Abstract
Purpose
The aim of this study was to establish whether laparoscopic RAMPS (L-RAMPS) is a safe procedure with better oncological outcomes compared to laparoscopic distal pancreatectomy (LDP) with splenectomy among patients with distal pancreatic ductal adenocarcinoma (PDAC).
Methods
This is a retrospective study performed on consecutive patients who underwent L-RAMPS and LDP with splenectomy for resectable or borderline resectable PDAC of the body and tail. In this paper, we presented our technique of laparoscopic RAMPS and analyzed intraoperative and perioperative complications, oncological efficacy, and long-term survival.
Results
The study included 12 patients in the L-RAMPS group and 13 patients in the LDP with splenectomy. L-RAMPS was associated with significantly higher rates of R0 resection (91.7% vs. 69.2%, p = 0.027). There were no differences between the L-RAMPS and LDP with splenectomy groups in intraoperative blood loss (400 mL vs 400 mL, p = 0.783) and median operative time (250 min vs 220 min, p = 0.785). No differences were found in terms of perioperative complications, including the incidence of pancreatic fistula.
Conclusion
Laparoscopic RAMPS is a feasible and safe procedure. It provides higher radicality as compared with LDP with splenectomy, without increasing the risk of complications. Further studies are necessary to evaluate long-term outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma of the body and tail is characterized by an aggressive course. It is commonly diagnosed during advanced stage when the tumor has spread beyond the margins of the pancreas to adjacent or distant organs [1]. The only available curative therapy is surgical treatment if possible. The most important goal in pancreatic cancer surgery is to achieve complete resection, sufficient lymphadenectomy, and tumor-free margins. The rate of positive (R1) resection margins after standard distal pancreatectomy with splenectomy (SDP) remains high, which is the main cause of local recurrence and metastasis. In order to achieve radical operation with more extensive lymphadenectomy, Strasberg proposed the radical antegrade modular pancreatosplectomy (RAMPS) technique in 2003 [2], based on the studies on pancreatic lymph drainage conducted by O’Morchoe [3]. This technique provides better posterior margin and possibility to adjust the dissection margin depending on the depth of tumor infiltration. Studies comparing RAMPS and SDP with splenectomy have shown that it is correlated with higher R0 resection rates and harvest of more lymph nodes [4,5,6,7,8,9,10].
Laparoscopic left pancreatectomy has become the standard for pancreatic body and tail tumors. Similarly to many other laparoscopic procedures, laparoscopic approach was implemented for RAMPS. Laparoscopic RAMPS (L-RAMPS) is a technically more demanding, and due to the lack of proven superiority, it is rather rarely performed. Minimally invasive RAMPS was initially performed in carefully selected patients [8, 11,12,13,14]. Early results showed that L-RAMPS was feasible and safe. Currently, the is no clear evidence whether it improves oncological outcomes or whether it increases the risk of perioperative complications. In this paper, we present our laparoscopic RAMPS technique and its short-term results compared to laparoscopic distal pancreatectomy (LDP) with splenectomy among patients with left-sided pancreatic ductal adenocarcinoma.
Material and methods
The following study is a retrospective cohort study of consecutive patients that underwent laparoscopic radical antegrade modular pancreatosplenectomy (RAMPS) for adenocarcinoma of the body and tail of the pancreas between June 2021 and December 2022. The inclusion criteria to the RAMPS group were as follows: ≥ 18 years old patients, qualified and consent for RAMPS due to clinical suspicion for adenocarcinoma of the pancreas, or confirmed adenocarcinoma of body or tail of pancreas.
Control group consisted of retrospective cohort of consecutive, adult patients that underwent laparoscopic distal pancreatectomy with splenectomy due to pancreatic ductal adenocarcinoma between August 2018 and December 2022. Follow-up for patients’ survival was completed in February 2023.
Preoperative counseling and preparations
Preoperative diagnostic workout included serum Ca 19–9, transaminases, albumin and protein levels, and other standard blood tests required for general anesthesia. Assessment of tumor staging and resectability was carried out with use of computed tomography (CT) with three-dimensional (3D) angiography and contrast-enhanced magnetic resonance imaging of abdomen (MRI). Endoscopic ultrasonography (EUS) with biopsy was performed in case of doubt whether the tumor is malignant. In case of a high suspicion of dissemination, the PET-CT scan was performed.
