minerals, proteins, enzymes, and starches (Sarkhel and Roy 2022). In a plant-based diet, PA is thought to be the most potent inhibitor of the bioavailability of Fe and Zn (Kumar et al. 2017a, b). Zn deficiency, called "hidden hunger," is a widespread public issue worldwide and brings many health problems (Waqeel and Khan 2022). Additionally, recent research has used CRISPR/Cas9 to target-related genes in the inositol pathway to develop the bioavailability of zinc. Ibrahim et al. (2022) created mutations on three TaIPK1 homologs, TaIPK1.A, TaIPK1.B, and TaIPK1.D, with the CRISPR/Cas9 technique in wheat. An examination of the sequences of T0 plants revealed the deletion of 1–23 nt and even an addition of 1 nt in different lines. Conventional breeding methods cannot lower gluten concentration in bread wheat due to 100 genes and pseudogenes placed in tandem at the Gli-2 loci of 6A, -B, and -D chromosomes for α-gliadins (Jouanin et al. 2019). Using the CRISPR/Cas9 technique to target a conserved section of the α-gliadin genes, the researchers designed two sgRNAs and obtained wheat lines with low-gluten (Sánchez‐León et al. 2018). In addition, low-gluten wheat mutants have been developed by knockout of the γ-gliadin genes (Gli-γ1 and Gli-γ2) (Liu et al. 2023). Hu et al. (2022) used CRISPR/Cas9 to target wheat’s protein disulfide isomerase (PDI) gene. As a result, seven homozygous TaPDI mutants were obtained, and it was reported that the mutants significantly reduced glutenin macro polymers, especially PDI-abd-6. Regulation of this gene may be suggested to improve the glutenin macro polymers accumulation and processing features of wheat dough.
Resistant starch (RS) and amylose-rich foods have considerable potential to benefit human health and decrease the risk of serious noninfectious sicknesses (Kim et al. 2021). Li et al. (2020) induced mutations in the TaSBEIIa gene in two different wheat varieties, Zhengmai 7698 (winter) and Bobwhite (spring–summer), with the CRISPR/Cas9 technique, and wheat with high amylose content was produced. Wheat with improved grain quality such as dough color, grain hardness, and starch quality has been developed by knockout of the Pinb, Waxy, Ppo, and Psy genes using the CRISPR/Cas9 technique by Zhang et al. (2021a, b, c).
Nitrogen (N) as fertilizer has been used widely in agriculture to develop wheat yield to meet the increasing demands for food needs (Anas et al. 2020). Unfortunately, current wheat cultivars have a low N-use effectiveness (NUE), therefore the overuse of nitrogen fertilizer in agriculture contributes to ecological degradation and environmental pollution. For this purpose, Zhang et al. (2021a, b, c) extracted and identified three TaARE1 (abnormal cytokinin response1 repressor1) homeologs from wheat cv. ZhengMai 7698. The authors next used CRISPR/Cas9-mediated targeted mutagenesis to generate a panel of transgene-free mutant lines with either double- or triple-null taare1 alleles. These lines included greater tolerance to N absence, delayed senility, and improved grain production under field conditions. ARE1 orthologs can be used to grow high-yielding wheat that can also be expanded to other important grain crops. Free asparagine is the precursor for acrylamide, which is produced when meals made from wheat are baked, toasted, or subjected to other high-temperature processes (Baskar et al. 2018). The authors knocked out the asparagine synthetase gene (TaASN2) in wheat using the CRISPR/Cas9 technique, revealing that the content of free asparagine in all 6 TaASN2 alleles is diminished compared to wild species (Raffan et al. 2021). Upadhyay et al. (2013) targeted TaINOX and TaPDS genes, which encode enzymes involved in the cellulose production and carotenoid biosynthetic pathway respectively, with CRISPR/Cas9 technology using one expression construct in wheat. In addition to obtaining a sequence with a 24 bp deletion in the TaINOX gene among the editing spectra, the authors found that the mutagenesis efficiency was 18–22%.
Enhancement resistance to diseases caused by biotic stresses in wheat
Although the high diversity of wheat in the world, there has not been an important rise in yield and production in late years because of biotic/abiotic stress conditions (Arslan et al. 2021). Various organisms including bacteria, fungi, viruses, and insects are the factors of biotic stresses (Maharajan et al. 2022). Wheat is susceptible to infection from a wide variety of pathogens, which can result in several diseases (Wulff and Krattinger 2022). Diseases like these can be managed with the use of a variety of fungicides, herbicides, and insecticides, but these chemicals pose serious threats to both humans and animals, as well as the environment (Aydin et al 2021; Tănăsescu and Lite 2022). Therefore, a better way CRISPR/Cas9 technology can target genes sensitive to biotic stress and induce plant resistance.
One of the most damaging wheat diseases, wheat stripe rust induced by the Puccinia striiformis f. sp. tritici (Pst) causes major losses in wheat output around the world (Esmail et al. 2023). Calcineurin B-like protein (CBL)-interacting protein kinases (CIPKs) has been shown to be related to resistance throughout plant-pathogen interactions (Xiao et al. 2022). Research on wheat has shown that the knockdown of the TaCIPK14 gene can lead to wheat developing resistance to Pst. Conversely, overexpression of TaCIPK14 increased whea’s sensitivity to Pst by reducing the defense response by suppressing reactive oxygen species (ROS) accumulation and pathogenesis-relative genes (He et al. 2022). The MILDEW LOCUS O (MLO) gene was first recognized in barley and later emerged as a susceptibility (S) gene in mono-dicotyledons (Borrelli et al. 2018; Langner et al. 2018). Degrement of function mutations on MLO genes leads to resistance to powdery mildew in both monocot and dicot plants (Shan et al. 2013; Wang et al. 2014; Gil-Humanes et al. 2017; Li et al. 2022). Modification of the TaMLOX gene using CRISPR/Cas9 provided resistance to powdery mildew in four elite wheat cultivars without loss of crop growth and yield (Li et al. 2022). CRISPR/Cas9 technique-mediated knockout of improving disease resistance1 (TaEDR1) showed enhanced resistance in hexaploid wheat to powdery mildew caused (Blumeria graminis) (Zhang et al. 2017). Fusarium head blight (Fusarium graminearum) is another devastating disease of wheat (Xu et al. 2022). A mycotoxin virulence factor called deoxynivalenol (DON) encourages the growth of the Fusarium graminearum in the floral tissues of wheat (McLaughlin et al. 2021). Brauer et al. (2020) showed DON-induced transcriptional alterations in wheat spikelets to reveal the effects of DON exposure on plant cell function. For this purpose, they targeted the TaNFXL1 gene using the CRISPR/Cas9 method and showed that this gene increased fusarium head blight resistance in wheat.
In addition to fungal diseases, virus infections are also fundamental problems in wheat farming. That's why scientists have started using CRISPR/Cas9 to crop tolerance to viruses. The genomes of viruses evolve rapidly and may include double-stranded DNA/RNA (dsDNA/dsRNA) and single-stranded DNA/RNA (ssDNA/ssRNA) (Nasir and Caetano-Anollés 2015). Both Ta-eIF4E and Ta-eIF(iso)4E genes encode translation initiation factors that are well conserved. They also act as S factors necessary for the life cycle of plant viruses, which are members of the Potyviridae family. Bread wheat mutant’s knockout of Ta-eIF4E and Ta-eIF(iso)4E by CRISPR/Cas9 technique is susceptible to wheat spindle streak mosaic virus (WSSMV) and wheat yellow mosaic virus (WYMV) is predicted to be resistant (Hahn et al. 2021).
Enhancement of abiotic stress tolerance in wheat
Among the most common abiotic stresses plants face are those brought on by drought, salinity, temperature extremes, lack of nutrients, and heavy metals (Taspinar et al. 2018). In plants under abiotic stresses, changes such as Ca2+ concentrations, ROS, and abscisic acid (ABA) activation occur (Lee et al. 2022; Waseem et al. 2022). Several studies have shown that abiotic stressors have a significant negative impact on the growth and production of grains. In some cases, abiotic stress factors can reduce a plant's output by more than 50% (Yigider et al. 2021). Many recent studies have reported that specific target genes such as S and tolerance (T) genes are used to improve wheat tolerance to abiotic stress by CRISPR/Cas9 application. By targeting these genes, activation of specific signal transduction pathways and transcriptional rearrangement that can activate protecting mechanisms (i.e., cell detoxification, osmotic regulation, modulation of stress signaling, and improved stress-induced damage) can be induced.
Regarding wheat's ability to tolerate drought, Abdallah et al. (2022) used multiplex CRISPR/Cas9 techniques to edit the TaSal1 gene; the results showed that five Sal1 mutant seedlings grew better under drought stress than the wild-type control. Polyploidy is common in plants and has a vital role in both the process of plant evolution and the differentiation of species (Sabooni et al. 2022). Since polyploids are known to be more adaptable to environmental stresses against diploid plants, it is thought that they can develop new properties of diploids through gene editing studies. Zheng et al. (2021) mutated the TaHAG1, an important regulator of salt tolerance in wheat using CRISPR/Cas9. Mutations on the TaHAG1 gene increase salt tolerance by controlling ROS production and signal specificity. Kim et al. (2018) published their findings that two genes (TaDREB2 and TaERF3) related to stress were edited via the CRISPR/Cas9 in wheat protoplasts. Consequently, demonstrated the ability to generate new stress-resistant variants in plant strains expressing CRISPR/Cas9 with this modification. Wheat harvests are being negatively impacted by climate change because the plant's reproductive system is being damaged by the increased temperatures (Ullah et al. 2022). Therefore, heat stress significantly reduces global wheat output and results in significant yield losses worldwide (Wu et al. 2022). When wheat is subject to high temperatures, the TaMBF1c gene is activated. Tian et al. (2022) employed the CRISPR/Cas9 technique to understand the role of Multiprotein Binding Factor 1 (MBF1) in heat stress tolerance in wheat. The finding display that TaMBF1c is evolutionarily conserved in di-, tetra-, and hexaploid wheat species, and knockdown-out mutants are heat sensitive.
Speeding wheat hybrid seed production
As a result of heterosis, hybrid crop types frequently exhibit superior features in comparison to their homozygous parents (Schnable and Springer 2013). Wheat hybrids have up to 20% yield advantages compared to elite wheat varieties and are more resistant to environmental stress factors (Mühleisen et al. 2014). The creation of male-sterile and double-haploid wheat plants can significantly accelerate the process of creating hybrid seeds. Wheat's high genetic redundancy because of the allohexaploid makes it challenging to create nuclear recessive male sterile mutants through spontaneous mutation, chemical or physical mutagenesis technique. Therefore, CRISPR/Cas9 systems have also been utilized to develop other crop qualities such as fertility leading to the improvement of new plant species and haploid lines (Liu et al. 2022). Li et al. (2020) have investigated the editing of TaNP1 pollen fertility genes using CRISPR/Cas9 systems. In the study, various RNA polymerase III promoters, namely TaU3p-U6p and OsU3p-U6p, were used and the TaU3 promoter was determined as the most effective. Editing three homologous alleles encoding the TaNP1 gene resulted in completely male-sterile mutants in wheat. The male sterile 45 (Ms45) gene homolog in wheat was knockout out using the CRISPR/Cas9 technique, and eventually, male sterile plant mutants were produced (Singh et al. 2018). Recently, completely male sterile mutants have been obtained by creating frameshift mutations in the MS1 gene in wheat via the CRISPR/Cas9 technique (Okada et al. 2019). Editing the CENTROMERIC HISTONE (CENH3) gene by genetic engineering induces paternal haploid induction (HI) (Wang et al. 2019a, b). Lv et al. (2020) use the CRISPR/Cas9 technology to create a commercially viable paternal HI line in wheat with a 7% HI ratio and TaCENH3 gene alterations. The TaPLA gene was knockout using the CRISPR/Cas9 technique in the A and D genomes of wheat, resulting in an HI ratio of 2–3% (Liu et al. 2020a, b). The TaWaxy and TaMTL genes in wheat were genome editing with CRISPR/Cas9 for haploid plant production, and the highest mutation frequency of 80.5% was obtained using the TaU3 promoter (Liu et al. 2020a, b).
