Abstract
Purpose
Adjuvant chemotherapy is accepted as a standard treatment after surgical resection of pancreatic carcinoma; however, the optimal timing between surgery and initiation of adjuvant chemotherapy has not been reported. The aim of this study was to determine the optimal timing of adjuvant chemotherapy after surgical resection of pancreatic carcinoma.
Methods
Records of 104 patients who received adjuvant chemotherapy after curative surgical resection of pancreatic carcinoma were reviewed retrospectively. Patients were grouped according to whether they received initial adjuvant chemotherapy within 20 days after surgery (≦20 days, n = 57) or more than 20 days after surgery (>20 days, n = 47). Relationships between time to initiation of adjuvant chemotherapy, other clinicopathological factors, and survival were analyzed.
Results
The rate of postoperative complication was significantly lower than in the ≦20 days group compared with the >20 days group (P = 0.003); no significant difference in other clinicopathological factors was found. Multivariate analysis revealed that time to initiation of adjuvant chemotherapy was an independent prognostic factor of disease-free survival (P = 0.009) and overall survival (P = 0.037). The ≦20 days group had longer 5-year overall survival rates than did the >20 days group (52 vs. 26 %, P = 0.013) as well as longer 5-year disease-free survival rates (53 vs. 22 %, P = 0.007).
Conclusions
Adjuvant chemotherapy for patients with resected pancreatic carcinoma should be initiated as soon as possible after surgical resection. Prevention of postoperative complication is needed to enable early initiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prognosis for pancreatic carcinoma remains dismal in the United States. The overall 5-year survival rate is 6 % [1]. For the subpopulation of patients who have resectable tumors, the five-year survival rate after complete tumor resection has been reported to be less than 20 % [2–5]. Therefore, effective adjuvant therapy is clearly needed for long-term survival of patients after pancreatic carcinoma resection. Since the 1980s, several investigators have conducted randomized controlled studies of adjuvant treatment for patients after pancreatic carcinoma resection [6–15], and more recently, several large-scale randomized controlled studies were conducted, including the European Study Group for Pancreatic Cancer 1 or 3 (ESPAC-1 or 3) [6, 11] and the Charite Onkologie 001 (CONKO-001) [10]. These studies demonstrated that adjuvant chemotherapy using gemcitabine or fluorouracil plus folinic acid significantly improved overall survival of patients with pancreatic carcinoma after surgical resection, compared with surgery alone. Based on these results, adjuvant chemotherapy is now regarded as a standard treatment and is routinely recommended after curative resection of pancreatic carcinoma.
The adjuvant chemotherapy described in the more recent studies typically commenced within 8–10 weeks after surgical resection [6–11]. However, to our knowledge, there have been no published reports regarding the optimal timing between surgical resection of pancreatic carcinoma and initiation of adjuvant chemotherapy. Several studies have demonstrated that early initiation of adjuvant chemotherapy after primary surgery was associated with better survival in patients with colorectal cancer [16–20] or breast cancer [21–23]. In 2002, our institution implemented adjuvant chemotherapy using gemcitabine plus S-1 for patients with resected pancreatic carcinoma, and the beneficial survival effect of this adjuvant chemotherapy has been reported [24–26]. Our institutional policy in these cases is that adjuvant chemotherapy commences as soon as practical after surgical resection. The aim of this study was to determine the optimal timing of adjuvant gemcitabine plus S-1 chemotherapy after surgical resection of pancreatic carcinoma by using retrospective medical record analysis of patients treated in a single institution.
Patients and methods
Study design
One hundred and sixty-seven patients with pancreatic carcinoma underwent surgical resection with curative intent at the Department of Surgery, Hiroshima University Hospital between May 2002 and December 2010. Of these 167 patients, 63 either refused postoperative adjuvant chemotherapy or did not meet other eligibility criteria for this study. All patients had confirmed pathological diagnosis. Patients with distal common bile duct, ampullary carcinoma, duodenal carcinoma, pancreatic ductal adenocarcinoma derived from an intraductal papillary-mucinous neoplasm, or a mucinous cystic neoplasm were excluded [27–29]. The presence of distant metastases, even if resected, was also an exclusion criterion. However, patients who had para-aortic lymph node metastasis detected only by final pathological investigation, and not by preoperative imaging examinations, were included [30]. Therefore, 104 patients who underwent R0 and R1 resection and received postoperative adjuvant chemotherapy with gemcitabine plus S-1 were enrolled in this study.