Nutritional interventions were implemented in all cases. Oral protein and immunomodulative supplementation was administered. In case more advanced malnutrition, patients were admitted 2 weeks prior of surgery for parenteral nutritional treatment. All patients were scheduled for laparoscopic procedures, regardless of tumor size or suspicion of large vessel infiltration.
Surgical technique
Patient was positioned in the supine position with legs and arms abducted. Legs were flexed in hips, and patient was positioned in slight reverse Trendelenburg position. Surgeon was standing between patients’ legs, and assistants were at both sides of patients. However, the position of the operator may change depending on the situation in the operating field. Scrub nurse was standing on the patient’s right. Ten- to 12-mm trocars were inserted in the abdominal wall 3–4 cm above the umbilicus and then in the left midclavicular line slightly above the first one. After careful visual exclusion of peritoneal neoplastic dissemination, additional 5-mm trocars were inserted: subcostal trocar in the left anterior axillary line; right midclavicular line; and if needed subcostal trocar on the right side between the midline and right midclavicular line (Fig. 1).
Two monitors were used. CO2 pneumoperitoneum was set at a pressure of 12 mm Hg. A 30° laparoscope was used routinely.
Laparoscopic radical antegrade modular pancreatosplenectomy (L-RAMPS) adapted the same principles as described by Strasberg [2]. The gastrocolic ligament was widely opened to visualize the body and tail of the pancreas. The splenocolic ligament was divided using a harmonic sealer/divider and clips. The splenic flexure of the colon was mobilized downward as much as needed. The anterior aspect of the pancreas was exposed by dividing the adhesions between the posterior surface of the stomach and the pancreas. Transverse colon mesentery was divided from the inferior pancreatic border. Usually at the level of the tumor, some part of the transverse mesentery was also resected, in most cases with preservation of important vascular structures. The neck of the pancreas was dissected below the inferior pancreatic border until the superior mesenteric vein was exposed. Small branches of the superior mesenteric vein were controlled either with harmonic sealer/divider or with titanium clips. At this stage, the left and anterior wall of the SMA was safely exposed. Next, the surgeon approached the superior border of the pancreas. Lymph nodes along the common hepatic artery, proper hepatic artery, and portal vein were dissected. The left gastric vein and artery were identified and followed toward the origin of the splenic artery. The left gastric lymph nodes were dissected until the splenic artery was exposed. The splenic artery was isolated. Splenic vessels were isolated from the pancreas, and the neck of the pancreas was divided using an endoscopic stapler. Pancreatic stump was oversewn with barbed horizontal mattress suture. After transection of the pancreas, the splenic artery was isolated and divided between clips close to the celiac artery, the same as the splenic vein at confluence with the superior mesenteric vein. Celiac axis lymph nodes and nodes along the anterior and the left side of the superior mesenteric artery (SMA) were dissected. The block of tissue, frequently referred to as “mesopancreas” located between SMA and celiac axis (“Heidelberg’s triangle”), is removed together with specimen. The lateral and posterior sides of the SMA were dissected further until the left renal vein was identified. Retroperitoneal dissection continued along the anterior surface of the renal vein laterally. After reaching the anterior wall of the renal vein, the surgeon was able to accurately assess the extent of tumor penetration and decide whether anterior or posterior RAMPS is optimal. The posterior plane of the dissection runs along the anterior wall of the left renal vein. If anterior RAMPS is performed, the dissection plane continues along the anterior wall of the left adrenal vein and the anterior aspect of the left adrenal gland. For tumors extended beyond the posterior margin of the pancreas, a posterior RAMPS should be performed. The dissection should begin deeper and form the lateral wall of the aorta, along the posterior wall of the body and diaphragm, with excision of the left adrenal gland. Posterior and lateral dissection is continued to the anterior surface of the kidney (Fig. 2).
The superior and inferior attachments of the pancreas were divided as dissection proceeds toward the spleen. The short gastric vessels were then divided, usually without clips. Finally, the lienorenal ligament was divided. When spleno-pancreatic block was fully mobilized, it was inserted into a large retrieval endo-bag and then retracted through Pfannenstiel incision (with use of the wound protection system). Drain was placed near the pancreatic stump.
Postoperative course, discharge from hospital
Perioperative care was based on ERAS protocol [15]. One day prior to surgery and on the day of surgery, each patient received a prophylactic dose of low-molecular-weight heparin and was allowed to drink clear fluids. On the first day after surgery, the patient returned to the oral diet if there are no symptoms of postoperative ileus.
On the third postoperative day, the level of amylase in the discharge from the drain was assessed. The decision to remove the drain was made individually, depending on the volume and concentration of amylase in the discharge.