Potential obstacles and the laboratory experience-based solution alternatives for wheat CRISPR studies
Wheat has much less genome editing research than other plant species because critical components of the CRISPR system, such as the Cas9 enzyme, are still being developed to fit the properties of the wheat genome (Dayani et al. 2019). Recently, the CRISPR/Cas9 system has been successfully used to increase the characteristics of agricultural crops, but there are still some problems with the CRISPR/Cas9 editing technology that is currently available (Fig. 1). These problems include off-target impacts, the scope of editing, and problems with plant genetic transformation methods.
The targeting specificity of CRISPR/Cas9 is still an ongoing debate in research. Cas9's ability to cleave genomic regions that are outside of its intended target results in off-target that are not intended (Liu et al. 2022). The targeting accuracy of Cas9 endonuclease is known to be heavily influenced by PAM (3–5 bp) sequences positioned at the 3' end of the target region of the genome and the 20 nt sgRNA (Manghwar et al. 2020). Even though there are mismatches in the target sequence, the system can still work normally (Mahfouz et al. 2014). The presence of off targeting can cause chromosomal rearrangements that may lead to damage to functional gene activity, resulting in damage to incorrectly matched genomic loci (Manghwar et al. 2020). Therefore, it should be recognized that there will always be some risk of off-target mutagenesis in CRISPR/Cas9 studies and new strategies should be considered to minimize this possibility. There are essentially two approaches to solving this problem. The first strategy involves improving specificity to decrease the likelihood of off-target impacts, the second strategy requires experimenting to minimize the effects of false results, reducing the potential for off-target mutagenesis.
Correctly designing the sgRNA is a vital stage in maximizing the effectiveness of the CRISPR/Cas9 technique. Many biotech companies are developing software for sgRNA design, and CRISPy-web, CRISPR-P, CRISPR-ERA, Cas Designer WheatCRISPR, and CHOPCHOP are some of them (Lei et al. 2014; Liu et al. 2015; Park et al. 2015; Blin et al. 2016; Labun et al. 2016; Cram et al. 2019). Badhan et al. (2021) two sets of sgRNA were designed using the CHOPCHOP tool. Off-target effects can only be predicted with accurate information about the sequence of the genome. The wheat genome is complicated because of its allohexaploid structure or containment of different genomes (AABBDD) (Dayani et al. 2019). sgRNAs designed for wheat genome editing studies can bind to off-target regions in other subgenomes in addition to the targeted region. Therefore, it is required to design sgRNA from conserved regions in all genomes of wheat and to identify non-target regions. For this, various software is available that predict whether the sgRNA genome is the target of the CRISPR/Cas9 and, if possible, the off-target regions in the genome to which it can possibly bind. The sgRNAs can be evaluated using tools such as CasOFFinder, CasOT, CCTop, and CRISPR Multitargeter (Bae et al. 2014; Xiao et al. 2014; Prykhozhij et al. 2015; Stemmer et al. 2015). Lee et al. (2019) employed the CasOFFinder tool, which could identify probable off-target regions throughout the genome, to assist decrease the off-target proportion. Cas9 enzyme cuts three nucleotides upstream of the PAM site in the CRISPR/Cas9 mechanism. The cutting effectiveness of Cas9 varies in some cases with the specialty of the PAM sequences, so selecting the correct PAM sequence can decrease the rate of off-target mutations (Yuan et al. 2017; Zhu 2022). Scientists have shown that by mutating some regions of the enzyme, they can limit Cas9 off-target impacts (Kleinstiver et al. 2016; Liu et al. 2022). This restriction was made easy with the development and application of the engineered SpCas9 variant SpRY, which can target DNA PAM-free in plant cells (Ren et al. 2021).
Although CRISPR technology gives several opportunities to modify the wheat genome, the fact that wheat is a monocotyledonous plant makes vector transfer to cells and the regeneration of transgene cells more challenging. The plant transformation process is the transfer of foreign genetic material into plant cells, tissues, or organs to change the plant's trait(s) or phenotype (Chanyalew et al. 2019). When compared to dicotyledons, monocotyledons have fewer explant sources from which somatic embryos can be formed. Dicotyledonous plants frequently use hypocotyls and leaf fragments for transformation, while monocotyledonous plants use seed embryos and scutellum (Hiei et al. 1997). Also, an important rise in callus formation has been suggested after mechanical injury to tissue surfaces (Aydin et al. 2011).
An effective and stable genetic transformation mechanism is required for gene editing of agricultural crops with the CRISPR/Cas9 technique. For crop genetic enhancement, frequently used methods include Agrobacterium and the gene gun (microprojectile or particle) technique (Kapusi and Stoger 2022; Wijerathna-Yapa et al. 2022). Most wheat CRISPR/Cas9 investigations have used biolistic gene transfer since the genetic transformation is difficult (Zhang et al. 2018). Biolistic is a DNA transformation technique in which metal particles coated with foreign genes (multiple genes, RNA sequences, or proteins) are blasted with high-pressure gas and incorporated into recipient cells (Twyman and Christou 2004; Liu et al. 2019; Zhi et al. 2022). The first effective genetic transformation of bread wheat using biolistic was performed by researchers at the University of Florida (USA) (Vasil et al. 1992). The application of the biolistic technique makes it possible to add very large DNA fragments to wheat. According to Partier et al. (2017), a 53-kilobyte linear cassette was successfully inserted into the bread wheat genome through the biolistic transformation technique. It has proven to be more effective than Agrobacterium-mediated techniques while less dependent on the specific genotype used (Wijerathna-Yapa et al. 2022). However, the efficacy of biolistic approaches is very limited because of constraints in current tissue culture regeneration systems and multiple-copy insertions, and their transformation capacity is low (Sparks and Jones 2004; Liu et al. 2022). Although other techniques for crop transformation have been tried and tested (such as polyethylene glycol, liposomes, silicon carbide, and microinjections), their drawbacks prevent their widespread application. For example, these methods are time-consuming and costly, and they rely on complex infrastructures (tissue culture regeneration systems) and specific genotypes (plants) (Que et al. 2014).
Agrobacterium-mediated techniques, on the other hand, often show a higher frequency of single-site insertions (Dai et al. 2001). Agrobacterium (gram-negative soil bacterium) is successfully and widely used for plant genetic transformation. Agrobacterium strains have plasmids called Ti plasmid (tumor-inducing) (Aglawe 2018). Agrobacterium is inherently capable of infecting injured dicotyledonous plants, and then bacterial T-DNA is integrated into the host genome and expressed owing to the host DNA repair mechanism (Hiei et al. 1997). Additionally, the Monsanto researchers were the first to paper on the generation of transgenic wheat via the Agrobacterium-dependent transformation method (Cheng et al. 1997).
Each genetic transformation process includes three important steps: susceptibility of infected tissue to Agrobacterium, the regeneration capacity of the target tissue, and an effective selection system for the recovery of transformed plants (Wijerathna-Yapa et al. 2022). Although genome editing represents an unprecedented technological advance, its use for agricultural enhancement is hampered by low plant regeneration frequencies and genotype dependency. The efficiency of Agrobacterium-mediated gene transformation is affected by a variable of factors. Different genetic backgrounds, as well as physical and metabolic factors, cause various results in Agrobacterium infection (Das et al. 2002). Genotype dependence is the most well-known obstacle among variants of the same plant species. ‘Fielder’ and ‘Bobwhite’ are two examples of wheat varieties bred with the purpose of working in wheat gene transformation experiments (Ishida et al. 2015). Especially, the Bobwhite SH98-26 variety shows a TF of over 70% in gene transfer with the biolistic method (Pellegrineschi et al. 2002; Ye et al. 2022). The Agrobacterium-mediated transfer method is not appropriate for all agricultural crops and tissues, as it depends on the plant tissue culture regeneration system (Liu et al. 2022). The transformation frequency (TF) of every plant, including wheat, is significantly influenced by the conditions under which it was grown in tissue culture. Several significant factors in tissue culture that impact TF in various species or plant organs are known to be associated with the basal media, including macro and microelements and vitamins (Hesami et al. 2021). Multiple reports on wheat transformation have demonstrated that MS (Murashige and Skoog 1962) or a modified MS-based medium is appropriate for inoculation and co-cultivation (Ding et al. 2009; Alikina et al. 2016; Hayta et al. 2021). Greer et al. (2009) enhanced the biolistic transformation efficiency of elite wheat (cv. Superb) calli sevenfold by adding 62.56 mM ammonium nitrate to the callus production medium. However, there are also some studies that show that high amounts of salts in the environment during transformation reduce the effectiveness of Agrobacterium. Therefore, reducing MS salts to 1/10 at the transformation stage is recommended (Carvalho et al. 2004; Wang et al. 2022a, b, c).
The utilization of genes involved in regeneration throughout in vitro culture can increase plant transformation's efficiency. The authors indicated that overexpression of the wheat gene TaWOX5 importantly rises immature embryo transformation efficiency while being less genotype dependent (Wang et al. 2022a, b, c). Overexpression of the TaCB1 gene, from the same gene family, intensely enhanced the transformation efficiency of numerous wheat cultivars without genotype dependence (Ke et al. 2021). Furthermore, two studies stated that the overexpression of TaCB1 and TaWOX5 in wheat callus did not inhibit both shoot differentiation and root development. The findings of Qiu et al. (2022) showed that the modulation of TaGRF4 and TaGIF1 genes in wheat led to an increase in both the frequency of genome editing and the rate of regeneration. Raman et al. (2022) described a method for delivering proteins that reduce plant defense and/or boost transformation using A. tumefaciens that has been engineered with a T3SS. In practice, T-DNA was co-transfected with P. syringae effectors such AvrPto, AvrPtoB, or HopAO1, which have been indicated to increase transformation efficiency in Arabidopsis, alfalfa, branchy grass, wheat, and Nicotiana benthamiana.
Some specific metabolites such as salicylic acid, ethylene, isoflavones, and γ-aminobutyric acid can prevent the growth and gene transfer efficiency of Agrobacterium (Nonaka et al. 2008; Hasan Nudin et al. 2015; Zhang et al. 2015; Park et al. 2010). Salicylic acid attenuates the kinase activity of the Vir A protein, which prevents the activation of vir genes (Yuan et al. 2007). On other hand, the addition of surfactants, polyamines, and amino acids to the culture medium has proven to improve TF positively. One of them, polyamines promote cell division, totipotency, and plant cell differentiation (Aydin et al. 2016; Jangra et al. 2022). The addition of polyamines to the transformation process leads to the induction of vir genes and T-DNA transfer, thereby increasing the efficiency of transformation (Xu et al. 2022). When 0.1 M spermidine was supplemented to the regeneration medium to help get transformants from pHK21/LBA4404-infected calli, the TF went up from 1.2% to 3.9% (Khanna and Daggard 2003).
Agrobacterium strain, Agrobacterium density, acetosyringone (AS) concentration, explant type, pre-culture process, co-cultivation conditions, antibiotics used in plant selection and against Agrobacterium growth, hormone types and concentrations used are all among the factors that affect gene transfer in wheat. For every transformation system, the optimum level of each of these components must be established. The most widely used Agrobacterium strains for wheat transformation include AGL0, AGL1, C58C1, EHA101, EHA105, GV3101, and LBA4404 (Hao et al. 2021; Upadhyaya et al. 2021; Ahansal et al. 2022; Sing and Kumar et al. 2022; Wang et al. 2022a, b, c). Khurana et al. (2002) used Agrobacterium LBA4404 to develop transgenic tetraploid wheat (Triticum dicoccum Schuble) plants from mature embryo-derived calli. Studies with diverse wheat explants revealed that a high Agrobacterium density boosted transitory GUS expression but was not related to a higher persistent transformation frequency (Cheng et al. 1997). Amoah et al. (2001) used Agrobacterium cells with a cell density of OD600 1–1,5 and showed a significant rise in the number of explants showing high β-Glucuronidase (GUS) activity. According to Kumar et al. (2019), a bacterial density of OD600 = 1 resulted in the highest explant viability (83.6%).
