Abstract
Purpose
Although numerous studies have highlighted the potential value of indocyanine green (ICG) imaging in lymph node dissection of cancer surgery, its efficacy and optimal method remain to be clarified. This study aimed to investigate how lymphatic flow observation via ICG fluorescence could contribute to colon cancer surgery.
Methods
From October 2018 to March 2021, a total of 56 patients with colon cancer who underwent laparoscopic complete mesocolic excision with intraoperative ICG imaging were analyzed. Lymphatic flow was examined at the following time points following ICG injection: within 5 min, 30–60 min, and over 60 min. We also evaluated the distribution of ICG fluorescence per each vascular pedicle.
Results
Lymphatic flow was observed within 5 min following ICG injection in 6 cases (10.7%), and at 30–60 min following ICG injection in 43 cases (76.8%). ICG-stained vascular pedicles were variable especially in hepatic flexural, transverse, and splenic flexural colon cancer. Lymph node metastases were observed in 14 cases. Although metastatic lymph nodes were present only in the area along the ICG-stained vascular pedicles in 12 of the 14 cases, two patients exhibited lymph node metastasis in areas along the ICG-unstained vascular pedicles. ICG fluorescence was observed outside the standard range of lymph node dissection in 9 cases (20.9%: 9/43). Although addition of the proposed resection areas was made in 8 of these 9 cases, there was no pathologically positive lymph node.
Conclusion
Real-time ICG fluorescence imaging of lymph nodes may improve the performance of laparoscopic colon cancer surgery, although its oncological benefit is not yet clear.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Based on the concept of total mesorectal excision for rectal cancer proposed by Heald [1], complete mesocolic excision (CME) with central vascular ligation (CVL) was proposed by Hohenberger as the surgical options used in the treatment for colon cancer [2]. Currently, these techniques are widely accepted for improving oncological outcomes after colon cancer surgery [3]. These techniques focus on en bloc resection of the tumor, its main feeding vessels and lymphatics. However, in colon cancer, it is sometimes difficult to determine the range of sufficient lymph node dissection because of the variability of blood flow or the complexity of lymphatic flow. In the left-sided colon, lymphatic flow may be distributed along the path of various vessels, such as the left branch of middle colic artery (lt-MCA), left colic artery (LCA), and accessory middle colic artery (AccMCA) [4, 5]. Similarly, in the right-sided colon, lymphatic vessels may flow along various vessels, including the ileocolic artery (ICA), right colic artery (RCA), and right branch of middle colic artery (Rt-MCA) [6]. Recently, the application of indocyanine green (ICG) fluorescence has been investigated as a potential method of determining the appropriate range of lymph node dissection.
Numerous studies have discussed the relationship between ICG fluorescence within sentinel lymph nodes and lymph node metastasis [7,8,9]. Sensitivity was reported to range widely from 0 to 100% [10]. Meanwhile, intraoperative observation of the lymphatic flow via ICG fluorescence has also been reported [11, 12]. To maximize the removal of metastatic lymph nodes and minimize the leftover of metastatic lymph nodes are critical for patients with colon cancer. However, owing to the limited research published, the optimal method to use for the determination of the extent of lymph node dissection or the method’s feasibility itself is still controversial.
The purpose of this study was to investigate the optimal timing of fluorescence investigation after ICG injection, and to evaluate how lymphatic flow observation via ICG fluorescence could contribute to colon cancer surgery.
Materials and methods
Study design and participants
This prospective study was conducted at the Kyoto University Hospital, Japan. From October 2018 to March 2021, patients who underwent CME with CVL for colon cancer were enrolled in this study, regardless of the clinical stage or tumor site. Patients with a history of ICG or iodine allergies were excluded from this study. The study protocol was approved by the Ethics Committee of Kyoto University (Y0001), and conformed to the Declaration of Helsinki. Written informed consent was obtained from all of the study participants.