Patients were divided into two groups according to time from the date of surgery to the date of first chemotherapy session: patients who received initial adjuvant chemotherapy within 20 days after surgery (n = 57) or more than 20 days after surgery (n = 47). Relationships between time to initiation of adjuvant chemotherapy and patient demographics, perioperative factors, tumor characteristics, or patient survival were analyzed retrospectively with univariate or multivariate analyses. Written informed consent was obtained from all patients for surgical treatment and for adjuvant chemotherapy according to institutional guidelines.
Preoperative workup and surgical procedures
Preoperative workup including ultrasonography, computed tomography, endoscopic sonography, endoscopic retrograde cholangiopancreatography, or percutaneous transhepatic cholangiography was performed to assess local or distant tumor extension. A tumor was regarded as unresectable if distant metastases, peritoneal dissemination, apparent para-aortic lymph node metastasis or the presence of invasion of the celiac or superior mesenteric artery was found by these preoperative examinations. Involvement of the portal or superior mesenteric vein alone was not a contraindication to radical resection.
Pylorus-preserving pancreatoduodenectomy was usually performed in patients with carcinoma in the pancreatic head. However, if the tumor was close to the duodenal bulb in the superior pancreatic head, pancreatoduodenectomy with anterectomy was performed. All patients with carcinoma in the pancreatic body or tail underwent distal pancreatectomy with splenectomy. All patients underwent dissection of regional and para-aortic lymph nodes. However, to prevent severe postoperative diarrhea, the nerve plexus around the superior mesenteric artery was not dissected. Partial resection of the portal or superior mesenteric vein was done if the surgeon observed tumor invasion of the vein. Intraoperative pathological assessment of the proximal or distal pancreatic margins was performed using frozen tissue sections. If any pancreatic margin was positive for cancerous cells, further resection of the pancreas was performed to the maximum extent possible.
Pathological examinations
After tumor resection, all specimens were examined pathologically, and each tumor was classified as well-differentiated tubular adenocarcinoma, moderately differentiated tubular adenocarcinoma, poorly differentiated tubular adenocarcinoma or adenosquamous carcinoma according to the predominant pathological findings. Specimens were also examined pathologically for anterior serosal invasion, retropancreatic tissue invasion, choledochal invasion, duodenal invasion, portal vein invasion, and lymph node metastasis. Surgical margins were considered positive if infiltrating adenocarcinoma was present at the proximal or distal pancreatic transection line or in dissected peripancreatic soft tissue margins. The final stage of pancreatic carcinoma was determined pathologically according to the TNM classification system published by the International Union Against Cancer (UICC), seventh edition [31].
Adjuvant gemcitabine plus S-1 chemotherapy
Use of adjuvant gemcitabine plus S-1 chemotherapy has been reported [24–26]. Clinical eligibility criteria for initiation of chemotherapy included 20 years of age or older, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, adequate bone marrow reserve (white blood cell count >3,000/mm3; platelet count >100,000/mm3; hemoglobin level >8 g/dL), and adequate renal function (serum creatinine concentration <1.5 mg/dL) and liver function (total serum bilirubin concentration <3 mg/dL). Patients with high postoperative levels of serum carbohydrate antigen 19-9 (CA19-9) were eligible [32]. Chemotherapy was started as soon as practical between 1 and 6 weeks after surgery. Patients received adjuvant chemotherapy with 10 cycles of gemcitabine plus S-1 every 2 weeks. Each cycle consisted of intravenous gemcitabine (700 mg/m2) on day one and oral S-1 (50 mg/m2) for seven consecutive days, followed by a 1-week pause. Neither external beam radiation nor intraoperative irradiation was given to any patient during the study period. Patients were withdrawn from adjuvant gemcitabine plus S-1 chemotherapy for any of the following reasons: recurrent disease, patient’s decision, or unacceptable treatment toxicity. Toxicity was assessed according to the National Cancer Institute (NCI) Common Toxicity Criteria (CTC) scale Version 2.0. An additional course of chemotherapy was withheld if toxicity of grade 3 or above was observed or if the patient’s condition did not improve sufficiently to fit the clinical eligibility criteria.