Agrobacterium has a broad host range, infecting at least 41 plant groups, most of which are woody (De Saeger et al. 2021). Although cereals are not Agrobacterium’s native host, numerous investigations have been conducted to find host strains that are compatible with different wheat genotypes (Pérez-Piñeiro et al. 2012). Besides, AS has been used to induce Agrobacterium in wheat gene transfer studies. The phosphorylation of the VirG protein is triggered when the VirA protein is activated by phenolic chemicals such as AS. This in turn promotes the inducement of other Vir genes contained in the Ti plasmid (Chen et al. 2022a, b). Aadel et al. (2021) described that the addition of 0, 100, 200, and 400 µM AS to the Agrobacterium culture, especially of 200 µM AS increased the callus resistance frequency (44%) in the Achtar (from intact-immature cultured) wheat cultivar. Sing and Kumar (2021) successfully used 150 µM AS concentrations in several bread wheat genotypes such as HD 2967–3086 and Bobwhite for gene transfer studies. Kharb et al. (2022) supplemented the bacterial suspension with 200 µM AS during co-cultivation to facilitate the transformation of wheat and rice.
A dilemma in the planning of laboratory studies is the selection of mature or immature embryos. Successful studies of Agrobacterium-mediated transformation in wheat have been performed both using immature embryos and mature embryos to produce fertile transgenic plants (Kuluev et al. 2022; Liang et al. 2022; Sing and Kumar 2022). When we look at gene transfer studies with wheat tissue culture, it is understood that gene transfer to immature embryos is still the best approach. In contrast, gene transfer to tissues derived from mature embryos has a significantly low TF and can only be applied to a limited number of germplasms. Wang et al. (2009) transformed longitudinally cut mature embryos and found that the TF for the cultivars Bobwhite, Yumai 66, and Lunxuan 208 was, respectively, 0.06%, 0.67%, and 0.89%. Although immature embryos are frequently used for gene transfer studies in wheat, they are difficult to obtain throughout the year and their suitable stage for culture is severely limited (Yu et al. 2008; Ding et al. 2009). Therefore, correctly identifying the study material will increase the TF. However, endosperm-supported mature embryos had a larger percentage of embryogenic callus and a much higher rate of regeneration (Chauhan et al. 2017).
The next and another principal subject is the selection and concentration determination of surfactants. Surfactants such as Silwet, Pluronic F-68 -127, and Tween 20–80 have been reported to significantly affect T-DNA delivery during inoculation and co-cultivation of wheat transformation. Also, these surfactants are used for the hold of Agrobacterium to the surfaces of plant tissues in gene transfer. Pluronic F-68 (0.03% w/v) was added to the IM medium to increase transformation efficiency (Cheng et al. 1997). Researchers found that Pluronic F-68 worked well at 0.01% concentration (Ding et al. 2009). Parmar et al. (2015) evaluated the transformation efficiency of Pluronic F-68 at diverse doses (0, 0.015, 0.05, 0.1, 0.15, and 0.2%) in a gene transformation study in mature embryos. As a result, it was revealed that the maximum GUS expression was 0.15% of Pluronic F-68. Maximum Silwet L-77 concentrations of 0.05% were found to negatively impact the survival and callus formation of newly isolated immature embryos, leading to the determination that a concentration of 0.01% was optimal (Wu et al. 2003). In wheat gene transfer, 0.05% Silwet L-77 and 100 μM AS were prepared and added to the medium just before starting the study and successfully carrying out gene transfer (Hayta et al. 2021).
Drying can be vital to preventing some serious problems from occurring, but how?
After infection with Agrobacterium, it is essential to dry pre-cultured wheat embryos or embryogenic calluses before being placed in the co-cultivation medium. When using filter paper for co-cultivation, Agrobacterium overgrowth is limited, leading to an 82.3% explant survival rate. This compares to a survival rate of only 22.7% when using a medium-based method (Kumar et al. 2019). The effect of temperature on T-DNA delivery during co-cultivation is known. Bacterial conjugation studies determined that the most suitable temperature for T-DNA transfer was 19 °C (Salas et al. 2001). The ideal temperature for vir gene induction (25 °C) is typically lower than the ideal temperature for Agrobacterium vegetative development (28–30 °C) (Allahi et al. 2014). The co-cultivation temperature of monocot plants such as wheat is 24–25 °C, but the co-cultivation temperature of some crops reaches 28 °C (Opabode 2006). Following all transformations, the tissues are kept in the co-cultivation medium at 25 °C for 2–5 days (Wu et al. 2003; Zhao et al. 2006; Hayta et al. 2019). Transformed tissues frequently become sternly necrotic after co-cultivation during Agrobacterium-mediated transformation processes. It is known that this is because cultured wheat immature embryos rapidly produce H2O2 and that most tissues turn brown when these tissues come into contact with Agrobacterium (Tao et al. 2011). Browning and necrosis of infected tissues following Agrobacterium infection are significant barriers to gene delivery in wheat (Shrawat and Lörz 2006). Browning and necrosis tissues do not subsequently form somatic embryos and die quickly (Shrawat et al. 2007). Ascorbic acid, glutathione, lipoic acid, selenite, and cysteine are all antioxidants that have been shown to improve plant regeneration and TF while going through genetic transformation by reducing necrosis and darkening of the tissues (Jones et al. 2005; Dan 2008).
Using antibiotics does really help? If so, which one is the true one, and what is the effective dose of that?
For calli that are 4–20 days old, post-co-cultivation Agrobacterium removal or growth suppression using antibiotics is challenging (Kumar et al. 2017a, b). The dose of cefotaxime antibiotic used to kill Agrobacterium after gene transfer is very important. Cefotaxime is penicillin and contains the β-lactam group and lyses and kills the bacteria by inhibiting the biosynthesis of a component of the bacterial cell wall called peptidoglycan (Kumar et al. 2017a, b). It has the least adverse effect on most plant tissues. However, Ishida et al. (1996) found that high concentrations of antibiotics like cefotaxime were harmful to explants and reduced the transformation frequency by several folds. Li et al. (2002) stated that embryogenic callus could be encouraged on media with either 250 mg l–1 or 500 mg l–1 of cefotaxime, but that the frequency was much lower than on a medium without antibiotics. Before beginning the gene transfer, determine the lethal dose of cefotaxime on the plant based on the genotype to be used.
Herbicide resistance and antibiotic tolerance genes are often utilized as selectable markers in wheat transformation because they drastically decrease the escape rate. hpt II (hygromycin phosphotransferase) gene which confers hygromycin B antibiotic resistance (Sing and Kumar 2022), npt II (neomycin phosphotransferase II) gene which confers kanamycin antibiotic resistance (Mushke et al. 2019), bla (β-lactamase) gene which confers resistance to ampicillin antibiotic (Qin et al. 2022), EPSPS (5-enolpyruvylshikimate-3-phosphate synthase) gene which confers glyphosate herbicide resistance (Arndell et al. 2019) and bar (bialaphos resistance) and pat (phosphinothricin acetyltransferase) genes which confer resistance to phosphinothricin herbicide (Thiyagarajan et al. 2022) were used commonly as selection marker genes.
Antibiotics in excessive amounts destroy non-transformed cells and slow the growth of transformed cells and plants, delaying the regeneration process. Interestingly, some of the wheat seeds in gene transfer studies initially germinate under antibiotic selection, but these plants do not survive any longer. The effectiveness of antibiotic selection is influenced by a variety of factors, including the species of plant, the types of tissue, the cultivar type, and organs present, and even the stages of development present within the same species (Tran and Sanan-Mishra 2015). Kanamycin and hygromycin have been effectively employed as selectable markers in the transformation of various legume crops. According to Abdul et al. (2011) wheat cv. The GA-2002 experiments used the Agrobacterium strain (LBA4404) with the binary vector pBI121. Plant selection was done with 125 mg/l of kanamycin, and the resistant plants that were chosen were put through PCR testing and GUS histochemical staining. For this reason, before starting gene transfer studies in wheat, the doses of selective antibiotics to be used should be determined by preliminary trials.
Future perspective
Uncovering the mechanism of the immune system developed by bacteria against phages and plasmids has formed the basis of various genome editing tools (Mahler et al. 2022). Researchers are constantly working towards improving better technologies for making our lives easier. CRISPR/Cas is based on the natural protection mechanism found in bacteria and has been continuously developed since its emergence. Despite significant efforts by researchers, progress in the genetic engineering of wheat continues to lag other important agricultural crops such as maize and rice. This can be related to the genetic characteristics of wheat, such as its extremely large about 17,000 Mbp and complicated genome structure, and the significant dependence of most wheat species on growth and regeneration in vitro. It is thought that genetic engineering studies will mostly continue with CRISPR/Cas systems. Even though wheat's huge genome and complicated polyploid nature have previously slowed the progress of wheat genetic engineering, effective CRISPR/Cas9 systems are now accessible for the development of wheat biology (Li et al. 2020). NHEJ is plants’ predominant repair pathway to create gene replacements and gene chains. However, the researchers believe that precision gene editing through HR will be used in wheat breeding soon by improving the efficiency of HR through some mechanisms. Strategies applied to increase genetic transformation efficiency in wheat go beyond simply optimizing culture medium composition and growth conditions. Understanding the problems of gene transfer to wheat is necessary for solving the related mechanisms with the developing genetic engineering tools. In addition, the researchers believe that precision gene editing through HR will be used in wheat breeding soon by improving the efficiency of HR through some mechanisms.
Recent advancements in CRISPR/Cas9 technology have led to the development of base editing and prime editing techniques that can more precisely modify the wheat genome. Although these techniques are relatively new, they show promise for improving wheat quality in the future. For instance, base editing could be used to introduce point mutations to specific genes that are associated with desirable traits, while prime editing could be used to make more complex edits to the genome without the need for double-strand breaks. Further research is needed to fully explore the potential of these techniques for improving wheat production.
Targeted genome editing has the potential to completely revolutionize plant breeding by allowing for the creation of wheat cultivars with improved resistance to environmental challenges generated by climate change. The use of the CRISPR/Cas9 system in drought stress tolerance or signaling engineering has not been enough studied in wheat but may demonstrate to be an important tool in the future to understand the functions of genes involved in signaling pathways. Additionally, when the studies on the nutritional quality of wheat are examined, it is understood that there are not enough studies on this subject, and in the future, researchers should accelerate their studies to raise the quality of the wheat grain to food the growing population and reduce hidden hunger.
Today, in many laboratories around the world, scientists are focused on understanding, developing CRISPR technology, and contributing knowledge to the “Science Pool”, by this way, have begun to produce developments and patents to make it more effective and efficient every day.
All new developments have brought fear and criticism throughout human history. Does CRISPR technology also come with risks? What are the ethical issues? Can editing the gene pool lead to unpredictable results? Could CRISPR lead to off-target mutations in plants? What kind of damage can be caused at partially matched genomic loci through chromosomal rearrangements? If gene activity is lost, what physiological or signal abnormalities can occur? As plant scientists, while concentrating on these ideas, we also must listen to society, understand their concerns, and produce satisfactory answers. CRISPR is one of the most promising technologies for crop production, particularly given the widespread public concerns about GMO crops.
GMO and Genome Editing are fundamentally different methods. CRISPR technology is a good opportunity to tackle anti-GMO approaches. Small mutations such as deletions or insertions provide a great occasion for us to make changes to agronomic traits without leaving any foreign DNA behind. In the future, as society's needs change, plant breeders would increasingly use CRISPR resemble technologies instead of transgenic plants to produce high-quality and safe plants.
Global-scale evaluations should be made for the future of CRISPR technologies. As a part of the planned CRISPR goals, it is crucial that people living in low-income, low-R&D potential geographies be included so that they will have access to agricultural products (particularly essential nutrients like wheat) that are of sufficient quality and quantity in the future. There is also a need to establish a clear framework for all the legal regulations regarding intellectual property rights, R&D, production, and trade of CRISPR patents.
Considering agricultural purposes, CRISPR technology has the potential to lead to improvements in the extent of our obligations such as diversification of crops, improvement of their quality, herbicide tolerance, and resistance to diseases and pests through metabolic engineering and editing of host genes in plants. To achieve efficient genome editing, this technology must be improved, including the elimination of its current problems, such as low regulatory efficiency, loss of function genes, physiological or signal abnormalities. Insomuch, CRISPR technology is still considered to be in its infancy by some scientific assessments. More effective DNA-cutting tools and repairs removed from randomness are the goals for the renewed CRISPR technology of the future while eliminating random outputs for therapeutic effect is among the goals of developing the technique as well. As a result of these improvements, more complex targets would be achievable.
Author contribution statement
The article was conceived by EY, MST, and GA, and they all contributed to the literature review and drafting of the article. All authors read and confirmed the last manuscript.