We used the revised 2016 International Study Group on Pancreatic Fistula (ISGPF) definition of pancreatic fistula [16].
Statistical analysis
Data were analyzed with Statistica 13.3 PL (TIBCO Software, Palo Alto, USA). Continuous data are presented as means with standard deviation (SD) or as medians with first and third quartiles (Q1–Q3) when appropriate. Groups were compared with chi-square test with or without Yates correction or Fisher’s test, when appropriate. Quantitative data were compared in Mann–Whitney’s test. Survival was assessed after Kaplan–Meier’s curve analysis with Cox–Mantel’s test.
Ethics
All procedures have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent for surgical treatment was obtained from all patients before surgery. Study was approved by the local bioethics committee.
Material
Twenty-five patients were in total included in this study. The median age was 67 (61–72) years. Study population included 8 males (32%) and 17 females (68%).
Results
L-RAMPS group included 12 (48%) patients while control group 13 (52%) who underwent laparoscopic distal pancreatectomy (LDP) with splenectomy. Patients’ characteristics are presented in Table 1. Both groups did not differ in terms of age, sex, BMI, and tumor localization (respectively, p = 0.086; 0.202; 0.183; 0.593). Localization of tumors in the body or tail of the pancreas did not differ groups significantly (p = 0.593). The tumor size in L-RAMPS groups was significantly smaller (median 26.5 mm, Q1–Q3 21–44) than in LDP with splenectomy (median 45 mm, Q1–Q3 35–50). Table 2 demonstrates perioperative outcomes. One patient in LDP with splenectomy required conversion due to necessity of stomach and splenic flexure en bloc resection. There was no difference in the median operative time in the L-RAMPS group (median 250 min, Q1–Q3 150–300) and LDP with splenectomy group (median 220 min, Q1–Q3 178–260). The blood loss in both group was comparable.
The were no significant differences between the two groups in the incidence of pancreatic fistulas. Clinically relevant pancreatic fistulas (grade B and C) were diagnosed in 3 patients (25%, 2 grade B, 1 grade C) in L-RAMPS group and in 3 patients in LDP with splenectomy group (23.1%, 1 grad B, 2 grade C). Biochemical leakage (grade A) was confirmed in 1 patient (8.3%) in L-RAMPS group and in 2 patients (15.4%) in LDP with splenectomy group. One patient in L-RAMPS group died due to postoperative cardiopulmonary failure, secondary to congestive heart failure and other comorbidities, without surgical complications.
Table 3 presents results of histopathological evaluation. L-RAMPS was associated with significantly higher rates of R0 resection (91.7% vs. 69.2%, p = 0.027).
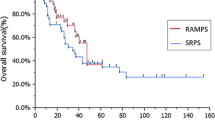
Outcomes of follow-up are demonstrated in Table 4. Cancer-related death (> 90 days) rate was higher in LDP with splenectomy group than in L-RAMPS (61.5% vs. 41.7.0%, p = 0.320), which might be related to noticeably longer follow-up in the first group. Patients’ survival is presented in Kaplan–Meier curve in Fig. 3. Cox–Mantel’s test revealed no significant differences in patients’ survival depending on groups with p = 0.333.
Discussion
LDP is a safe and feasible procedure for benign and borderline left-sided pancreatic tumors [17,18,19,20]. For malignant lesions in the body or tail of pancreas, RAMPS has now become the standard procedure. It has been proven that RAMPS provides higher chance for radical resection and increases the number of harvested lymph nodes [4,5,6,7,8,9]. However, we do not have high-quality evidence of increased overall survival.
Intuitively, minimally invasive surgical approach is expected to provide benefits such as lower blood loss, less morbidity, and shorter hospital stay as compared to open surgery. All efforts aimed at decrease of postoperative complications is of outmost important in patients with adenocarcinoma of the pancreas as it increases the chance for early initiation of chemotherapy and thus increased overall survival [21]. The number of reports on L-RAMPS is growing, but there is still no clear evidence of it advantage over open radical antegrade modular pancreatosplenectomy (O-RAMPS) [22,23,24,25,26].
In this paper, we present the result of 12 L-RAMPS compared with 13 LDP with splenectomy performed in patients with left-sided pancreatic ductal adenocarcinoma. We do not compare L-RAMPS with O-RAMPS in our study, because with the growing experience in minimally invasive surgery, all patients with suspected or diagnosed with ductal adenocarcinoma of the pancreatic body and tail were scheduled for laparoscopic surgery. Infiltration of the adjacent major veins was not exclusion criteria for L-RAMPS. Minimally invasive RAMPS with major venous resection has been shown to be safe and feasible [27, 28].