Surgical procedure
After a thorough observation of the abdominal cavity and the tumor location, injections of ICG (Diagnogreen, Daiichi-Sankyo, Tokyo, Japan), consisting of 0.2−0.5 ml at a concentration of 2.5 mg/mL, were administered into the subserosal layer at the both proximal and distal points of the tumor using a 27-gauge winged needle inserted into the abdominal cavity (Online Resource 1). The dose of ICG or the number of injection points was recorded depending on the case. The time required for ICG injection was about 1−2 min. Afterwards, surgical procedures (i.e., complete mesocolic excision with central ligation) were started. As a rule, the lymphatic flow was examined at the following time points using a near infrared camera system (1588 AIM camera system, Stryker Japan K. K., Tokyo, Japan; infrared endoscopic camera system, Olympus, Tokyo, Japan; D-Light P, Karl Storz, Tuttlingen, Germany): within 5 min following ICG injection, 30−60 min following ICG injection, and over 60 min following ICG injection. Within each time frame, ICG fluorescence was measured several times while ensuring that there were no interruptions in the middle of surgical procedures. The extent of intestinal resection and lymph node dissection was based on the surgical treatment strategy described in the guidelines of the Japanese Society for Cancer of the Colon and Rectum [13, 14]. However, if ICG fluorescence was observed to be outside the standard range of lymph node dissection, the extent of resection was changed at the discretion of the participating surgeons, based on the tumor’s location and stage as well as the potential risks of surgical complications.
Outcome measures
The number of patients with ICG-fluorescent lymphatic flow and lymph node visualization was calculated based on the multiple time points after ICG injection. In addition, for patients with ICG fluorescence, we evaluated the distribution of ICG fluorescence per vascular pedicle. Subsequently, patients in whom ICG fluorescence was observed outside the standard range of lymph node dissection were examined to determine whether these lymph nodes had been resected or not, and their pathological results were also documented. The harvested lymph nodes were examined for a pathological diagnosis. After formalin fixation, metastatic foci within the lymph node were evaluated using a cross-section with hematoxylin and eosin-staining.
Statistical analysis
The discrete and continuous variables were described as number and percentage and median (interquartile range). Data were compared with Fisher’s exact test for categorical variables and Wilcoxon rank sum test for continuous variables. A P value of <0.05 was considered statistically significant. All statistical analyses were conducted using R version 3.6.2 software.
Results
Patient characteristics
The flow chart used for patient enrollment is shown in Fig. 1. Of the 67 patients enrolled in the study, ICG was used in 62 patients. Since the aim of this study was to explore the timing of ICG fluorescence observation and its availability in laparoscopic surgery, three cases of laparotomy and one case of endoscopic submucosal ICG injection were excluded from this analysis. After exclusion of another two cases with missing data, 56 patients were included in the final analysis. Patient characteristics are shown in Table 1. The tumor locations were the cecum in 10 cases (17.9%), ascending colon in 18 cases (32.1%), hepatic flexure in 7 cases (12.5%), transverse colon in 7 cases (12.5%), splenic flexure in 7 cases (12.5%), descending colon in 5 cases (8.9%), and sigmoid colon in 2 cases (3.6%). The surgical procedures were ileocecal resection in 8 cases (14.3%), right hemicolectomy in 30 cases (53.6%), transverse colon resection in 4 cases (7.1%), left hemicolectomy in 11 cases (19.6%), sigmoid colon resection in 2 cases (3.6%), and subtotal colectomy in 1 case (1.8%). Based on the TNM Classification [15], there were 20 cases (35.7%) with stage I, 18 cases (32.1%) with stage II, 14 cases (25%) with stage III, and 4 cases (7.1%) with stage IV.
The visualized lymphatic flow
The results of the lymphatic flow visualized via ICG fluorescence at multiple time points are shown in Table 2. Lymphatic flow and lymph nodes could be visualized within 5 min following ICG injection in 6 cases (10.7%), and at 30−60 min following ICG injection in 43 cases (76.8%). There was no case in which lymphatic flow and lymph nodes could be identified only after 60 min following ICG injection. In all cases, there were no adverse events related to ICG injection. Lymphatic flow visualized via ICG fluorescence was not observed in 13 cases (23.2%). In total, the median number of total harvested lymph nodes was 29.5 [18.8−41.3]. In this cohort, the numbers of total harvested lymph nodes and central N3 lymph nodes in the ICG-stained group were not different from those in the ICG-unstained group (28.0 vs. 30.0, P = 0.78; 8.0 vs. 8.0, P = 0.86, respectively).