Data availability
Because no data sets were generated or analyzed during the current study, data sharing does not apply to this article.
References
Aadel H, Udupa S, Abdelwahd R, Gaboun F, Diria G, Douira A, Iraqi D (2021) Agrobacterium-mediated genetic transformation of bread wheat (Triticum aestivum L.) using immature embryos. Rom Agric Res 38:99–107
Abdallah NA, Elsharawy H, Abulela HA, Thilmony R, Abdelhadi AA (2022) Elarabi NI (2022) Multiplex CRISPR/Cas9-mediated genome editing to address drought tolerance in wheat. GM Crops Food. https://doi.org/10.1080/216456982120313
Abdul R, Ishfaq AH, Imran M, Azhar H (2011) Development of in planta transformation protocol for wheat. Afr J Biotechnol 10:740–750
Abe F, Haque E, Hisano H, Tanaka T, Kamiya Y, Mikami M, Kawaura K, Endo M, Onishi K, Hayashi T, Sato K (2019) Genome-edited triple-recessive mutation alters seed dormancy in wheat. Cell Rep 28:1362–1369
Abudayyeh OO, Gootenberg JS, Konermann S, Joung J, Slaymaker IM, Cox DB, Shmakov S, Makarova KS, Semanova E, Minakhin L, Severinov K, Regev A, Lander ES, Koonin EV, Zhang F (2016) C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Sci. https://doi.org/10.1126/science.aaf5573
Achary V, Reddy MK (2021) CRISPR-Cas9 mediated mutation in GRAIN WIDTH and WEIGHT2 (GW2) locus improves rice’s aleurone layer and grain nutritional quality. Sci Rep 11:1–13
Aglawe SB, Barbadikar KM, Mangrauthia SK, Madhav MS (2018) New breeding technique “genome editing” for crop improvement: applications, potentials and challenges. 3 Biotech 8:1–20
Ahansal K, Abdelwahd R, Udupa S, Aadel H, Gaboun F, Ibriz M, Iraqi D (2022) Effect of type of mature embryo explants and acetosyringone on Agrobacterium-mediated transformation of moroccan durum wheat. J Biosci 38:e38007
Ahmad HM, Iqbal MS, Abdullah M, El-Tabakh MA, Oranab S, Mudassar M, Shimira F, Zahid G (2023) Recent Trends in Genome Editing Technologies for Agricultural Crop Improvement. Sustainable Agriculture in the Era of the OMICs Revolution. Springer International Publishing, Cham, pp 357–379
Alikina O, Chernobrovkina M, Dolgov S, Miroshnichenko D (2016) Tissue culture efficiency of wheat species with different genomic formulas. Crop Breed Appl Biotechnol 16:307–314
Allahi S, Khodaparast SA, Sohani MM (2014) Agrobacterium-mediated transformation of Indica rice: A non-tissue culture approach. Int J Agric Innov Res 3:2319–1473
Amoah BK, Wu H, Sparks C, Jones HD (2001) Factors influencing Agrobacterium-mediated transient expression of uid A in wheat inflorescence tissue. J Exp Bot 52:1135–1142
Anas M, Liao F, Verma KK, Sarwar MA, Mahmood A, Chen ZL, Li Q, Zeng XP, Li YR (2020) Fate of nitrogen in agriculture and environment: agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol Res 53:1–20
Anders C, Niewoehner O, Duerst A, Jinek M (2014) Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature 513:569–573
Appels R, Eversole K, Feuille C, Keller B, Rogers J, Stein N (2018) The International Wheat Genome Sequencing Consortium (IWGSC). Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361:10–1126
Arndell T, Sharma N, Langridge P, Baumann U, Watson-Haigh NS, Whitford R (2019) gRNA validation for wheat genome editing with the CRISPR-Cas9 system. BMC Biotechnol 19:1–12
Arslan E, Agar G, Aydin M (2021) Humic acid as a biostimulant in improving drought tolerance in wheat: The expression patterns of drought-related genes. Plant Mol Biol Rep 39:508–519
Awan MJA, Pervaiz K, Rasheed A, Amin I, Saeed NA, Dhugga KS, Mansoor S (2022) Genome edited wheat-current advances for the second green revolution. Biotechnol Adv 60:108006
Aydin M, Tosun M, Haliloglu K (2011) Plant regeneration in wheat mature embryo culture. Afr J Biotechnol 10:15749–15755
Aydin M, Pour AH, Haliloğlu K, Tosun M (2016) Effect of polyamines on somatic embryogenesis via mature embryo in wheat. Turk J Biol 40:1178–1184
Aydin M, Arslan E, Yigider E, Taspinar MS, Agar G (2021) Protection of Phaseolus vulgaris L. from Herbicide 2, 4-D results from exposing seeds to humic acid. Arab J Sci Eng 46:163–173
Badhan S, Ball AS, Mantri N (2021) First report of CRISPR/Cas9 mediated DNA-free editing of 4CL and RVE7 genes in chickpea protoplasts. Int J Mol Sci 22:396
Bae S, Park J, Kim JS (2014) Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30:1473–1475
Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712
Baskar G, Aiswarya R (2018) Overview on mitigation of acrylamide in starchy fried and baked foods. J Sci Food Agric 98:4385–4394
Bettgenhaeuser J, Krattinger SG (2019) Rapid gene cloning in cereals. Theor Appl Genet 132:699–711
Blin K, Pedersen LE, Weber T, Lee SY (2016) CRISPy-web: an online resource to design sgRNAs for CRISPR applications. Synth Syst Biotechnol 1:118–121
Borrelli VM, Brambilla V, Rogowsky P, Marocco A, Lanubile A (2018) The enhancement of plant disease resistance using CRISPR/Cas9 technology. Front Plant Sci 9:1245
Brauer EK, Balcerzak M, Rocheleau H, Leung W, Schernthaner J, Subramaniam R, Ouellet T (2020) Genome editing of a deoxynivalenol-induced transcription factor confers resistance to Fusarium graminearum in wheat. Mol Plant Microbe Interact 33:553–560
Caruso SM, Quinn PM, da Costa BL, Tsang SH (2022) CRISPR/Cas therapeutic strategies for autosomal dominant disorders. Clin Drug Investig 132:e158287
Carvalho CHS, Zehr UB, Gunaratna N, Anderson J, Kononowicz HH, Hodges TK, Axtell JD (2004) Agrobacterium-mediated transformation of sorghum: factors that affect transformation efficiency. Genet Mol Biol 27:259–269
Chang H, Yi B, Ma R, Zhang X, Zhao H, Xi Y (2016) CRISPR/cas9, a novel genomic tool to knock down microRNA in vitro and in vivo. Sci Rep 6:1–12
Chanyalew S, Assefa K, Tadele Z (2019) Tef [Eragrostis tef (Zucc.) Trotter] Breeding. Adv Plant Breed Strateg Cereals 5:373–403
Chaudhary J, Alisha A, Bhatt V, Chandanshive S, Kumar N, Mir Z, Kumar A, Yadav SK, Shivaraj SM, Sonah H, Deshmukh R (2019) Mutation breeding in tomato: Advances, applicability and challenges. Plants 8:128
Chauhan H, Khurana P (2017) Wheat genetic transformation using mature embryos as explants. In Wheat Biotechnology. Humana Press, New York pp. 153–167
Chavez M, Chen X, Finn PB, Qi LS (2022) Advances in CRISPR therapeutics. Nat Rev Nephrol 1–14
Chen K, Wang Y, Zhang R, Zhang H, Gao C (2019a) CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu Rev Plant Biol 70:667–697
Chen W, Zhang H, Zhang Y, Wang Y, Gan J, Ji Q (2019b) Molecular basis for the PAM expansion and fidelity enhancement of an evolved Cas9 nuclease. PLoS Biol 17:e3000496
Chen Z, Ke W, He F, Chai L, Cheng X, Xu H, Wang X, Du D, Zhao Y, Chen X, Xing J, Xin M, Guo W, Hu Z, Su Z, Liu J, Peng H, Yao Y, Sun Q, Ni Z (2022) A single nucleotide deletion in the third exon of FT-D1 increases the spikelet number and delays heading date in wheat (Triticum aestivum L.). Plant Biotechnol J 20:920
Chen Z, Debernardi JM, Dubcovsky J, Gallavotti A (2022a) Recent advances in crop transformation technologies. Nat Plants 1–9
Cheng M, Fry JE, Pang S, Zhou H, Hironaka CM, Duncan DR, Conner TW, Wan Y (1997) Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol 115:971–980
Cheng X, Gao C, Liu X, Xu D, Pan X, Gao W, Yan S, Yao H, Cao J, Min X, Lu J, Chang C, Zhang H, Ma C (2022) Characterization of the wheat VQ protein family and expression of candidate genes associated with seed dormancy and germination. BMC Plant Biol 22:1–18
Chylinski K, Le Rhun A, Charpentier E (2013) The tracrRNA and Cas9 families of type II CRISPR-Cas immunity systems. RNA Biol 10:726–737
Cram D, Kulkarni M, Buchwaldt M, Rajagopalan N, Bhowmik P, Rozwadowski K, Parkin İAP, Sharpe AG, Kagale S (2019) WheatCRISPR: a web-based guide RNA design tool for CRISPR/Cas9-mediated genome editing in wheat. BMC Plant Biol 19:1–8
Curtis BC, Rajaram S, Gómez Macpherson H (2002) Bread wheat: improvement and production. Food and Agriculture Organization of the United Nations (FAO)
Dai SH, Zheng P, Marmey P, Zhang SP, Tian WZ, Chen SY, Beachy RN, Fauquet C (2001) Comparative analysis of transgenic rice plants obtained by Agrobacterium-mediated transformation and particle bombardment. Mol Breed 7:25–33
Dan Y (2008) Biological functions of antioxidants in plant transformation. In Vitro Cell Dev Biol 44:149–161
Das D, Reddy M, Upadhyaya K, Sopory S (2002) An efficient leaf-disc culture method for the regeneration via somatic embryogenesis and transformation of grape (Vitis vinifera L.). Plant Cell Rep 20:999–1005
Dayani S, Sabzalian MR, Mazaheri-Tirani M (2019) CRISPR/Cas9 genome editing in bread wheat (Triticum aestivum L.) genetic improvement. Adv Plant Breed Strateg Cereals 5:453–469
De Saeger J, Park J, Chung HS, Hernalsteens JP, Van Lijsebettens M, Inzé D, Montagu MV, Depuydt S (2021) Agrobacterium strains and strain improvement: Present and outlook. Biotechnol Adv 53:107677
Ding L, Li S, Gao J, Wang Y, Yang G, He G (2009) Optimization of Agrobacterium-mediated transformation conditions in mature embryos of elite wheat. Mol Biol Rep 36:29–36
Ding Y, Li H, Chen LL, Xie K (2016) Recent advances in genome editing using CRISPR/Cas9. Front Plant Sci 7:703
Doudna JA, Charpentier E (2014) The new frontier of genome engineering with CRISPR-Cas9. Science 346:1258096
Erturk FA, Agar G, Arslan E, Nardemir G (2015) Analysis of genetic and epigenetic effects of maize seeds in response to heavy metal (Zn) stress. Environ Sci Pollut Res 22:10291–10297
Esmail SM, Omar GE, Mourad AM (2023) In-Depth Understanding of the Genetic Control of Stripe Rust Resistance (Puccinia striiformis f. sp. tritici) Induced in Wheat (Triticum aestivum) by Trichoderma asperellum T34. Plant Dis 107:457–472