The most important prognostic factors for recurrence and overall survival after pancreatic resections for cancer are margin status and lymph node invasion [29,30,31].
One of the main principles of the RAMPS is modular setting of the posterior plane of dissection to increase negative posterior margins rates. The decision for an anterior or posterior L-RAMPS is most often made preoperatively on the basis of posterior margin of the tumor identified in CT scans. If the tumor penetrates the left adrenal gland or the posterior surface of the pancreas is involved with the tumor, a posterior RAMPS (with left adrenalectomy) should be performed [2]. None of our patient required a posterior L-RAMPS.
Based on the results from the analysis of our small group of patients, we cannot draw strong conclusions about oncologic and perioperative outcomes. In addition, the two groups we analyzed differed significantly in terms of tumor size (the tumor in the LDP group was about 20mm larger)—a confounding factor for resection margins.
The R0 resection rate in our study in L-RAMPS group is 91.7%, compared to 69.2% in the LDP with splenectomy. Our result are comparable to those achieved with O-RAMPS [4, 32]. The use of a minimally invasive technique in RAMPS does not adversely affect these results, as confirmed by the data already available [22, 24, 25, 33]. Sato S. et al. showed in a retrospective cohort study comparing L-RAMPS with O-RAMPS that the use of laparoscopy resulted in a 100% R0 resection rate compared to 90.7% in the O-RAMPS group [34]. They also showed that among patients eligible for adjuvant chemotherapy, the L-RAMPS group showed a favorable induction rate (100.0 vs. 89.6%, p = 0.037).
The randomized controlled trial (DIPLOMA) comparing oncological outcomes specifically in pancreatic ductal adenocarcinoma (PDAC) has been recently published [35]. This is an international, multicenter, patient- and pathologist-blind randomized controlled trial with the primary end point of radical resection. This study compared the results of minimally invasive left radical “no-touch” pancreatosplenectomy [36] with radical antegrade modular pancreatosplenectomy for open procedures [2]. In this study, R0 resection occurred in 73% of patients in the minimally invasive group and in 69% of patients in the open procedure group.
To determine the quality of surgical treatment, we should refer to the results of benchmark studies [37,38,39,40]. Müller PC et al. [39] analyzed the results of four multicenter retrospective studies defining benchmark cut-off values for distal pancreatectomy (open, laparoscopic, and robotic). Two studies reported on oncological outcomes for patients with PDAC with cut-offs for R0 rate of 83% [40] and 76% [37].
Another main principle of RAMPS is complete lymphadenectomy along the body and tail of the pancreas and around the celiac axis and the anterior left wall of the superior mesenteric artery. As intended, RAMPS allows for a more extensive lymphadenectomy [6, 41]. There is no evidence of deterioration of the quality of lymphadenectomy in L-RAMPS [24, 25, 33]. In our study, half of the patients (6 L-RAMPS, 7 LDP with splenectomy) were diagnosed with lymph node metastases.
Up to date, open and laparoscopic RAMPS have not been shown to have any effect on overall survival [6, 23, 25, 26, 42, 43]. However, a recent report showed that it reduces the risk of local recurrence [9]. This suggest that RAMPS is an appropriate procedure for patients with advanced tumors, as it enables early adjustment of the resection plane depending on the depth of tumor infiltration. In combination with adjuvant chemotherapy, it may have beneficial effect on local disease control.
The greatest concern in the postoperative course is the risk of postoperative pancreatic fistula (POPF). The prevalence of POPF after distal pancreatectomy ranges widely from 5 to 40% [44,45,46]. Currently, many risk factors for POPF are known [47,48,49]. In particular, these are mainly the consistency and thickness and the pancreas [50, 51]. Pancreatic transection using a laparoscopic endostapler is the most commonly used and convenient method, but the rate of POPF is still worrisome. The use of endoscopic staplers with a prolonged peri-firing compression during laparoscopic surgery may decrease the risk of POPF [52, 53]. To reinforce the staple line, we additionally cover it with a continuous suture [54, 55]. In our study, 23% patients in the LDP with splenectomy and 25% in the L-RAMPS group were diagnosed with relevant POPF (grade B and C). Biochemical leakage (grade A) occurred in 15% patients in the LDP with splenectomy group and in 8% in the L-RAMPS group. In benchmark studies, the incidence of clinically significant pancreatic fistula (grade B/C) ranges from 24 to 32% [39].