Distribution of ICG fluorescence with respect to vascular pedicles
The distribution of ICG fluorescence was evaluated with respect to vascular pedicles in relevant cases (Fig. 2). In the cecum and ascending colon cancer (n = 20), ICG-stained vascular pedicles were confined to ICA, RCA, and Rt-MCA. In 13 cases (65%; 13/20), only one vascular pedicle was ICG-stained. In the hepatic flexural colon cancer (n = 5), ICG-stained vascular pedicles were mainly confined to RCA and Rt-MCA, while ICG fluorescence was observed at the region of right gastroepiploic artery (RGEA) in one case (20%; 1/5). In the transverse colon cancer (n = 6), ICG-stained vascular pedicles were extended to Rt-MCA, Lt-MCA, AccMCA, and LCA. Of note, two vascular pedicles were ICG-stained in 4 cases (67%; 4/6). In the splenic flexural colon cancer (n = 5), ICG-stained vascular pedicles were extended to Lt-MCA, AccMCA, LCA, and sigmoidal branch. In 3 cases (60%; 3/5), two vascular pedicles were ICG-stained. In the descending colon cancers (n = 5), ICG-stained vascular pedicles were confined to LCA and AccMCA. Although the distribution of ICG-stained vascular pedicles essentially depended on the tumor location, there were some variations that did not match the tumor location. For example, in two cases, ICG fluorescence was visualized within the lt-MCA region, even in tumors on the right side of the transverse colon. In one case, ICG fluorescence was also visualized within the LCA region, when the tumor itself was located at the middle part of the transverse colon.
Among the 43 cases showing ICG fluorescence, lymph node metastasis was observed in 14 cases (32.5%). In 12 of the 14 cases, metastatic lymph nodes were present only in the area along the ICG-stained vascular pedicles. In contrast, two patients exhibited lymph node metastasis in areas along the ICG-unstained vascular pedicles. In the two cases, multiple lymph nodes were pathologically positive (pN1b, pN2).
ICG fluorescence outside the standard range of lymph node dissection
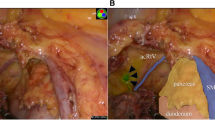
Of the 43 cases showing ICG fluorescence, 9 cases (20.9%) showed ICG fluorescence outside the standard range of lymph node dissection (Table 3). Examples are shown in Fig. 3 (the region of the small bowel mesentery) and Fig. 4 (the region of the RGEA). In 8 of these 9 cases, additions of the proposed resection areas were made. No adverse events, such as vascular injury or conversion to laparotomy, occurred during these procedures. In all these cases, there was no pathologically positive lymph node.
Case of laparoscopic right hemicolectomy for ascending colon cancer. Five minutes following ICG injection, the lymph flow of ICG fluorescence was directed toward the small bowel mesentery. The separation line of the mesentery was determined to include the area of ICG fluorescence. a Normal light view. b ICG fluorescence-mediated view
Discussion
Intraoperative ICG fluorescence was originally developed to assess the quality of coronary artery bypass grafts and sentinel lymph node navigation in breast cancer surgery [16, 17]. Currently, it is also used in various surgical fields, such as hepatobiliary and plastic surgeries [18,19,20]. In colorectal surgery, there are many studies on the use of ICG angiography and sentinel lymph node mapping [7,8,9, 21,22,23,24]. Assessing blood flow at the anastomotic site using ICG angiography can reduce the rate of postoperative anastomotic leakage [21,22,23,24]. Regarding the relationship between ICG fluorescence within sentinel lymph nodes and lymph node metastasis, sensitivity has been reported to range widely from 0 to 100%, and so is still inconclusive [10].