FAO (2022) The state of food security and nutrition in the world 2020: transforming food systems for affordable healthy diets. Food & Agriculture Org. 2022. Available from: https://www.fao.org/3/ca9692en/online/ca9692en.html
Fedorova I, Vasileva A, Selkova P, Abramova M, Arseniev A, Pobegalov G, Kazalov M, Musharova O, Goryanin I, Artamonova D, Zyubko T, Shmakov S, Artamonova T, Khodorkovskii M, Severinov K (2020) PpCas9 from Pasteurella pneumotropica—a compact Type II-C Cas9 ortholog active in human cells. Nucleic Acids Res 48:12297–12309
Gao XQ, Wang N, Wang XL, Zhang XS (2019) Architecture of wheat inflorescence: insights from rice. Trends Plant Sci 24:802–809
Garneau JE, Dupuis M-E, Villion M, Romero DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadán AH, Moineau S (2010) The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468:67–71
Gasiunas G, Barrangou R, Horvath P, Siksnys V (2012) Cas9–crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. PNAS 109:E2579–E2586
Gil-Humanes J, Wang Y, Liang Z, Shan Q, Ozuna CV, Sánchez-León S, Baltes NJ, Starker C, Barro F, Gao C, Voytas DF (2017) High-efficiency gene targeting in hexaploid wheat using DNA replicons and CRISPR/Cas9. Plant J 89:1251–1262
González Castro N, Bjelic J, Malhotra G, Huang C, Alsaffar SH (2021) Comparison of the feasibility, efficiency, and safety of genome editing technologies. Int J Mol Sci 22:10355
Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F (2018) Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 360:439–444
Gostimskaya I (2022) CRISPR–Cas9: A History of Its Discovery and Ethical Considerations of Its Use in Genome Editing. Biochem (Moscow) 87:777–788
Greer MS, Kovalchuk I, Eudes F (2009) Ammonium nitrate improves direct somatic embryogenesis and biolistic transformation of Triticum aestivum. N Biotechnol 26:44–52
Guha TK, Edgell DR (2017) Applications of alternative nucleases in the age of CRISPR/Cas9. Int J Mol Sci 18:2565
Gupta D, Bhattacharjee O, Mandal D, Sen MK, Dey D, Dasgupta A, Kazi TA, Gupta R, Sinharoy S, Acharya K, Chattopadhyay D, Ravichandiran V, Roy S, Ghosh D (2019) CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sci 232:116636
Gupta A, Hua L, Zhang Z, Yang B, Li W (2022) CRISPR‐induced miRNA156‐recognition element mutations in TaSPL13 improve multiple agronomic traits in wheat. Plant Biotechnol J
Hahn F, Sanjurjo Loures L, Sparks CA, Kanyuka K, Nekrasov V (2021) Efficient CRISPR/Cas-mediated targeted mutagenesis in spring and winter wheat varieties. Plants 10:1481
Hajiahmadi Z, Movahedi A, Wei H, Li D, Orooji Y, Ruan H, Zhuge Q (2019) Strategies to increase on-target and reduce off-target effects of the CRISPR/Cas9 system in plants. Int J Mol Sci 20:3719
Hao X, Song S, Zhong Q, Hajano JUD, Guo J, Wu Y (2021) Rescue of an Infectious cDNA Clone of Barley Yellow Dwarf Virus-GAV. Phytopathology 111:2383–2391
Hasan Nudin NF, van Kronenburg B, Tinnenbroek I, Krens F (2015) The importance of salicylic acid and an improved plant condition in determining success in Agrobacterium-mediated transformation. In XXV International EUCARPIA Symposium Section Ornamentals: Crossing Borders 1087:65–69
Hashemi A (2020) CRISPR–Cas9/CRISPRi tools for cell factory construction in E. coli. World J Microbiol Biotechnol 36:1–13
Hayta S, Smedley MA, Demir SU, Blundell R, Hinchliffe A, Atkinson N, Harwood WA (2019) An efficient and reproducible Agrobacterium-mediated transformation method for hexaploid wheat (Triticum aestivum L.). Plant Methods 15:1–15
Hayta S, Smedley MA, Clarke M, Forner M, Harwood WA (2021) An efficient Agrobacterium-mediated transformation protocol for hexaploid and tetraploid wheat. Curr Protoc 1:e58
He Y, Zhu M, Wang L, Wu J, Wang Q, Wang R, Zhao Y (2018) Programmed self-elimination of the CRISPR/Cas9 construct greatly accelerates the isolation of edited and transgene-free rice plants. Mol Plant 11:1210–1213
He F, Wang C, Sun H, Tian S, Zhao G, Liu C, Wan C, Guo J, Huang X, Zhan G, Yu X, Kang Z, Guo J (2022) Simultaneous editing of three homoeologs of TaCIPK14 confers broad-spectrum resistance to stripe rust in wheat. Plant Biotechnol J 21:354–368
Hesami M, Jones AMP (2021) Modeling and optimizing callus growth and development in Cannabis sativa using random forest and support vector machine in combination with a genetic algorithm. Appl Microbiol Biotechnol 105:5201–5212
Hiei Y, Komari T, Kubo T (1997) Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol Biol 35:205–218
Hillary VE, Ceasar SA (2023) A Review on the Mechanism and Applications of CRISPR/Cas9/Cas12/Cas13/Cas14 Proteins Utilized for Genome Engineering. Mol Biotechnol 65:311–325
Hu J, Yu M, Chang Y, Tang H, Wang W, Du L, Wang K, Yan Y, Ye X (2022) Functional analysis of TaPDI genes on storage protein accumulation by CRISPR/Cas9 edited wheat mutants. Int J Biol Macromol 196:131–143
Ibrahim S, Saleem B, Rehman N, Zafar SA, Naeem MK, Khan MR (2022) CRISPR/Cas9 mediated disruption of Inositol Pentakisphosphate 2-Kinase 1 (TaIPK1) reduces phytic acid and improves iron and zinc accumulation in wheat grains. J Adv Res 37:33–41
Ilhan E, Kasapoğlu AG, Muslu S, Macit M, Sezer B, Mevlütoğulları A, Guler D, Aydın M, Eksi F, Aydın M (2021) CRISPR/Cas9 and its Application in Plant Biotechnology. Nat pro Biotech 1:118–143
Isaacson W (2021) The Code Breaker: Jennifer Doudna, Gene Editing, and the Future of the Human Race, Simon & Schuster (New York, USA)
Ishida Y, Saito H, Ohta S, Hiei Y, Komari T, Kumashiro T (1996) High efficiency transformation of maize (Zea mays) mediated by Agrobacterium tumefaciens. Nat Biotechnol 4:745–750
Ishida Y, Tsunashima M, Hiei Y, Komari T (2015) Wheat (Triticum aestivum L.) transformation using immature embryos. Agrobacterium Protocols: 1:189–198
Ishino Y, Shinagawa H, Makino K, Amemura M, Nakatura A (1987) Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isoenzyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol 169:5429–5433
Jangra A, Chaturvedi S, Kumar N, Singh H, Sharma V, Thakur M, Tiwari S, Chhokar V (2022) Polyamines: The Gleam of Next-Generation Plant Growth Regulators for Growth, Development, Stress Mitigation, and Hormonal Crosstalk in Plants—A Systematic Review. J Plant Growth Regul 1–25
Janik E, Niemcewicz M, Ceremuga M, Krzowski L, Saluk-Bijak J, Bijak M (2020) Various aspects of a gene editing system—crispr–cas9. Int J Mol Sci 21:9604
Jarvis P (2020) Environmental technology for the sustainable development goals (SDGs). Environ Technol 41:2155–2156
Jayavaradhan R, Pillis DM, Goodman M, Zhang F, Zhang Y, Andreassen PR, Malik P (2019) CRISPR-Cas9 fusion to dominant-negative 53BP1 enhances HDR and inhibits NHEJ specifically at Cas9 target sites. Nat Commun 10:2866
Jiang F, Doudna JA (2017) CRISPR-Cas9 structures and mechanisms. Annu Rev Biophys 46:505–529
Jiang W, Zhou H, Bi H, Fromm M, Yang B, Weeks DP (2013a) Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res 41:e188
Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA (2013b) RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat Biotechnol 31:233–239
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
Jones HD, Doherty A, Wu H (2005) Review of methodologies and a protocol for the Agrobacterium-mediated transformation of wheat. Plant Methods 1:1–9
Jouanin A, Borm T, Boyd LA, Cockram J, Leigh F, Santos BA, Visser RGF, Smulders MJM (2019) Development of the GlutEnSeq capture system for sequencing gluten gene families in hexaploid bread wheat with deletions or mutations induced by γ-irradiation or CRISPR/Cas9. J Cereal Sci 88:157–166
Kapusi E, Stoger E (2022) Molecular farming in seed crops: gene transfer into barley (Hordeum vulgare) and wheat (Triticum aestivum). Methods Mol Biol 2480:49–60
Karakas FP, Keskin CN, Agil F, Zencirci N (2022) Phenolic composition and antioxidant potential in Turkish einkorn, emmer, durum, and bread wheat grain and grass. S Afr J Bot 149:407–415
Ke W, Shi L, Liang X, Zhao P, Wang W, Liu J, Chang Y, Hiei Y, Yanagihara C, Du P, Ishida Y, Guo Y (2021) The gene TaCB1 overcomes genotype dependency in wheat genetic transformation
Khanna H, Daggard G (2003) Agrobacterium tumefaciens-mediated transformation of wheat using a superbinary vector and a polyamine-supplemented regeneration medium. Plant Cell Rep 21:429–436
Kharb P, Chaudhary R, Tuteja N, Kaushik P (2022) A Genotype-Independent, Simple, Effective and Efficient in Planta Agrobacterium-Mediated Genetic Transformation Protocol. Methods Protoc 5:69
Khurana J, Chugh A, Khurana P (2002) Regeneration from mature and immature embryos and transient gene expression via Agrobacterium mediated transformation in emmer wheat (Triticum diccocum Schuble). Indian J Exp Biol 40:1295–1303
Kim KH, Kim JY (2021) Understanding Wheat Starch Metabolism in Properties, Environmental Stress Condition, and Molecular Approaches for Value-Added Utilization. Plants 10:2282
Kim E, Koo T, Park SW, Kim D, Kim K, Cho HY, Song DW, Lee KJ, Jung MH, Kim S, Kim JH, Kim JH, Kim JS (2017) In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat Commun 8:1–12
Kim D, Alptekin B, Budak H (2018) CRISPR/Cas9 genome editing in wheat. Funct Integr Genom 18:31–41
Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, Joung JK (2016) High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529:490–495
Koonin EV, Makarova KS, Zhang F (2017) Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol 37:67–78