Survival
Patients were followed regularly every 3–6 months using blood tests or computed tomography. Diagnosis of recurrence was made based on imaging findings. Overall and disease-free survival times for each patient were calculated from time of surgery. For patients who died, survival time after surgery and cause of death were recorded. For surviving patients, postoperative survival time and recurrence status were recorded. Median follow-up duration after surgery was 47.1 months (range 6–123 months) for the 104 patients.
Statistical analysis
Factors between the two groups were compared using the Chi-squared test or Fisher exact test. Survival curves were constructed using the Kaplan–Meier method, and differences in survival curves were compared by univariate log-rank (Mantel–Cox) testing. Factors found to be significant on univariate analysis were subjected to multivariate analysis using a Cox proportional hazards model. P < 0.05 was considered statistically significant. Statistical analysis was performed with JMP statistical software version 5.1 (SAS Institute, Cary, NC, USA).
Results
Patient demographics and tumor characteristics
The 104 eligible patients included 48 men and 56 women, with a median age of 69 years (range 42–83 years). Of these, 66 patients (63 %) and 15 (14 %) had preoperative and postoperative CA19-9 levels of more than 90 units/mL, respectively. Of 69 patients with carcinoma in the pancreatic head, 63 received pylorus-preserving pancreatoduodenectomy. All 35 patients with carcinoma in the pancreatic body or tail underwent distal pancreatectomy with splenectomy. Partial resection of the portal or superior mesenteric vein was performed for 36 patients (35 %) because of tumor involvement. Postoperative complications occurred in 21 patients (20 %). The leading complication was pancreatic fistula (n = 12), followed by surgical site infection (n = 3), intraabdominal abscess (n = 2), biliary fistula (n = 2), and miscellaneous complications (n = 2). Eighty-seven patients (84 %) received the full number of adjuvant gemcitabine plus S-1 chemotherapy. However, adjuvant chemotherapy was not continued for 17 patients because of recurrent disease (n = 7) or patient refusal (n = 10). The tumor was located in the head of the pancreas in 69 patients (66 %) and in the pancreatic body or tail in 35 patients (34 %). Median tumor size was 3.0 cm (range 0.8–7.0 cm). Tumors were identified as well-differentiated tubular adenocarcinoma in 50 patients (48 %), moderately differentiated tubular adenocarcinoma in 40 patients (38 %), poorly differentiated tubular adenocarcinoma in 10 patients (10 %), and adenosquamous carcinoma in 4 patients (4 %). Anterior serosal invasion, retropancreatic tissue invasion, choledochal invasion, duodenal invasion, and portal or superior mesenteric vein invasion were found in 73 patients (70 %), 81 patients (78 %), 43 patients (41 %), 35 patients (34 %), and 30 patients (29 %), respectively. Sixty-seven patients (64 %) had lymph node metastasis, and 11 patients (11 %) had involvement of the para-aortic lymph nodes. Eighty (77 %) and 24 (23 %) patients underwent R0 and R1 resections, respectively. According to the TNM system, 7 (7 %), 6 (6 %), and 91 patients (87 %) had pT1, pT2, and pT3 tumors, respectively; 5 (5 %), 3 (3 %), 29 (28 %), 56 (53 %), and 11 patients (11 %) were diagnosed with stage IA, IB, IIA, IIB, and IV disease, respectively. All patients with stage IV disease had para-aortic lymph node metastasis that was found only by final pathological investigation (Table 1).
Comparison of clinicopathological factors according to time to initiation of adjuvant chemotherapy
Adjuvant chemotherapy for all patients started between postoperative day 11 and day 56 (median day 20). Of the 104 patients, 57 patients received initial adjuvant chemotherapy within 20 days after surgery (median time, 17 days) and 47 patients received initial adjuvant chemotherapy more than 20 days after surgery (median time, 30 days). Clinicopathological characteristics of the two groups are shown in Table 2. There were no significant differences in gender, age, preoperative or postoperative CA19-9 levels, portal or superior mesenteric vein resection, completion of adjuvant chemotherapy, tumor location, tumor size, tumor differentiation, lymph node status, resection margin status, UICC pT factor, and UICC stage between the two groups. However, the rate of postoperative complication in patients whose adjuvant chemotherapy was initiated within 20 days after surgery was significantly lower than that in patients whose adjuvant chemotherapy was initiated more than 20 days after surgery (P = 0.003).