Recently, the use of ICG in the observation of lymphatic flow has been recognized [11, 12]. Lymphatic flow observation may contribute to determine the separation line of the mesentery and to identify the central vessels to be dissected [11, 12]. However, studies pertaining to lymphatic flow observation using ICG fluorescence are limited in number. The method of ICG injection and the timing of ICG fluorescence observation vary in published literature, and its usefulness remains a matter of contention.
We examined the patients with ICG fluorescence visualization, based on the timing of observed fluorescence. To the best of our knowledge, previous reports on lymphatic flow observation assessed the outcome of a single time point observation, and this is the first study to examine the outcome using multiple time point observations. In this study, there was no case in which lymphatic flow and lymph nodes could be identified only after 60 min following ICG injection, which suggests that an evaluation within 30–60 min following ICG injection can provide some information about lymphatic flow. In our study, lymphatic flow was observed in 76.8% (43/56), consistent with the frequency reported in previous studies [25, 26]. Although some studies reported the influencing factors for detection of visualized lymphatic flow [25, 26], no apparent associated factors were identified in our study (Online Resource 2). Technical problems, such as insufficient injection of ICG into the subserosal layer, might have prevented the observation of lymphatic flow.
We also examined the frequency of ICG fluorescence in each pedicle, and found that ICG-stained vascular pedicles were variable especially in hepatic flexural, transverse, and splenic flexural colon cancer (Fig. 2). In addition, we found some variations that did not match the tumor location. The ability of ICG fluorescence to detect such differences in lymphatic flow has the potential to be an invaluable asset for surgeons, in determining the range of sufficient lymph node dissection. In contrast, the relationship between ICG fluorescence accumulation and the pathological findings of lymph nodes has been discussed in terms of obstruction of lymphatic vessels or lymph nodes [12]. ICG generally binds to macrophages within the lymph nodes. Some groups reported that the areas occupied by cancer cells within metastatic lymph nodes emitted no fluorescence, and that partially deformed fluorescence was observed in the nodes where some lymphoid tissues still remained [27, 28]. In this study, two patients exhibited lymph node metastasis in areas along the vascular pedicles where ICG fluorescence was not visible. Considering that both patients had multiple pathologically positive lymph nodes, it is possible that a bypass due to lymphatic vessels or lymph node obstruction could have led to this result. Therefore, even if there is no ICG fluorescence accumulation along the vascular pedicle that is intended to be dissected, the lymph node dissection around that pedicle should not be omitted.
It is also unclear what the best action is when fluorescent lymph nodes are observed outside the standard range of lymph node dissection. In previous reports, ICG fluorescence contributed to changes regarding the central vessels to be dissected or the surgical plan itself, in about 20–30% of patients [12, 29, 30]. In our study, lymph nodes were fluorescent outside the planned range of lymph node dissection in 9 (20.9%) of 43 cases with ICG fluorescence. Some studies described that metastatic spread to the lymph node along the RGEA was found in about 4–5% of the patients with hepatic flexural colon cancer [2, 31, 32]. However, these lymph nodes are outside of the mesocolic area, and classified as sites of distant metastasis (stage IV) according to the TNM classification [15]. As a variation in the branching pattern of the RCA, branching from the RGEA was reported [33]. Considering our results, it is possible that there are some cases where lymphatic flow along the colon mesentery could be also connected to that along the RGEA. Although a discussion on the necessity of dissection in this region is not within the purview of this study, future research is needed in that avenue, especially regarding ICG fluorescence. On pathological examination, none of the eight patients in whom the resection boundaries had been changed exhibited lymph node metastasis. Although ICG fluorescence can provide surgeons with information about lymphatic flow, a relationship between ICG fluorescence and the lymph node metastases remains yet to be established. Although ICG fluorescence may be quite useful in fine adjustment of the mesenteric dissection line, substantial expansion or change of lymph node dissection area should be determined on a case-by-case basis.