Typically, a more extensive surgery is expected to result in higher blood loss, increased risk of infection (SSI), longer hospital stay, and worse complication rate. With increasing experience and development of technique, surgeon can more accurately utilize the advantages of laparoscopic access, such as magnification and different exposure of the operating field. This results in a less traumatic dissection and reduced blood loss. Despite the complexity of the L-RAMPS procedure, blood loss and the transfusions rate in this group are acceptable and significantly lower than those in the O-RAMPS [22,23,24, 26, 33, 34, 56]. In our study, the median blood loss was 400 ml in both groups. We did not find that L-RAMPS was significantly longer operation as compared to LDP with splenectomy. With regard to open surgery, we have not noticed that laparoscopic approach increase the time of RAMPS [10]. Regarding intraoperative outcomes, benchmark cut-offs for blood loss ranged from 150 to 195 ml and operative times from 232 to 300 min [39]. No surgical site infections (SSI) were noted in both compared groups. Typically, as in most laparoscopic operations, we observed shortening of the recovery time of gastrointestinal function. As compared with data from literature about O-RAMPS, the first flatus and first oral intake occurred significantly earlier [26].
Limitations
The analyzed data are from a single center and for the L-RAMPS group are collected from 2020. The most important limitation of our study is the small size of the study group. The compared groups also differ significantly in terms of tumor size and length of follow-up. These limitations implement need for further research to provide more comparability of perioperative and oncologic outcomes.
Conclusion
The introduction of laparoscopic RAMPS for pancreatic cancer did not increased morbidity and mortality of patients as compared with patients after LDP with splenectomy. Laparoscopic RAMPS provided higher rate of microscopically radical operations as compared with LDP with splenectomy but did not improve patients’ survival in our cohort. Further studies are necessary to evaluate long-term outcomes.
References
Brennan MF, Moccia RD, Klimstra D (1996) Management of adenocarcinoma of the body and tail of the pancreas. Ann Surg 223:506–512. https://doi.org/10.1097/00000658-199605000-00006
Strasberg SM, Drebin JA, Linehan D (2003) Radical antegrade modular pancreatosplenectomy. Surgery 133:521–527. https://doi.org/10.1067/msy.2003.146
O’Morchoe CCC (1997) Lymphatic system of the pancreas. Microsc Res Tech 37:456–477. https://doi.org/10.1002/(SICI)1097-0029(19970601)37:5/6%3c456::AID-JEMT9%3e3.0.CO;2-B
Cao F, Li J, Li A, Li F (2017) Radical antegrade modular pancreatosplenectomy versus standard procedure in the treatment of left-sided pancreatic cancer: a systemic review and meta-analysis. BMC Surg 17:1–10. https://doi.org/10.1186/s12893-017-0259-1
Huo Z, Zhai S, Wang Y et al (2019) Comparison of radical antegrade modular pancreatosplenectomy with standard retrograde pancreatosplenectomy for left-sided pancreatic cancer: a meta-analysis and experience of a single center. Med Sci Monit 25:4590–4601. https://doi.org/10.12659/MSM.914540
Chun YS (2018) Role of radical antegrade modular pancreatosplenectomy (RAMPS) and pancreatic cancer. Ann Surg Oncol 25:46–50. https://doi.org/10.1245/s10434-016-5675-4
Abe T, Ohuchida K, Miyasaka Y et al (2016) Comparison of surgical outcomes between radical antegrade modular pancreatosplenectomy (RAMPS) and standard retrograde pancreatosplenectomy (SPRS) for left-sided pancreatic cancer. World J Surg 40:2267–2275. https://doi.org/10.1007/s00268-016-3526-x
Grossman JG, Fields RC, Hawkins WG, Strasberg SM (2016) Single institution results of radical antegrade modular pancreatosplenectomy for adenocarcinoma of the body and tail of pancreas in 78 patients. J Hepatobiliary Pancreat Sci 23:432–441. https://doi.org/10.1002/jhbp.362