Survival and prognostic factors
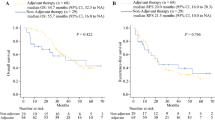
Disease-free survival rates for all 104 patients were 65 % at 1 year postsurgery, 52 % at 2 years, and 39 % at 5 years. Median disease-free survival time was 24.8 months (Fig. 1). Among the 104 patients, prognostic impact of time to initiation of adjuvant chemotherapy was investigated by evaluating factors associated with disease-free survival using univariate and multivariate analyses. Univariate analysis showed that the following eight prognostic factors significantly correlated with long-term, disease-free survival: preoperative CA19-9 level (P = 0.015), postoperative CA19-9 level (P = 0.006), portal or superior mesenteric vein resection (P = 0.002), completion of adjuvant chemotherapy (P < 0.001), time to initiation of adjuvant chemotherapy (P = 0.007), tumor size (P = 0.001), tumor differentiation (P = 0.046), and lymph node status (P < 0.001). These eight factors were entered into multivariate analysis using a Cox proportional hazards model. Completion of adjuvant chemotherapy (P = 0.001), time to initiation of adjuvant chemotherapy (P = 0.009), tumor size (P = 0.046), and lymph node metastasis (P = 0.002) remained independently associated with long-term survival (Table 3). The 5-year disease-free survival rates of patients whose adjuvant chemotherapy was initiated within 20 days after surgery and patients whose adjuvant chemotherapy was initiated more than 20 days after surgery were 53 and 22 %, respectively (P = 0.007, Fig. 2a).
Similar prognostic factor analyses were performed for association with overall survival. Overall survival rates were 87 % at 1 year postsurgery, 67 % at 2 years, and 39 % at 5 years with median overall survival time of 43.4 months (Fig. 1). Univariate analysis showed that the following seven prognostic factors significantly correlated with long-term overall survival: preoperative CA19-9 level (P = 0.029), portal or superior mesenteric vein resection (P = 0.001), completion of adjuvant chemotherapy (P < 0.001), time to initiation of adjuvant chemotherapy (P = 0.013), tumor size (P = 0.001), tumor differentiation (P = 0.025), and lymph node status (P < 0.001). Using multivariate analysis, time to initiation of adjuvant chemotherapy (P = 0.037), tumor differentiation (P = 0.036), and lymph node metastasis (P = 0.003) remained independently associated with long-term survival (Table 4). The 5-year overall survival rates of patients whose adjuvant chemotherapy was initiated within 20 days after surgery and patients whose adjuvant chemotherapy was initiated more than 20 days after surgery were 52 and 26 %, respectively (P = 0.013, Fig. 2b).
Discussion
Many investigators have demonstrated the survival benefits of adjuvant chemotherapy following surgical resection of pancreatic carcinoma [6, 9–11]. However, there have been no reports concerning the optimal timing for postsurgical initiation of adjuvant chemotherapy for these patients. Results of this study showed that patients who received initial adjuvant chemotherapy within 20 days after surgery had significantly better disease-free and overall survival rates than patients whose adjuvant chemotherapy was initiated more than 20 days after surgery. In addition, earlier initiation of adjuvant chemotherapy was an independent prognostic factor of long-term disease-free and overall survival in this cohort. Based on these results, we believe that adjuvant chemotherapy for patents with resected pancreatic carcinoma should be initiated earlier after surgical resection.
In several large-scale randomized controlled trials of adjuvant treatment after resection of pancreatic carcinoma, adjuvant chemotherapy was initiated about 1 month after surgery. The median time from surgery to initiation of adjuvant chemotherapy was reported as 46 days (range 34–67 days) in the ESPAC-1 study [11], 36 days (range 28–43 days) in the CONKO-001 study [10], and 45 days (range 29–57 days) in the ESPAC-3 study [6]. Patients in these trials had overall survival times of 20.1–23.6 months and five-year overall survival rates of 21–23 % [6, 10, 11]. In contrast, the median time from surgery to initiation of adjuvant chemotherapy in the current study was 20 days (range 10–42 days). This difference reflects our policy that adjuvant chemotherapy should be initiated as soon as possible after surgical resection. Our patients had a longer median overall survival time of 43.4 months and a higher 5-year overall survival rate of 39 %. Early initiation of adjuvant chemotherapy may have contributed to better overall survival in this cohort.