In this study, we investigated ICG fluorescence at multiple time points following ICG injection, and prospectively recorded the visualization for each pedicle. The potential advantages of intraoperative ICG injection are that it eliminates the need for preoperative colonoscopy several days before surgery and allows for intraoperative decision-making based on the differences in fluorescence at each point. Despite this possibility, to the best of our knowledge, very few studies have reported intraoperative laparoscopic ICG injection, not under preoperative colonoscopy, for observing lymphatic flow. This is the first study to consider fluorescence imaging observations with respect to the time course following ICG injection. However, there are some limitations in this study. First, this was an exploratory analysis of a single cohort at a single-center, and statistical considerations were difficult due to the small sample size. There was a discrepancy between the tumor localization and staging. Subgroup analysis might have been possible with a larger number of cases. Second, ICG fluorescence imaging was not quantitative; therefore, the uncertainty of detection cannot be denied. Individual differences in the presence of RCA and AccMCA may have influenced the results. However, we collected data prospectively and also checked the collected data via a video review (conducted by H.K and K.K). Third, long-term outcomes are beyond the scope of this study. Further large-scale studies with objective measurements are required to confirm these findings.
Conclusion
In conclusion, an evaluation within 30–60 min following ICG injection can provide surgeons with information about lymphatic flow. ICG fluorescence may also contribute to the determination of the appropriate separation line of the mesentery, and the identification of the central vessels to be dissected. A clearcut relationship between ICG fluorescence and the lymph node metastases remains elusive, and large-scale studies are required in the future.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
16 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00423-023-02824-5
References
Heald RJ (1988) The 'Holy Plane' of rectal surgery. J R Soc 81:503–508
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis 11:354–364
West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P (2010) Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 28:272–278
Rusu MC, Vlad M, Voinea LM, Curcă GC, Sişu AM (2022) Detailed anatomy of a left accessory aberrant colic artery. Surg Radiol Anat 30:595–599
Murono K et al (2022) Vascular anatomy of the splenic flexure: a review of the literature. Surg Today 52:727–735
Gamo E et al (2016) The superior mesenteric artery and the variations of the colic patterns. A new anatomical and radiological classification of the colic arteries. Surg Radiol Anat 38:519–527
Hirche C et al (2012) Ultrastaging of colon cancer by sentinel node biopsy using fluorescence navigation with indocyanine green. Int J Colorectal Dis 27:319–324
Currie AC et al (2017) A pilot study to assess near infrared laparoscopy with indocyanine green (ICG) for intraoperative sentinel lymph node mapping in early colon cancer. Eur J Surg Oncol 43:2044–2051
Villegas-Tovar E et al (2020) Performance of Indocyanine green for sentinel lymph node mapping and lymph node metastasis in colorectal cancer: a diagnostic test accuracy meta-analysis. Surg Endosc 34:1035–1047
Liberale G et al (2018) Indocyanine green fluorescence imaging for sentinel lymph node detection in colorectal cancer: a systematic review. Eur J Surg Oncol 44:1301–1306
Watanabe J, Ota M, Suwa Y, Ishibe A, Masui H, Nagahori K (2017) Evaluation of lymph flow patterns in splenic flexural colon cancers using laparoscopic real-time indocyanine green fluorescence imaging. Int J Colorectal Dis 32:201–207
Nishigori N et al (2016) Visualization of lymph/blood flow in laparoscopic colorectal cancer surgery by ICG fluorescence imaging (Lap-IGFI). Ann Surg Oncol 23(Suppl 2):S266–S274
Watanabe T et al (2018) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 23:1–34
Hashiguchi Y et al (2020) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25:1–42
Brierley JD, Gospodarowicz MK, Wittekind C (eds) (2016) TNM classification of malignant tumours, 8th edn. Wiley-Blackwell, NJ
Murawa D, Hirche C, Dresel S, Hünerbein M (2009) Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg 96:1289–1294