Takahashi A, Mise Y, Watanabe G et al (2023) Radical antegrade modular pancreatosplenectomy enhances local control of the disease in patients with left-sided pancreatic cancer. HPB 25:37–44. https://doi.org/10.1016/j.hpb.2022.08.004
Zhou Y, Shi B, Wu L, Si X (2017) A systematic review of radical antegrade modular pancreatosplenectomy for adenocarcinoma of the body and tail of the pancreas. HPB 19:10–15. https://doi.org/10.1016/j.hpb.2016.07.014
Choi SH, Kang CM, Lee WJ, Chi HS (2011) Laparoscopic modified anterior RAMPS in well-selected left-sided pancreatic cancer: technical feasibility and interim results. Surg Endosc 25:2360–2361. https://doi.org/10.1007/s00464-010-1556-2
Kim EY, Hong TH (2017) Initial experience with laparoscopic radical antegrade modular pancreatosplenectomy for left-sided pancreatic cancer in a single institution: technical aspects and oncological outcomes. BMC Surg 17:1–7. https://doi.org/10.1186/s12893-016-0200-z
Ome Y, Hashida K, Yokota M et al (2017) Laparoscopic radical antegrade modular pancreatosplenectomy for left-sided pancreatic cancer using the ligament of Treitz approach. Surg Endosc 31:4836–4837. https://doi.org/10.1007/s00464-017-5561-6
Kim S, Yoon YS, Han HS, Cho JY (2019) Laparoscopic subtotal pancreatectomy with radical antegrade modular pancreatosplenectomy for left-sided pancreatic cancer. Surg Oncol 28:150. https://doi.org/10.1016/j.suronc.2018.12.006
Melloul E, Lassen K, Roulin D et al (2020) Guidelines for perioperative care for pancreatoduodenectomy: enhanced recovery after surgery (ERAS) recommendations 2019. World J Surg 44:2056–2084. https://doi.org/10.1007/s00268-020-05462-w
Bassi C, Marchegiani G, Dervenis C et al (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surg (United States) 161:584–591. https://doi.org/10.1016/j.surg.2016.11.014
Yi X, Chen S, Wang W et al (2017) A systematic review and meta-analysis of laparoscopic and open distal pancreatectomy of nonductal adenocarcinomatous pancreatic tumor (NDACPT) in the pancreatic body and tail. Surg Laparosc Endosc Percutaneous Tech 27:206–219. https://doi.org/10.1097/SLE.0000000000000416
Björnsson B, Larsson AL, Hjalmarsson C et al (2020) Comparison of the duration of hospital stay after laparoscopic or open distal pancreatectomy: randomized controlled trial. Br J Surg 107:1281–1288. https://doi.org/10.1002/bjs.11554
Lyu Y, Cheng Y, Wang B et al (2020) Assessment of laparoscopic versus open distal pancreatectomy: a systematic review and meta-analysis. Minim Invasive Ther Allied Technol 0:1–9. https://doi.org/10.1080/13645706.2020.1812664
Song SH, Kim HJ, Park E-K et al (2020) Comparison of laparoscopic versus open distal pancreatectomy for benign, pre-malignant, and low grade malignant pancreatic tumors. Ann Hepato-Biliary-Pancreatic Surg 24:57. https://doi.org/10.14701/ahbps.2020.24.1.57
Murakami Y, Uemura K, Sudo T et al (2013) Early initiation of adjuvant chemotherapy improves survival of patients with pancreatic carcinoma after surgical resection. Cancer Chemother Pharmacol 71:419–429. https://doi.org/10.1007/s00280-012-2029-1
Tang W, Zhang Y-F, Zhao Y-F et al (2022) Comparison of laparoscopic versus open radical antegrade modular pancreatosplenectomy for pancreatic cancer: a systematic review and meta-analysis. Int J Surg 103:106676. https://doi.org/10.1016/j.ijsu.2022.106676
Wu EJ, Kabir T, Zhao JJ, Goh BKP (2022) Minimally invasive versus open radical antegrade modular pancreatosplenectomy: a meta-analysis. World J Surg 46:235–245. https://doi.org/10.1007/s00268-021-06328-5
Larkins K, Rowcroft A, Pandanaboyana S, Loveday BPT (2021) A systematic scoping review of the initial experience with laparoscopic radical antegrade modular pancreatosplenectomy for pancreatic malignancy. Surg Endosc 35:4930–4944. https://doi.org/10.1007/s00464-021-08528-5
Zhang H, Li Y, Liao Q et al (2021) Comparison of minimal invasive versus open radical antegrade modular pancreatosplenectomy (RAMPS) for pancreatic ductal adenocarcinoma: a single center retrospective study. Surg Endosc 35:3763–3773. https://doi.org/10.1007/s00464-020-07938-1