The prognostic impact of early initiation of adjuvant chemotherapy has been reported in patients with colorectal cancer [16–20] or breast cancer [20–22] by several investigators. Lima et al. [16] reported that in an analysis of 1,053 patients with stage III colon cancer, patients whose adjuvant chemotherapy was initiated 12–16 weeks after surgery had a 43 % greater risk of dying than those whose adjuvant chemotherapy was initiated within 8 weeks after surgery; they concluded that patients with stage III colon cancer should receive adjuvant chemotherapy within 12 weeks after surgery. Biagi et al. [17] reported in a meta-analysis of 10 studies involving 15,410 patients, that longer time from surgery to adjuvant chemotherapy was associated with worse survival among patients with resected colorectal cancer. A different meta-analysis of 14 studies that included 17,645 patients with colorectal cancer demonstrated that delaying adjuvant chemotherapy more than 8 weeks after surgery significantly decreased overall survival [18] In a retrospective analysis of 2,594 patients with early stage breast cancer, Lohrisch et al. [22] reported that overall survival was significantly worse for patients whose adjuvant chemotherapy was initiated more than 12 weeks after surgery compared with patients who received chemotherapy within 12 weeks. Initiation of adjuvant chemotherapy more than 12 weeks after surgery remained an independent prognostic factor for poorer overall survival by multivariate analysis. Another study included analyses of 402 patients with breast cancer and demonstrated that overall survival was significantly better in patients who started adjuvant treatment within 44 days after surgery compared with patients who started adjuvant treatment more than 44 days postsurgically [21]. Based on these reports, treatment recommendations for patients with colorectal cancer or breast cancer included initiation of adjuvant chemotherapy within 8–12 weeks after surgery. However, pancreatic carcinoma is a more aggressive malignancy than either colorectal or breast cancer [1]. In the current study, we demonstrated that patients with pancreatic carcinoma whose adjuvant chemotherapy was initiated within 20 days after surgical resection had a more favorable prognosis than those whose adjuvant chemotherapy was initiated more than 20 days after surgery. Earlier initiation of adjuvant chemotherapy may be needed for patients with pancreatic carcinoma compared with those with colorectal or breast cancer.
The aim of adjuvant chemotherapy is thought to be eradication of the residual cancer cells or micrometastatic deposits which may develop cancer recurrence. Studies in animal models have demonstrated that surgery enhanced angiogenesis [33] and production of oncogenic growth factor [34], either or both of which may lead to tumor growth. A longer interval between surgery and adjuvant chemotherapy yields a higher risk of proliferation of any residual cancer cells. Chemotherapy is more effective when tumor burden is lower [17]. Therefore, early initiation of adjuvant chemotherapy should more effectively eradicate residual cancer cells.
Our guidelines for use of adjuvant chemotherapy for patients with pancreatic carcinoma are to initiate therapy as soon as possible after surgical resection. However, early initiation of adjuvant chemotherapy is difficult for patients who undergo major surgery such as pancreatoduodenectomy because of prolonged recovery from surgery. In addition, postoperative complications including pancreatic fistula and delayed gastric emptying frequently occur in patients who undergo pancreatic surgery, which also delays initiation of adjuvant chemotherapy [35]. Several investigators reported that the reasons for delay of adjuvant chemotherapy included older age, postoperative complication, more advanced disease stage, and longer recovery from surgery [16, 18–20]. In the current study, patients whose adjuvant chemotherapy was initiated more than 20 days after surgery had a significantly higher rate of postoperative complication than those whose adjuvant chemotherapy was initiated within 20 days after surgery, although no significant differences were found in age or tumor stage between the two groups. Prevention of postoperative complication is quite important for early initiation of adjuvant chemotherapy.