Kou H, Zhang Z, Yang Y, Wei C, Xu L, Zhang G (2023) Advances in the Mining of Disease Resistance Genes from Aegilops tauschii and the Utilization in Wheat. Plants 12:880
Kuluev BR, Mikhailova EV, Kuluev AR, Galimova AA, Zaikina EA, Khlestkina EK (2022) Genome Editing in Species of the Tribe Triticeae with the CRISPR/Cas System. Mol Biol 56:885–901
Kumar V, Jain M (2015) The CRISPR-Cas system for plant genome editing: Advances and opportunities. J Exp Bot 66:47–57
Kumar R, Mamrutha HM, Kaur A, Grewal A (2017a) Synergistic effect of cefotaxime and timentin to suppress the Agrobacterium overgrowth in wheat (Triticum aestivum L.) transformation. Asian J Microbiol Biotechnol Environm Sci 19:961–967
Kumar A, Lal MK, Kar SS, Nayak L, Ngangkham U, Samantaray S, Sharma SG (2017b) Bioavailability of iron and zinc as affected by phytic acid content in rice grain. J Food Biochem 41:e12413
Kumar R, Mamrutha HM, Kaur A, Venkatesh K, Sharma D, Singh GP (2019) Optimization of Agrobacterium-mediated transformation in spring bread wheat using mature and immature embryos. Mol Biol Rep 46:1845–1853
Labun K, Montague TG, Gagnon JA, Thyme SB, Valen E (2016) CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res 44:W272–W276
Langner T, Kamoun S, Belhaj K (2018) CRISPR crops: plant genome editing toward disease resistance. Annu Rev Phytopathol 56:479–512
Lawrenson T, Shorinola O, Stacey N, Li C, Østergaard L, Patron N, Uauy C, Harwood W (2015) Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol 16:258
Lee K, Zhang Y, Kleinstiver BP, Guo JA, Aryee MJ, Miller J, Malzahn A, Zarecor S, Lawrence-Dill CJ, Joung JK, Qi Y, Wang K (2019) Activities and specificities of CRISPR/Cas9 and Cas12a nucleases for targeted mutagenesis in maize. Plant Biotechnol J 17:362–372
Lee BR, La VH, Park SH, Mamun MA, Bae DW, Kim TH (2022) Dimethylthiourea Alleviates Drought Stress by Suppressing Hydrogen Peroxide-Dependent Abscisic Acid-Mediated Oxidative Responses in an Antagonistic Interaction with Salicylic Acid in Brassica napus Leaves. Antioxidants 11:2283
Lei Y, Lu L, Liu HY, Li S, Xing F, Chen LL (2014) CRISPR-P: a web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol Plant 7:1494–1496
Li X, Krasnyanski SF, Korban SS (2002) Optimization of the uidA gene transfer into somatic embryos of rose via Agrobacterium tumefaciens. Plant Physiol Biochem 40:453–459
Li JF, Norville JE, Aach J, McCormack M, Zhang D, Bush J, Church GM, Sheen J (2013) Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol 31:688–691
Li GL, Quan R, Wang HQ, Ruan XF, Mo JX, Zhong CL, Yang HQ, Li ZC, Gu T, Liu D, Wu ZF, Cai GY, Zhang XW (2019a) Inhibition of KU70 and KU80 by CRISPR interference, not NgAgo interference, increases the efficiency of homologous recombination in pig fetal fibroblasts. J Integr Agric 18:438–448
Li J, Li Y, Ma L (2019b) CRISPR/Cas9-based genome editing and its applications for functional genomic analyses in plants. Small Methods 3:1800473
Li J, Wang Z, He G, Ma L, Deng XW (2020) CRISPR/Cas9-mediated disruption of TaNP1 genes results in complete male sterility in bread wheat. J Genet Genom 47:263–272
Li J, Jiao G, Sun Y, Chen J, Zhong Y, Yan L, Jiang D, Ma Y, Xia L (2021a) Modification of starch composition, structure and properties through editing of TaSBEIIa in both winter and spring wheat varieties by CRISPR/Cas9. Plant Biotechnol J 19:937–951
Li J, Li Y, Ma L (2021) Recent advances in CRISPR/Cas9 and applications for wheat functional genomics and breeding. aBIOTECH 2:1–11
Li S, Lin D, Zhang Y, Deng M, Chen Y, Lv B, Li B, Lei Y, Wang Y, Zhao L, Liang Y, Liu J, Chen K, Liu Z, Xiao J, Qiu JL, Gao C (2022) Genome-edited powdery mildew resistance in wheat without growth penalties. Nature 602:455–460
Liang X, Bie X, Qiu Y, Wang K, Yang Z, Jia Y, Xu Z, Yu M, Du L, Lin Z, Ye X (2022) Development of powdery mildew resistant derivatives of wheat variety Fielder for use in genetic transformation. Crop J
Liu L, Fan XD (2014) CRISPR–Cas system: a powerful tool for genome engineering. Plant Mol Biol 85:209–218
Liu H, Wei Z, Dominguez A, Li Y, Wang X, Qi LS (2015) CRISPR-ERA: a comprehensive design tool for CRISPR-mediated gene editing, repression and activation. Bioinformatics 31:3676–3678
Liu X, Wu S, Xu J, Sui C, Wei J (2017) Application of CRISPR/Cas9 in plant biology. Acta Pharm Sin B 7:292–302
Liu J, Nannas NJ, Fu FF, Shi J, Aspinwall B, Parrott WA, Dawe RK (2019) Genome-scale sequence disruption following biolistic transformation in rice and maize. Plant Cell 31:368–383
Liu C, Zhong Y, Qi X, Chen M, Liu Z, Chen C, Tian X, Li J, Jiao Y, Wang D, Wang Y, Li M, Xin M, Liu W, Jin W, Chen S (2020a) Extension of the in vivo haploid induction system from diploid maize to hexaploid wheat. Plant Biotechnol J 18:316
Liu H, Wang K, Jia Z, Gong Q, Lin Z, Du L, Pei X, Ye X (2020b) Efficient induction of haploid plants in wheat by editing of TaMTL using an optimized Agrobacterium-mediated CRISPR system. J Exp Bot 71:1337–1349
Liu Q, Yang F, Zhang J, Liu H, Rahman S, Islam S, Ma W, She M (2021) Application of CRISPR/Cas9 in crop quality improvement. Int J Mol Sci 22:4206
Liu H, Chen W, Li Y, Sun L, Chai Y, Chen H, Huang C (2022) CRISPR/Cas9 technology and its utility for crop improvement. Int J Mol Sci 23:10442
Liu D, Yang H, Zhang Z, Chen Q, Guo W, Rossi V, Xin M, Du J, Hu Z, Liu J, Peng H, Ni Z, Sun Q, Yao Y (2023) An elite γ-gliadin allele improves end-use quality in wheat. New Phytol. https://doi.org/10.1111/nph.18722
Loureiro A, da Silva GJ (2019) Crispr-cas: Converting a bacterial defence mechanism into a state-of-the-art genetic manipulation tool. Antibiotics 8:18
Lv J, Yu K, Wei J, Gui H, Liu C, Liang D, Wang Y, Zhou H, Carlin R, Rich R, Lu T, Que Q, Wang WC, Zhang X, Kelliher T (2020) Generation of paternal haploids in wheat by genome editing of the centromeric histone CENH3. Nat Biotechnol 38:1397–1401
Ma C, Ha K, Kim MS, Noh YW, Lin H, Tang L, Chen H, Han S, Zhang P (2018) The anaphase promoting complex promotes NHEJ repair through stabilizing Ku80 at DNA damage sites. Cell Cycle 17:1138–1145
Maharajan T, Krishna TP, Rakkammal K, Ceasar SA, Ramesh M (2022) Application of CRISPR/Cas system in cereal improvement for biotic and abiotic stress tolerance. Planta 256:1–17
Mahfouz MM, Piatek A, Stewart CN Jr (2014) Genome engineering via TALENs and CRISPR/Cas9 systems: challenges and perspectives. Plant Biotechnol J 12:1006–1014
Mahler M, Costa AR, van Beljouw SP, Fineran PC, Brouns SJ (2022) Approaches for bacteriophage genome engineering. Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2022.08.008
Makarova KS, Grishin NV, Shabalina SA, Wolf YI, Koonin EV (2006) A putative RNA-interferencebased immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol Direct 1:7
Makarova KS, Wolf YI, Alkhnbashi OS et al (2015) An updated evolutionary classification of CRISPR–Cas systems. Nat Rev Microbiol 13:722–736
Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, Brouns SJJ, Charpentier E, Cheng D, Haft DH, Horvath P, Moineau S, Mojica FJM, Scott D, Shah SA, Siksnys V, Terns MP, Venclovas Č, White MF, Yakunin AF, Yan W, Zhang F, Garrett RA, Backofen R, van der Oost J, Barrangou R, Koonin EV (2020) Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol 18:67–83
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM (2013) RNA-guided human genome engineering via Cas9. Sci 339:823–826
Manghwar H, Li B, Ding X, Hussain A, Lindsey K, Zhang X, Jin S (2020) CRISPR/Cas systems in genome editing: methodologies and tools for sgRNA design, off-target evaluation, and strategies to mitigate off-target effects. Adv Sci 7:1902312
Marraffini LA, Sontheimer EJ (2008) CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322:1843–1845
Marwein R, Debbarma J, Sarki YN, Baruah I, Saikia B, Boruah HPD, Velmurugan N, Chikkaputtaiah C, (2019) Genetic engineering/genome editing approaches to modulate signaling processes in abiotic stress tolerance. Plant Signal Mol s 63–82. Woodhead Publishing
McLaughlin JE, Darwish NI, Garcia-Sanchez J, Tyagi N, Trick HN, McCormick S, Macky RD, Tumer NE (2021) A lipid transfer protein has antifungal and antioxidant activity and suppresses Fusarium head blight disease and DON accumulation in transgenic wheat. Phytopathology 111:671–683
Mühleisen J, Piepho HP, Maurer HP, Longin CFH, Reif JC (2014) Yield stability of hybrids versus lines in wheat, barley, and triticale. Theor Appl Genet 127:309–316
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Mushke R, Yarra R, Kirti PB (2019) Improved salinity tolerance and growth performance in transgenic sunflower plants via ectopic expression of a wheat antiporter gene (TaNHX2). Mol Biol Rep 46:5941–5953
Nasir A, Caetano-Anollés G (2015) A phylogenomic data-driven exploration of viral origins and evolution. Sci Adv 1:e1500527
Nassar M, Nassar R, Maki H, Al-Yagoob A, Hachim M, Senok A, Williams D, Hiraishi N (2021) Phytic acid: Properties and potential applications in dentistry. Front Mater 8:638909
Natalini A, Acciarri N, Cardi T (2021) Breeding for nutritional and organoleptic quality in vegetable crops: The case of tomato and cauliflower. Agriculture 11:606
Nekrasov V, Staskawicz B, Weigel D, Jones JD, Kamoun S (2013) Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat Biotechnol 31:691–693
Nerkar G, Devarumath S, Purankar M, Kumar A, Valarmathi R, Devarumath R, Appunu C (2022) Advances in crop breeding through precision genome editing. Front Genet 13:880195
Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata S, Dohmae N, Ishitani R, Zhang F, Nureki O (2015) Crystal Structure of Cas9 in Complex with gRNA and target DNA. 156:935–949.