Reuthebuch O et al (2004) Novadaq SPY: intraoperative quality assessment in off-pump coronary artery bypass grafting. Chest 125:418–424
Cheung TT et al (2018) Pure laparoscopic hepatectomy with augmented reality-assisted indocyanine green fluorescence versus open hepatectomy for hepatocellular carcinoma with liver cirrhosis: a propensity analysis at a single center. Asian J Endosc Surg 11:104–111
Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N (2010) Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg 97:1369–1377
Pruimboom T, Schols RM, Van Kuijk SM, Van der Hulst RR, Qiu SS (2020) Indocyanine green angiography for preventing postoperative mastectomy skin flap necrosis in immediate breast reconstruction. Cochrane Database Syst Rev 4:Cd013280
Emile SH, Khan SM, Wexner SD (2022) Impact of change in the surgical plan based on indocyanine green fluorescence angiography on the rates of colorectal anastomotic leak: a systematic review and meta-analysis. Surg Endosc 36:2245–2257
Wada T et al (2019) The effects of intraoperative ICG fluorescence angiography in laparoscopic low anterior resection: a propensity score-matched study. Int J Clin Oncol 24:394–402
Kawada K et al (2017) Evaluation of intestinal perfusion by ICG fluorescence imaging in laparoscopic colorectal surgery with DST anastomosis. Surg Endosc 31:1061–1069
Wada T et al (2017) ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 31:4184–4193
Ushijima H et al (2020) Visualization of lymphatic flow in laparoscopic colon cancer surgery using indocyanine green fluorescence imaging. Sci Rep 10:14274
Ahn HM et al (2021) Optimal ICG dosage of preoperative colonoscopic tattooing for fluorescence-guided laparoscopic colorectal surgery. Surg Endosc 36:1152–1163
Kakizoe M et al (2021) The histopathological evaluation based on the indocyanine green fluorescence imaging of regional lymph node metastasis of splenic flexural colon cancer by near-infrared observation. Int J Colorectal Dis 36:717–723
Sato Y et al (2021) Snapshots of lymphatic pathways in colorectal cancer surgery using near-infrared fluorescence, in vivo and ex vivo. Eur J Surg Oncol 47:3130–3136
Watanabe J, Ota M, Suwa Y, Ishibe A, Masui H, Nagahori K (2016) Real-time indocyanine green fluorescence imaging-guided complete mesocolic excision in laparoscopic flexural colon cancer surgery. Dis Colon Rectum 59:701–705
Petz W, Bertani E, Borin S, Fiori G, Ribero D, Spinoglio G (2021) Fluorescence-guided D3 lymphadenectomy in robotic right colectomy with complete mesocolic excision. Int J Med Robot 17:e2217
Ye K, Lin J, Sun Y, Wu Y, Xu J, He S (2018) Variation and treatment of vessels in laparoscopic right hemicolectomy. Surg Endosc 32:1583–1584
Wang X, Huang S, Lu X, Huang Y, Chi P (2021) Incidence of and risk factors for gastroepiploic lymph node involvement in patients with cancer of the transverse colon including the hepatic flexure. World J Surg 45:1514–1525
Zoulamoglou M et al (2017) Anomalous origin of the right colic artery from the right gastroepiploic artery during complete mesocolic excision: a rare case report. J Surg Case Rep 2017:rjx204
Acknowledgements
The authors thank medical staffs and residents of Kyoto University Hospital gastrointestinal surgery for their participation in this study.
Author information
Authors and Affiliations
Contributions
K.K. and H.K. designed the study; contributed substantially to data acquisition, analysis, and interpretation; wrote the manuscript; and prepared the figures and tables. K.K., H.K., Y.I., R.O., N.O., T.O., and K.H. contributed to acquisition of the data. Y.I., R.O., N.O., T.O., K.H., and K.O. participated in drafting of the article. All authors revised the text critically for important intellectual content and gave approval of the final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the Ethics Committee of Kyoto University (Y0001), and conformed to the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all of the study participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary informations
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kinoshita, H., Kawada, K., Itatani, Y. et al. Timing of real-time indocyanine green fluorescence visualization for lymph node dissection during laparoscopic colon cancer surgery. Langenbecks Arch Surg 408, 38 (2023). https://doi.org/10.1007/s00423-023-02808-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-02808-5