In the current study, gemcitabine plus S-1 was used as adjuvant chemotherapy. S-1 is an oral anticancer drug consisting of tegafur as a prodrug of 5-fluorouracil (5-Fu), 5-chloro-2,4-dihydroxypyridine as a competitive inhibitor of dihydropyrimidine dehydrogenase, and potassium oxonate as a competitive inhibitor of orotate phosphoribosyltransferase [36]. A recent large-scale randomized phase III trial in patients with unresectable pancreatic carcinoma demonstrated that gemcitabine plus S-1 chemotherapy had an excellent response rate (29.3 %) compared with gemcitabine alone (13.3 %); however, no statistically significant difference was seen in overall survival between patients treated with gemcitabine plus S-1 (median survival time, 10.1 months) and patients treated with gemcitabine alone (median survival time, 8.8 months) [37]. Adjuvant chemotherapy using gemcitabine plus S-1 may have contributed to the excellent results for overall or disease-free survival in the current study.
This study has the limitations of being a retrospective analysis on a relatively small number of patients. Large, prospective, randomized controlled studies are needed to confirm the results of this study. However, prospective evaluations comparing survival of early versus late initiation of adjuvant chemotherapy for patients with resected pancreatic carcinoma are unlikely to be conducted for ethical reasons [18, 19, 22]. Meta-analyses using the published studies involving larger number of patients may be useful to resolve this question.
In conclusion, the current study demonstrated that earlier initiation of adjuvant chemotherapy after resection of pancreatic carcinoma improved overall or disease-free survival of these patients and postoperative complication delayed initiation of adjuvant chemotherapy. Adjuvant chemotherapy should be initiated as soon as possible after surgical resection and surgeons should pursue prevention of postoperative complication to enable early initiation of adjuvant chemotherapy.
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakashima A, Yuasa Y, Kondo N, Ohge H, Sueda T (2010) Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J Am Coll Surg 211:196–204
Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, Schulick RD, Choti MA, Lillemoe KD, Yeo CJ (2006) 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 10:1199–1211
Matsuno S, Egawa S, Fukuyama S, Motoi F, Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K, Nakao A, Hiraoka T, Hosotani R, Takeda K (2004) Pancreatic cancer registry in Japan: 20 years of experience. Pancreas 28:219–230
Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD (2000) Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 4:567–579
Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW, European Study Group for Pancreatic Cancer (2010) Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine followed pancreatic cancer resection: a randomized controlled trial. JAMA 304:1073–1081
Van Laethem JL, Hammel P, Mornex F, Azria D, Van Tienhoven G, Vergauwe P, Peeters M, Polus M, Praet M, Mauer M, Collette L, Budach V, Lutz M, Van Cutsem E, Haustermans K (2010) Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J Clin Oncol 28:4450–4456
Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML, Haddock MG, Schaefer P, Willett CG, Rich TA (2008) Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 299:1019–1026
Ueno H, Kosuge T, Matsuyama Y, Yamamoto J, Nakao A, Egawa S, Doi R, Monden M, Hatori T, Tanaka M, Shimada M, Kanemitsu K (2009) A randomized phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for pancreatic cancer. Br J Cancer 101:908–915
Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H (2007) Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297:267–277
Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler MW, European Study Group for Pancreatic Cancer (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350:1200–1210
Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, Nagakawa T, Nakayama T, Study Group of Surgical Adjuvant Therapy for Carcinomas of the Pancreas and Biliary Tract (2002) Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 95:1685–1695
Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A, Wils J (1999) Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 230:776–784
Bakkevold KE, Arnesjo B, Dahl O, Kambestad B (1993) Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater–results of a controlled, prospective, randomised multicentre study. Eur J Cancer 29A:698–703
Gastrointestinal Tumour Study Group (1987) Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer 59:2006–2010
Lima IS, Yasui Y, Scarfe A, Winget M (2011) Association between receipt and timing of adjuvant chemotherapy and survival for patients with stage III colon cancer in Alberta, Canada. Cancer 117:3833–3840
Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM (2011) Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 305:2335–2342
Des Guetz G, Nicolas P, Perret GY, Morere JF, Uzzan B (2010) Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer 46:1049–1055
Cheung WY, Neville BA, Earle CC (2009) Etiology of delays in the initiation of adjuvant chemotherapy and their impact on outcomes for Stage II and III rectal cancer. Dis Colon Rectum 52:1054–1063 (discussion 1064)
Hershman D, Hall MJ, Wang X, Jacobson JS, McBride R, Grann VR, Neugut AI (2006) Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer 107:2581–2588
Alkis N, Durnali AG, Arslan UY, Kocer M, Onder FO, Tokluoglu S, Celenkoglu G, Muallaoglu S, Utkan G, Ulas A, Altundag K (2011) Optimal timing of adjuvant treatment in patients with early breast cancer. Med Oncol 28:1255–1259
Lohrisch C, Paltiel C, Gelmon K, Speers C, Taylor S, Barnett J, Olivotto IA (2006) Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol 24:4888–4894
Colleoni M, Bonetti M, Coates AS, Castiglione-Gertsch M, Gelber RD, Price K, Rudenstam CM, Lindtner J, Collins J, Thürlimann B, Holmberg S, Veronesi A, Marini G, Goldhirsch A (2000) Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. The International Breast Cancer Study Group. J Clin Oncol 18:584–590
Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakagawa N, Ohge H, Sueda T (2008) Adjuvant gemcitabine plus S-1 chemotherapy after surgical resection for pancreatic adenocarcinoma. Am J Surg 195:757–762
Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Ohge H, Sueda T (2009) Impact of adjuvant gemcitabine plus S-1 chemotherapy after surgical resection for adenocarcinoma of the body or tail of the pancreas. J Gastrointest Surg 13:85–92
Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, Nakagawa N, Sueda T (2012) Long-term results of adjuvant gemcitabine plus S-1 chemotherapy after surgical resection for pancreatic carcinoma. J Surg Oncol 106:174–180
Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Ohge H, Sueda T (2007) Prognostic significance of lymph node metastasis and surgical margin status for distal cholangiocarcinoma. J Surg Oncol 95:207–212
Sudo T, Murakami Y, Uemura K, Hayashidani Y, Hashimoto Y, Ohge H, Shimamoto F, Sueda T (2008) Prognostic impact of perineural invasion following pancreatoduodenectomy with lymphadenectomy for ampullary carcinoma. Dig Dis Sci 53:2281–2286
Murakami Y, Uemura K, Ohge H, Hayashidani Y, Sudo T, Sueda T (2006) Intraductal papillary-mucinous neoplasms and mucinous cystic neoplasms of the pancreas differentiated by ovarian-type stroma. Surgery 140:443–453
Murakami Y, Uemura K, Sudo T, Hashimoto Y, Yuasa Y, Sueda T (2010) Prognostic impact of para-aortic lymph node metastasis in pancreatic carcinoma. World J Surg 34:1900–1907
Sobin LH, Gospodarowicz MK, Wittekind C (eds) (2010) International Union Against Cancer (UICC): TNM classification of malignant tumours, 7th edn. Wiley-Blackwell, New York
Kondo N, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Nakashima A, Sakabe R, Shigemoto N, Kato Y, Ohge H, Sueda T (2010) Prognostic impact of perioperative serum CA 19–9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol 17:2321–2329
Folkman J (1990) What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 82:4–6
Eggermont AM, Steller EP, Sugarbaker PH (1987) Laparotomy enhances intraperitoneal tumor growth and abrogates the antitumor effects of interleukin-2 and lymphokine-activated killer cells. Surgery 102:71–78
Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Nakagawa N, Ohge H, Sueda T (2008) No mortality after 150 consecutive pancreatoduodenctomies with duct-to-mucosa pancreaticogastrostomy. J Surg Oncol 97:205–209
Shirasaka T, Shimamoto Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, Fukushima M (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumour selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 7:548–557
Ioka T, Ikeda S, Ohkawa H, Yanagimoto H, Fukutomi A, Sugimori K, Baba H, Yamao K, Shimamura T, Chen JS, Mizumoto K, Furuse J, Funakoshi A, Hatori T, Yamaguchi T, Egawa S, Sato A, Ohashi Y, Cheng AL, Okusaka T (2011) Randomized phase III study of gemcitabine plus S-1 (GS) versus S-1 versus gemcitabine (GEM) in unresectable advanced pancreatic cancer (PC) in Japan and Taiwan: GEST study. J Clin Oncol 29 (suppl; abstr 4007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murakami, Y., Uemura, K., Sudo, T. et al. Early initiation of adjuvant chemotherapy improves survival of patients with pancreatic carcinoma after surgical resection. Cancer Chemother Pharmacol 71, 419–429 (2013). https://doi.org/10.1007/s00280-012-2029-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-2029-1