Huang J, Xiong C, Sheng Y et al (2021) Laparoscopic versus open radical antegrade modular pancreatosplenectomy for pancreatic cancer: a single-institution comparative study. Gland Surg 10:1057–1066. https://doi.org/10.21037/gs-21-56
Rosso E, Frey S, Zimmitti G et al (2020) Laparoscopic radical antegrade modular pancreatosplenectomy with vascular resection for pancreatic cancer: tips and tricks. J Gastrointest Surg 24:2896–2902. https://doi.org/10.1007/s11605-020-04695-3
Borys M, Wysocki M, Budzyński A (2022) Laparoscopic pancreatectomies for borderline tumors with major venous resections. Wideochirurgia i inne Tech maloinwazyjne = Videosurgery other miniinvasive Tech 17:680–687. https://doi.org/10.5114/wiitm.2022.116705
Buc E, Couvelard A, Kwiatkowski F et al (2014) Adenocarcinoma of the pancreas: does prognosis depend on mode of lymph node invasion? Eur J Surg Oncol 40:1578–1585. https://doi.org/10.1016/j.ejso.2014.04.012
Zacharias T, Jaeck D, Oussoultzoglou E et al (2007) Impact of lymph node involvement on long-term survival after R0 pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas. J Gastrointest Surg 11:350–356. https://doi.org/10.1007/s11605-007-0113-3
Kim KS, Kwon J, Kim K, Chie EK (2017) Impact of resection margin distance on survival of pancreatic cancer: a systematic review and meta-analysis. Cancer Res Treat 49:824–833. https://doi.org/10.4143/crt.2016.336
Chang YR, Han SS, Park SJ et al (2012) Surgical outcome of pancreatic cancer using radical antegrade modular pancreatosplenectomy procedure. World J Gastroenterol 18:5595–5600. https://doi.org/10.3748/wjg.v18.i39.5595
Kawabata Y, Hayashi H, Kaji S et al (2020) Laparoscopic versus open radical antegrade modular pancreatosplenectomy with artery–first approach in pancreatic cancer. Langenbeck’s Arch Surg 405:647–656. https://doi.org/10.1007/s00423-020-01887-y
Sato S, Oba A, Kato T et al (2023) Feasibility of laparoscopic radical antegrade modular pancreatosplenectomy (RAMPS) as a standard treatment for distal resectable pancreatic cancer. Langenbeck’s Arch Surg 408:217. https://doi.org/10.1007/s00423-023-02942-0
Korrel M, Jones LR, van Hilst J et al (2023) Minimally invasive versus open distal pancreatectomy for resectable pancreatic cancer (DIPLOMA): an international randomised non-inferiority trial. Lancet Reg Heal - Eur 31:1–15. https://doi.org/10.1016/j.lanepe.2023.100673
Abu Hilal M, Richardson JRC, de Rooij T et al (2016) Laparoscopic radical ‘no-touch’ left pancreatosplenectomy for pancreatic ductal adenocarcinoma: technique and results. Surg Endosc 30:3830–3838. https://doi.org/10.1007/s00464-015-4685-9
Durin T, Marchese U, Sauvanet A et al (2023) Defining benchmark outcomes for distal pancreatectomy: results of a french multicentric study. Ann Surg 278:103–109. https://doi.org/10.1097/SLA.0000000000005539
Giani A, van Ramshorst T, Mazzola M et al (2022) Benchmarking of minimally invasive distal pancreatectomy with splenectomy: European multicentre study. Br J Surg 109:1124–1130. https://doi.org/10.1093/bjs/znac204
Müller PC, Toti JMA, Guidetti C et al (2023) Benchmarking outcomes for distal pancreatectomy: critical evaluation of four multicenter studies. Langenbeck’s Arch Surg 408:1–7. https://doi.org/10.1007/s00423-023-02972-8
Müller PC, Breuer E, Nickel F et al (2023) Robotic distal pancreatectomy: a novel standard of care? Benchmark values for surgical outcomes from 16 international expert centers. Ann Surg 278:253–259. https://doi.org/10.1097/SLA.0000000000005601
Trottman P, Swett K, Shen P, Sirintrapun J (2014) Comparison of standard distal pancreatectomy and splenectomy with radical antegrade modular pancreatosplenectomy. Am Surg 80:295–300
Yin J, Huang XM, Lu ZP et al (2020) Comparison of radical antegrade modular pancreatosplenectomy with conventional distal pancreatectomy for pancreatic adenocarcinoma of the body and tail. Zhonghua Wai Ke Za Zhi 58:505–511. https://doi.org/10.3760/cma.j.cn112139-20200413-00301