Nonaka S, Yuhashi KI, Takada K, Sugaware M, Minamisawa K, Ezura H (2008) Ethylene production in plants during transformation suppresses vir gene expression in Agrobacterium tumefaciens. New Phytol 178:647–656
Nussenzweig PM, Marraffini LA (2020) Molecular mechanisms of CRISPR-Cas immunity in bacteria. Annu Rev Genet 93–120
Ober ES, Alahmad S, Cockram J, Forestan C, Hickey LT, Kant J, Watt M (2021) Wheat root systems as a breeding target for climate resilience. Theor Appl Genet 134:1645–1662
O’Connell MR (2019) Molecular mechanisms of RNA targeting by Cas13-containing type VI CRISPR–Cas systems. J Mol Biol 431:66–87
Okada A, Arndell T, Borisjuk N, Sharma N, Watson-Haigh NS, Tucker EJ, Baumann U, Langridge P, Whitford R (2019) CRISPR/Cas9-mediated knockout of Ms1 enables the rapid generation of male-sterile hexaploid wheat lines for use in hybrid seed production. Plant Biotechnol J 17:1905–1913
Opabode JT (2006) Agrobacterium-mediated transformation of plants: emerging factors that influence efficiency. Biotechnol Mol Biol Rev 1:12–20
Ozturk A, Erdem E, Aydin M, Karaoglu MM (2022) The effects of drought after anthesis on the grain quality of bread wheat depend on drought severity and drought resistance of the variety. Cereal Res Commun 50:105–116
Park DH, Mirabella R, Bronstein PA, Preston GM, Haring MA, Lim CK, Collmer A, Schuurink RC (2010) Mutations in γ-aminobutyric acid (GABA) transaminase genes in plants or Pseudomonas syringae reduce bacterial virulence. Plant J 64:318–330
Park J, Bae S, Kim JS (2015) Cas-Designer: a web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 31:4014–4016
Parmar SS, Jaiwal PK, Agarwal N, Kaushik SK (2015) Optimization and validation of Agrobacterium-mediated genetic transformation for commercial indian bread wheat (Triticum aestivum L.) Cultivars using mature embryo. Cell Tissue Res 15:5301
Parry MA, Madgwick PJ, Bayon C, Tearall K, Hernandez-Lopez A, Baudo M, Rakszegi M, Hamada W, Al-Yassin A, Ouabbou H, Labhilili M, Phillips AL (2009) Mutation discovery for crop improvement. J Exp Bot 60:2817–2825
Partier A, Gay G, Tassy C, Beckert M, Feuillet C, Barret P (2017) Molecular and FISH analyses of a 53-kbp intact DNA fragment inserted by biolistics in wheat (Triticum aestivum L.) genome. Plant Cell Rep 36:1547–1559
Pellegrineschi A, Noguera LM, Skovmand B, Brito RM, Velazquez L, Salgado MM, Hernandez R, Warburton M, Hoisington D (2002) Identification of highly transformable wheat genotypes for mass production of fertile transgenic plants. Genome 45:421–430
Pereira R, Oliveira J, Sousa M (2020) Bioinformatics and computational tools for next-generation sequencing analysis in clinical genetics. J Clin Med 9:132
Perez Rojo F, Nyman RKM, Johnson AAT, Navarro P, Ryan MH, Erskine W, Kaur P (2018) CRISPR-Cas systems: ushering in the new genome editing era. Bioengineered 9:214–221
Pérez-Piñeiro P, Gago J, Landín M, Gallego PP (2012) Agrobacterium-mediated transformation of wheat: general overview and new approaches to model and identify the key factors involved. Transgenic Plants-Advances and Limitations. Rijeka, Croatia: Intech Open Access Publisher 326
Pickar-Oliver A, Gersbach CA (2019) The next generation of CRISPR–Cas technologies and applications. Nat Rev Mol 20:490–507
Pinilla-Redondo R, Mayo-Muñoz D, Russel J, Garrett RA, Randau L, Sørensen SJ, Shah SA (2020) Type IV CRISPR–Cas systems are highly diverse and involved in competition between plasmids. Nucleic Acids Res 48:2000–2012
Prykhozhij SV, Rajan V, Gaston D, Berman JN (2015) Correction: CRISPR MultiTargeter: a web tool to find common and unique CRISPR single guide RNA targets in a set of similar sequences. PLoS ONE 10:e0138634
Puren H, Reddy BJ, Sarma A, Singh SK, Ansari WA (2023) Molecular Approaches for Biofortification of Cereal Crops. In Biofortification in Cereals: Progress and Prospects 21–58 Singapore: Springer Nature Singapore
Qin G, Wu S, Zhang L, Li Y, Liu C, Yu J, Deng L, Xiao G, Zhang Z (2022) An Efficient Modular Gateway Recombinase-Based Gene Stacking System for Generating Multi-Trait Transgenic Plants. Plants 11:488
Qiu F, Xing S, Xue C, Liu J, Chen K, Chai T, Gao C (2022) Transient expression of a TaGRF4-TaGIF1 complex stimulates wheat regeneration and improves genome editing. Sci China Life Sci 65:731–738
Que Q, Elumalai S, Li X, Zhong H, Nalapalli S, Schweiner M, Fei X, Nuccio M, Kelliher T, Gu W, Chen Z, Chilton MDM (2014) Maize transformation technology development for commercial event generation. Front Plant Sci 5:279
Raffan S, Sparks C, Huttly A, Hyde L, Martignago D, Mead A, Hanley SJ, Wilkinson PA, Barker G, Edwards KJ, Curtis TY, Usher S, Kosik O, Halford NG (2021) Wheat with greatly reduced accumulation of free asparagine in the grain, produced by CRISPR/Cas9 editing of asparagine synthetase gene TaASN2. Plant Biotechnol J 19:1602–1613
Raman V, Rojas CM, Vasudevan B, Dunning K, Kolape J, Oh S, Yun J, Yang L, Li G, Pant BD, Jiang Q, Mysore KS (2022) Agrobacterium expressing a type III secretion system delivers Pseudomonas effectors into plant cells to enhance transformation. Nat Commun 13:1–14
Ramesh P, Mallikarjuna G, Sameena S, Kumar A, Gurulakshmi K, Reddy BV, Reddy PCO, Sekhar AC (2020) Advancements in molecular marker technologies and their applications in diversity studies. J Biosci. https://doi.org/10.1007/s12038-020-00089-4
Rao MJ, Wang L (2021) CRISPR/Cas9 technology for improving agronomic traits and future prospective in agriculture. Planta 254:1–16
Reegan AD, Ceasar SA, Paulraj MG, Ignacimuthu S, Al-Dhabi NA (2016) Current status of genome editing in vector mosquitoes: a review. Biosci Trends 10:424–432
Ren Q, Sretenovic S, Liu S, Tang X, Huang L, He Y, Liu L, Guo Y, Zhong Z, Liu G, Cheng Y, Zheng X, Pan C, Yin D, Zhang Y, Li W, Qi L, Li C, Qi Y, Zhang Y (2021) PAM-less plant genome editing using a CRISPR–SpRY toolbox. Nat Plants 7:25–33
Riesenberg S, Maricic T (2018) Targeting repair pathways with small molecules increase precise genome editing in pluripotent stem cells. Nat Commun 9:2164
Rodríguez-Leal D, Lemmon ZH, Man J, Bartlett ME, Lippman ZB (2017) Engineering quantitative trait variation for crop improvement by genome editing. Cell 171:470–480
Rozov SM, Permyakova NV, Deineko EV (2019) The problem of the low rates of CRISPR/Cas9-mediated knock-ins in plants: approaches and solutions. Int J Mol Sci 20:3371
Russel J, Pinilla-Redondo R, Mayo-Muñoz D, Shah SA, Sørensen SJ (2020) CRISPRCasTyper: automated identification, annotation, and classification of CRISPR-Cas Loci. CRISPR J 3:462–469
Ryu SM, Hur JW, Kim K (2019) Evolution of CRISPR towards accurate and efficient mammal genome engineering. BMB Rep 52:475
Sabooni N, Gharaghani A (2022) Induced polyploidy deeply influences reproductive life cycles, related phytochemical features, and phytohormonal activities in blackberry species. Front Plant Sci. https://doi.org/10.3389/fpls.2022.938284
Saeed S, Usman B, Shim SH, Khan SU, Nizamuddin S, Saeed S, Shoaib Y, Jeon JS, Jung KH (2022) CRISPR/Cas-mediated editing of cis-regulatory elements for crop improvement. Plant Sci 324:111435
Sahu PK, Sao R, Mondal S, Vishwakarma G, Gupta SK, Kumar V, Sing S, Sharma D, Das BK (2020) Next generation sequencing based forward genetic approaches for identification and mapping of causal mutations in crop plants: A comprehensive review. Plants 9:1355
Salas M, Park S, Srivatanakul M, Smith R (2001) Temperature influence on stable T-DNA integration in plant cells. Plant Cell Rep 20:701–705
Samson JE, Magadan AH, Moineau S (2015) The CRISPR-Cas immune system and genetic transfers: reaching an equilibrium. Microbiol Spectr 3:3–1
Sánchez-León S, Gil-Humanes J, Ozuna CV, Giménez MJ, Sousa C, Voytas DF, Barro F (2018) Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol J 16:902–910
Sapranauskas R, Gasiunas G, Fremaux C, Barrangou R, Horvath P, Siksnys V (2011) The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res 39:9275–9282
Sarkhel S, Roy A (2022) Phytic acid and its reduction in pulse matrix: Structure–function relationship owing to bioavailability enhancement of micronutrients. J Food Process Eng 45:e14030
Savadi S, Prasad P, Kashyap PL, Bhardwaj SC (2018) Molecular breeding technologies and strategies for rust resistance in wheat (Triticum aestivum) for sustained food security. Plant Pathol 67:771–791
Scheben A, Wolter F, Batley J, Puchta H, Edwards D (2017) Towards CRISPR/Cas crops–bringing together genomics and genome editing. New Phytolog 216:682–698
Schindele P, Wolter F, Puchta H (2018) Transforming plant biology and breeding with CRISPR/Cas9, Cas12 and Cas13. FEBS Lett 592:1954–1967
Schnable PS, Springer NM (2013) Progress toward understanding heterosis in crop plants. Annu Rev Plant Biol 64:71–88
Scholz I, Lange SJ, Hein S, Hess WR, Backofen R (2013) CRISPR-Cas systems in the cyanobacterium Synechocystis sp. PCC6803 exhibit distinct processing pathways involving at least two Cas6 and a Cmr2 protein. PloS One 8:e56470
Shan Q, Wang Y, Li J, Zhang Y, Chen K, Liang Z, Zhang K, Liu J, Xi JJ, Jeff J, Qiu JL, Gao C (2013) Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol 31:686–688
Shmakov S, Smargon A, Scott D, Cox D, Pyzocha N, Yan W, Koonin EV (2017) Diversity and evolution of class 2 CRISPR–Cas systems. Nat Rev Microbiol 15:169–182
Shrawat AK, Lörz H (2006) Agrobacterium-mediated transformation of cereals: a promising approach crossing barriers. Plant Biotechnol J 4:575–603
Shrawat AK, Becker D, Lörz H (2007) Agrobacterium tumefaciens-mediated genetic transformation of barley (Hordeum vulgare L.). Plant Sci 172:281–290
Shreni Agrawal ER (2022) A Review: Agrobacterium-mediated gene transformation to increase plant productivity. J Phytopharmacol https://doi.org/10.31254/phyto.2022.11211
Shrivastav M, De Haro LP, Nickoloff JA (2008) Regulation of DNA double-strand break repair pathway choice. Cell Res 18:134–147
Singh P, Kumar K (2022) Agrobacterium-mediated In-planta transformation of bread wheat (Triticum aestivum L.). J Plant Biochem Biotechnol 31:206–212
Singh M, Kumar M, Albertsen MC, Young JK, Cigan AM (2018) Concurrent modifications in the three homeologs of Ms45 gene with CRISPR-Cas9 lead to rapid generation of male sterile bread wheat (Triticum aestivum L.). Plant Mol Biol 97:371–383
Singh P, Kumar K (2021) Agrobacterium-mediated In-planta transformation of bread wheat (Triticum aestivum L.). J Plant Biochem Biotechnol 1–7
Singha DL, Das D, Paswan RR, Chikkaputtaiah C, Kumar S (2022) Novel Approaches and Advanced Molecular Techniques for Crop Improvement. In Plant Microbe Interact 1–27. CRC Press.