Kim HS, Hong TH, You Y-K et al (2021) Radical antegrade modular pancreatosplenectomy (RAMPS) versus conventional distal pancreatectomy for left-sided pancreatic cancer: findings of a multicenter, retrospective, propensity score matching study. Surg Today 51:1775–1786. https://doi.org/10.1007/s00595-021-02280-y
Bassi C, Dervenis C, Butturini G et al (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138:8–13. https://doi.org/10.1016/j.surg.2005.05.001
Knaebel HP, Diener MK, Wente MN et al (2005) Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg 92:539–546. https://doi.org/10.1002/bjs.5000
Seeliger H, Christians S, Angele MK et al (2010) Risk factors for surgical complications in distal pancreatectomy. Am J Surg 200:311–317. https://doi.org/10.1016/j.amjsurg.2009.10.022
Yoshioka R, Saiura A, Koga R et al (2019) Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery. World J Gastroenterol 25:3722–3737. https://doi.org/10.3748/wjg.v25.i28.3722
Yoshioka R, Saiura A, Koga R et al (2010) Risk factors for clinical pancreatic fistula after distal pancreatectomy: analysis of consecutive 100 patients. World J Surg 34:121–125. https://doi.org/10.1007/s00268-009-0300-3
Zimmitti G, La Mendola R, Manzoni A et al (2021) Investigation of intraoperative factors associated with postoperative pancreatic fistula following laparoscopic left pancreatectomy with stapled closure: a video review-based analysis: video-review for predictors of pancreatic leak. Surg Endosc 35:941–954. https://doi.org/10.1007/s00464-020-07912-x
Eguchi H, Nagano H, Tanemura M et al (2011) A thick pancreas is a risk factor for pancreatic fistula after a distal pancreatectomy: selection of the closure technique according to the thickness. Dig Surg 28:50–56. https://doi.org/10.1159/000322406
Okano K, Oshima M, Kakinoki K et al (2013) Pancreatic thickness as a predictive factor for postoperative pancreatic fistula after distal pancreatectomy using an endopath stapler. Surg Today 43:141–147. https://doi.org/10.1007/s00595-012-0235-4
Nakamura M, Ueda J, Kohno H et al (2011) Prolonged peri-firing compression with a linear stapler prevents pancreatic fistula in laparoscopic distal pancreatectomy. Surg Endosc 25:867–871. https://doi.org/10.1007/s00464-010-1285-6
Ariyarathenam AV, Bunting D, Aroori S (2015) Laparoscopic distal pancreatectomy using the modified prolonged prefiring compression technique reduces pancreatic fistula. J Laparoendosc Adv Surg Tech A 25:821–825. https://doi.org/10.1089/lap.2015.0200
Aoki T, Mansour DA, Koizumi T et al (2020) Preventing clinically relevant pancreatic fistula with combination of linear stapling plus continuous suture of the stump in laparoscopic distal pancreatectomy. BMC Surg 20:1–7. https://doi.org/10.1186/s12893-020-00876-8
Yüksel A, Bostanc EB, Colakolu MK et al (2018) Pancreatic stump closure using only stapler is associated with high postoperative fistula rate after minimal invasive surgery. Turkish J Gastroenterol 29:191–197. https://doi.org/10.5152/tjg.2018.17567
Hirashita T, Iwashita Y, Fujinaga A et al (2022) Surgical and oncological outcomes of laparoscopic versus open radical antegrade modular pancreatosplenectomy for pancreatic ductal adenocarcinoma. Surg Today 52:224–230. https://doi.org/10.1007/s00595-021-02326-1
Author information
Authors and Affiliations
Contributions
MB, study conception and design, acquisition of data, and drafting of the manuscript. MW, study conception and design, analysis and interpretation of data, and drafting of the manuscript. KG, analysis and interpretation of data and acquisition of data. MS, study conception and design, acquisition of data, and drafting of the manuscript. AB, drafting of the manuscript and critical revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borys, M., Wysocki, M., Gałązka, K. et al. Laparoscopic radical antegrade modular pancreatosplenectomy (RAMPS) for adenocarcinoma of the body and tail of the pancreas — technical considerations with analysis of surgical outcomes. Langenbecks Arch Surg 409, 74 (2024). https://doi.org/10.1007/s00423-024-03265-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-024-03265-4