Sinkunas T, Gasiunas G, Fremaux C, Barrangou R, Horvath P, Siksnys V (2011) Cas3 is a single- stranded DNA nuclease and ATP- dependent helicase in the CRISPR/Cas immune system. The EMBO J 30:1335–1342
Soda N, Verma L, Giri J (2018) CRISPR-Cas9 based plant genome editing: Significance, opportunities and recent advances. Plant Physiol Biochem 131:2–11
Sparks CA, Jones HD (2004) Transformation of wheat by biolistics. Transgenic crops of the world: essential protocols, 19–34
Stemmer M, Thumberger T, del Sol KM, Wittbrodt J, Mateo JL (2015) CCTop: an intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS ONE 10:e0124633
Stevanovic M, Piotter E, McClements ME, MacLaren RE (2022) Crispr Systems Suitable for Single Aav Vector Delivery. Curr Gene Ther 22:1–14
Sun Y, Li J, Xia L (2016) Precise genome modification via sequence-specific nucleases-mediated gene targeting for crop improvement. Front Plant Sci 7:1928
Sun W, Liu H, Yin W, Qiao J, Zhao X, Liu Y (2022) Strategies for enhancing the homology-directed repair efficiency of CRISPR-cas systems. CRISPR J 5:7–18
Tamulaitis G, Venclovas Č, Siksnys V (2017) Type III CRISPR-Cas immunity: major differences brushed aside. Trends Microbiol 25:49–61
Tănăsescu EC, Lite MC (2022) Harmful health effects of pesticides used on museum textile artifacts-overview. Ecotoxicol Environ Saf 247:114240
Tao LL, Yin GX, Du LP, Shi ZY, She MY, Xu HJ, Ye XG (2011) Improvement of plant regeneration from immature embryos of wheat infected by Agrobacterium tumefaciens. Agr Sci China 10:317–326
Taspinar MS, Aydin M, Sigmaz B, Yildirim N, Agar G (2017) Protective role of humic acids against picloram-induced genomic instability and DNA methylation in Phaseolus vulgaris. Environ Sci Pollut Res 24:22948–22953
Taspinar MS, Sigmaz B, Aydin M, Arslan E, Güleray A (2018) Alleviative role of Β-Estradiol against 2, 4-dichlorophenoxyacetic acid genotoxicity on common bean genome. Yuzuncu Yil Univ J Agric Sci 28:1–9
Thiyagarajan K, Noguera LM, Pacheco M, Govindan V, Vikram P (2002) Agrobacterium mediated transformation and deciphering SNPs in TaLr67 gene homeologs for gene editing in wheat. bioRxiv 2022–03
Tian X, Qin Z, Zhao Y, Wen J, Lan T, Zhang L, Wang F, Qin D, Yu K, Zhao A, Hu Z, Yao Y, Ni Z, Sun Q, Smet ID, Peng H, Xin M (2022) Stress granule-associated TaMBF1c confers thermotolerance through regulating specific mRNA translation in wheat (Triticum aestivum). New Phytol 233:1719–1731
Tran TN, Sanan-Mishra N (2015) Effect of antibiotics on callus regeneration during transformation of IR 64 rice. Biotechnol Rep 7:143–149
Turhan S, Taspinar MS, Yigider E, Aydin M, Agar G (2021) The role of long terminal repeat (LTR) responses to drought in selenium-treated wheat. Environ Eng Manag J 20
Twyman RM, Christou P (2004) Plant transformation technology: particle bombardment. Handbook of Plant Biotechnology. John Wiley & Sons Ltd, Chichester
Ullah A, Nadeem F, Nawaz A, Siddique KH, Farooq M (2022) Heat stress effects on the reproductive physiology and yield of wheat. J Agron Crop Sci 208:1–17
Uniyal AP, Mansotra K, Yadav SK, Kumar V (2019) An overview of designing and selection of sgRNAs for precise genome editing by the CRISPR-Cas9 system in plants. 3 Biotech 9:1–19
Unniyampurath U, Pilankatta R, Krishnan M (2016) RNA interference in the age of CRISPR: Will CRISPR interfere with RNAi? Int J Mol Sci 17:291
Upadhyay SK, Kumar J, Alok A, Tuli R (2013) RNA-guided genome editing for target gene mutations in wheat. G3 Genes Genom Genet 3:2233–2238
Upadhyaya NM, Mago R, Panwar V, Hewitt T, Luo M, Chen J, Sperschneider J, Nguyen-Phuc H, Wang A, Ortiz D, Hac L, Bhatt D, Li F, Zhang J, Ayliffe M, Figueroa M, Kanyuka K, Ellis JG, Dodds PN (2021) Genomics accelerated isolation of a new stem rust avirulence gene–wheat resistance gene pair. Nat Plants 7:1220–1228
Usman B, Nawaz G, Zhao N et al (2021) Programmed editing of rice (Oryza sativa l.) osspl16 gene using crispr/cas9 improves grain yield by modulating the expression of pyruvate enzymes and cell cycle proteins. Int J Mol Sci 22:1–19
United States Department of Agriculture (2022) Wheat Data. https://www.ers.usda.gov/data-products/wheat-data/ (10.03.2023)
Vasil V, Castillo AM, Fromm ME, Vasil IK (1992) Herbicide resistant fertile transgenic wheat plants obtained by microprojectile bombardment of regenerable embryogenic callus. Nat Biotechnol 10:667–674
Vats S, Bansal R, Rana N, Kumawat S, Bhatt V, Jadhav P, Kale V, Sathe A, Sonah H, Jugdaohsingh R, Sharma TR, Deshmukh R (2022) Unexplored nutritive potential of tomato to combat global malnutrition. Crit Rev Food Sci Nutr 62:1003–1034
Waheed S, Zeng L (2020) The critical role of miRNAs in regulation of flowering time and flower development. Genes 11:319
Wang YL, Xu MX, Yin GX, Tao LL, Wang DW, Ye XG (2009) Transgenic wheat plants derived from Agrobacterium-mediated transformation of mature embryo tissues. Cereal Res Commun 37:1–12
Wang Y, Cheng X, Shan Q, Zhang Y, Liu J, Gao C, Qiu JL (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotech 32:947–951
Wang H, La Russa M, Qi LS (2016) CRISPR/Cas9 in genome editing and beyond. Annu Rev Biochem 85:227–264
Wang S, Jin W, Wang K (2019a) Centromere histone H3-and phospholipase-mediated haploid induction in plants. Plant Methods 15:1–10
Wang W, Pan Q, Tian B, He F, Chen Y, Bai G, Trick HN, Akhunov E (2019b) Gene editing of the wheat homologs of TONNEAU 1-recruiting motif encoding gene affects grain shape and weight in wheat. Plant J 100:251–264
Wang X, Aguirre L, Rodríguez-Leal D, Hendelman A, Benoit M, Lippman ZB (2021) Dissecting cis-regulatory control of quantitative trait variation in a plant stem cell circuit. Nat Plants 7:419–427
Wang K, Shi L, Liang X, Zhao P, Wang W, Liu J, Chang Y, Hiei Y, Yanagihara C, Ishida Y, Ye X (2022a) The gene TaWOX5 overcomes genotype dependency in wheat genetic transformation. Nat Plants 8:110–117
Wang SW, Gao C, Zheng YM, Yi L, Lu JC, Huang XY, Cai JB, Zhang PF, Cui YH, Ke AW (2022b) Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Mol Cancer 21:1–27
Wang Y, Du F, Wang J, Wang K, Tian C, Qi X, Jiao Y (2022c) Improving bread wheat yield through modulating an unselected AP2/ERF gene. Nat Plants 8:930–939
Waqeel J, Khan ST (2022) Microbial biofertilizers and micronutrients bioavailability: approaches to deal with zinc deficiencies. Microbial Biofertilizers and Micronutrient Availability. Springer, Cham, pp 239–297
Waseem M, Ahmad M, Saqib Naveed M, Pasha A, Hussain M, Ali Zafar S, Mujahid A, Rehman RS (2022) Abscisic acid mediated abiotic stress tolerance in plants. Asian J Crop Sci 1–17
Waters CA, Strande NT, Pryor JM, Strom CN, Mieczkowski P, Burkhalter MD, Oh S, Qaqish BF, Moore DT, Hendrickson EA, Ramsden DA (2014) The fidelity of the ligation step determines how ends are resolved during nonhomologous end joining. Nat Commun 5:1–11
Wijerathna-Yapa A, Ramtekey V, Ranawaka B, Basnet BR (2022) Applications of in vitro tissue culture technologies in breeding and genetic improvement of wheat. Plants 11:2273
Wolter F, Schindele P, Puchta H (2019) Plant breeding at the speed of light: the power of CRISPR/Cas to generate directed genetic diversity at multiple sites. BMC Plant Biol 19:176
World Health Organization (2020) The state of food security and nutrition in the world 2020: transforming food systems for affordable healthy diets. Food Agric Org
Wu H, Sparks C, Amoah B, Jones HD (2003) Factors influencing successful Agrobacterium-mediated genetic transformation of wheat. Plant Cell Rep 21:659–668
Wu C, Cui K, Fahad S (2022) Heat stress decreases rice grain weight: Evidence and physiological mechanisms of heat effects prior to flowering. Int J Mol Sci 23:10922
Wulff BB, Krattinger SG (2022) The long road to engineering durable disease resistance in wheat. Curr Opin Biotechno 73:270–275
Xiao A, Cheng Z, Kong L, Zhu Z, Lin S, Gao G, Zhang B (2014) CasOT: a genome-wide Cas9/gRNA off-target searching tool. Bioinformatics 30:1180–1182
Xiao C, Zhang H, Xie F, Pan ZY, Qiu WM, Tong Z, Wang ZQ, He XJ, Xu YH, Sun ZH (2022) Evolution, gene expression, and protein-protein interaction analyses identify candidate CBL-CIPK signalling networks implicated in stress responses to cold and bacterial infection in citrus. BMC Plant Biol 22:1–17
Xu M, Wang Q, Wang G, Zhang X, Liu H, Jiang C (2022) Combatting Fusarium head blight: advances in molecular interactions between Fusarium graminearum and wheat. Phytopathol Res 4:1–16
Xue C, Greene EC (2021) DNA repair pathway choices in CRISPR-Cas9-mediated genome editing. Trends Genet 37:639–656
Ye X, Wang K, Liu H, Tang H, Qiu Y, Gong Q (2022) Genome Editing Toward Wheat Improvement. Genome Editing Technologies for Crop Improvement. Springer, Singapore, pp 241–269
Yeh CD, Richardson CD, Corn JE (2019) Advances in genome editing through control of DNA repair pathways. Nat Cell Biol 21:1468–1478
Yigider E, Taspinar MS, Aydin M, Agar G (2021) Humic acid effects on retrotransposon polymorphisms caused by zinc and iron in the maize (Zea mays L.) genome. Cereal Res Commun 49:193–198
Yimam YT, Zhou J, Akher SA, Zheng X, Qi Y, Zhang Y (2021) Improving a Quantitative Trait in Rice by Multigene Editing with CRISPR-Cas9. Rice Genome Engineering and Gene Editing: Methods and Protocols, 205–219
Yu Y, Wang J, Zhu ML, Wei ZM (2008) Optimization of mature embryo-based high frequency callus induction and plant regeneration from elite wheat cultivars grown in China. Plant Breed 127:249–255
Yuan ZC, Edlind MP, Liu P, Saenkham P, Banta LM, Wise AA, Ronzone E, Binns AN, Kerr K, Nester EW (2007) The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc Natl Acad Sci 104:11790–11795
Yuan WX, Yun-Mei YU, Chun-Cai HU, Zhao ZG (2017) Current Issues and Progress in the Application of CRISPR/Cas9 Technique. Biotechnol Bull 33:70–77
Zafar K, Sedeek KE, Rao GS, Khan MZ, Amin I, Kamel R, Mukhtar Z, Zafar M, Mahfouz MS, MM, (2020) Genome editing technologies for rice improvement: progress, prospects, and safety concerns. Front Genome Ed 2:5
Zarei A, Razban V, Hosseini SE, Tabei SMB (2019) Creating cell and animal models of human disease by genome editing using CRISPR/Cas9. J Gene Med 21:e3082
Zhang F (2019) Development of CRISPR-Cas systems for genome editing and beyond. Q Rev Biophys 52:e6
Zhang F, Huang Z (2021) Mechanistic insights into the versatile class II CRISPR toolbox. Trends Biochem Sci 47:433–450
Zhang YM, Zhang HM, Liu ZH, Guo XL, Li HC, Li GL, Jiang CZ, Zhang MC (2015) Inhibition of isoflavone biosynthesis enhanced T-DNA delivery in soybean by improving plant–Agrobacterium tumefaciens interaction. Plant Cell Tissue Organ Cult 121:183–193
Zhang Y, Liang Z, Zong Y, Wang Y, Liu J, Chen K, Qui JL, Gao C (2016) Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat Commun 7:1–8
Zhang Y, Bai Y, Wu G, Zou S, Chen Y, Gao C, Tang D (2017) Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J 91:714–724
Zhang Y, Li D, Zhang D, Zhao X, Cao X, Dong L, Liu J, Chen K, Zhang H, Gao C, Wang D (2018) Analysis of the functions of Ta GW 2 homoeologs in wheat grain weight and protein content traits. Plant J 94:857–866
Zhang Z, Hua L, Gupta A, Tricoli D, Edwards KJ, Yang B, Li W (2019) Development of an Agrobacterium-delivered CRISPR/Cas9 system for wheat genome editing. Plant Biotechnol J 17:1623–1635
Zhang J, Zhang H, Li S, Li J, Yan L, Xia L (2021a) Increasing yield potential through manipulating of an ARE1 ortholog related to nitrogen use efficiency in wheat by CRISPR/Cas9. J Integr Plant Biol 63:1649–1663
Zhang S, Shen J, Li D, Cheng Y (2021b) Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics 11:614
Zhang S, Zhang R, Gao J, Song G, Li J, Li W, Qui Y, Li Y, Li G (2021c) CRISPR/Cas9-mediated genome editing for wheat grain quality improvement. Plant Biotechnol J 19:1684
Zhao TJ, Zhao SY, Chen HM, Zhao QZ, Hu ZM, Hou BK, Xia GM (2006) Transgenic wheat progeny resistant to powdery mildew generated by Agrobacterium inoculum to the basal portion of wheat seedling. Plant Cell Rep 25:1199–1204
Zheng M, Lin J, Liu X, Chu W, Li J, Gao Y, An K, Song W, Xin M, Yao Y, Peng H, Ni Z, Sun Q, Hu Z (2021) Histone acetyltransferase TaHAG1 acts as a crucial regulator to strengthen salt tolerance of hexaploid wheat. Plant Physiol 186:1951–1969
Zhi H, Zhou S, Pan W, Shang Y, Zeng Z, Zhang H (2022) The Promising Nanovectors for Gene Delivery in Plant Genome Engineering. Int J Mol Sci 23:8501
Zhu Y (2022) Advances in CRISPR/Cas9. Biomed Res Int 2022
Acknowledgements
The authors would like to thank Ataturk University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Gerhard Leubner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yigider, E., Taspinar, M.S. & Agar, G. Advances in bread wheat production through CRISPR/Cas9 technology: a comprehensive review of quality and other aspects. Planta 258, 55 (2023). https://doi.org/10.1007/s00425-023-04199-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-023-04199-9