Abstract
Purpose
Lymph node status is the most important prognostic factor in colon cancer, but the role of sentinel lymph node biopsy (SLNB) as a tool for identification of micrometastatic disease and extraanatomical lymph nodes for adjuvant strategies and a tailored approach still remains unclear. Indocyanine green (ICG) fluorescence detection is a new method for SLNB allowing real-time lymphography and lymph node detection. This study was designed to evaluate the feasibility of fluorescence-guided sentinel lymph node detection in colon carcinoma.
Methods
Twenty six patients with colon adenocarcinoma were prospectively included in this study. Intraoperatively, a peritumorous injection with a mean of 2.0 ml ICG was performed, followed by lymphatic mapping and SLNB. Clinical feasibility, detection rate, and sensitivity of the method were analyzed.
Results
No adverse reactions occurred due to the injection of ICG. Overall, ICG fluorescence imaging identified 1.7 sentinel lymph node (SLN) in average in 25 out of 26 patients (detection rate, 96%). Metastatic involvement of the SLN was found in nine out of 11 nodal positive patients by conventional histopathology. The sensitivity of the method was 82% for colon carcinoma, respectively.
Conclusion
ICG fluorescence imaging is a new, feasible method for SLNB of colon carcinoma and enables ultrastaging with improved accuracy but with limited validity due to the small number of cases. One advantage of this technique is real-time visualization of lymphatic vessels and SLNB without radiation exposure. Further, larger series are necessary to analyze the role of fluorescence-guided SLNB for colon cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sentinel lymph node (SLN) biopsy is a widely used and accurate method for the lymphonodal staging of breast cancer and melanoma [1, 2]. Recently, colon cancer has also been identified as a target for lymphatic navigation and SLN biopsy [3, 4]. It has been shown that lymph node status is an important prognostic factor as survival is correlated with node-positive status [5]. While several investigators have reported that SLN biopsy allows a minimally invasive access for individualized lymphadenectomy, thus reducing perioperative morbidity and improving the postoperative function in gastric cancer, the definitive role in colon cancer still needs to be defined [6–8]. In particular, colon cancer patients with early-stage tumor may benefit from an individual nodal approach, with the aim of ultrastaging in current strategies for the identification of nodal positive disease with indication for adjuvant chemotherapy [9]. Moreover, there are concerns about understaging the group of patients with colon cancer who develop recurrent, lymphonodal disease in up to 30% of patients [10].
SLNB for colon cancer is usually guided by blue dye, a radiocolloid, or a combination of both [4, 11]. The dye-guided method allows convenient proceeding, but there are application limitations due to a high false-negative node rate approaching 40% in some studies, a reduced tissue contrast, and a low but significant risk of anaphylactic reactions [12–15]. The radiocolloid-guided method enables increased detection rates, but the high radioactivity at the injection site may interfere with intraoperative gamma probe navigation to proximate lymph nodes [16]. Finally, the use of radioactive colloids involves additional expensive equipment and radiation protection measures, which is not available in all institutions [17].
Some investigators have focused on new approaches for SLN biopsy to conjoin the advantages of both the dye- and radiocolloid-guided methods. SLN biopsy by indocyanine green (ICG) fluorescence guidance has been shown to implement high detection rates and a low false-negative rate for breast cancer, skin cancer, anal cancer, and gastric cancer [18–22], but there is only limited experience with the application of fluorescence navigation in colon [23].
The aim of this study was to investigate the clinical application and feasibility of SLN biopsy guided by ICG fluorescence for colon cancer and to evaluate the application spectrum of fluorescence-guided SLN biopsy.
Patients and methods
Study design and patients
This study was prospectively designed and approved by the Institutional Review Board of the hospital. Preoperatively, informed consent was obtained from all patients. Inclusion criteria were histopathological diagnosis of colon cancer, image-guided tumor diameter without distinct evidence for serosal perforation, and exclusion of clinically and image-guided positive lymph node status. Nodal staging for colon cancer was performed with a 5–7.5-MHz endocavitary probe (GE Medical Systems Kretztechnik GmbH, Zipf, Austria) for EUS (GE Kretz Voluson 530 D, Munich, Germany) and contrast agent-enhanced computed tomography. Exclusion criteria comprised previous operations and/or radiation, definite lymph node metastases diagnosed preoperatively, preoperative tumor stage T4, pregnancy, and age <18 years.
Twenty six patients were enrolled in the study: 6 patients with cancer of the left colon, 7 patients of the right colon, 12 patients with sigmoid cancer, and 1 patient with multifocal colon cancer (1 carcinoma, 2 high-grade dysplasias) on the basis of hereditary nonpolyposis colorectal cancer. For this patient, as the definite analysis of pathology revealed one T3 tumor and two high-grade dysplasias, only the T3 tumor was recorded for analysis.
Fifteen patients with colon cancer underwent in vivo SLN biopsy after intraoperative dye injection with conventional approach, while 11 patients with colon cancer underwent laparoscopy-assisted surgery with SLN biopsy performed after mini-laparotomy. All patients underwent intraoperative injection of the fluorescent tracer followed by SLN biopsy, radical tumor resection, and lymph node dissection according to current oncological guidelines.
Detection rate, sensitivity, and false-negative rate were calculated by comparing the results of SLN biopsy and histopathology of the lymphatic basin.
-
Detection rate (%) = Number of successful attempts to retrieve a SLN × 100, divided by number of attempts to retrieve a SLN.
-
Sensitivity (%) = Number of patients with a positive SLN × 100, divided by number of patients with any positive lymph node after dissection and histopathological preparation of the lymph node basin.
-
Upstaging = Number of patients revealing micrometastases or isolated tumor cells in the SLN by immunohistochemistry × 100, divided by patients classified as lymphonodal negative after conventional histopathological examination.
Detailed clinicopathological data and tumor characteristics after histological analysis are summarized in Table 1.
ICG fluorescence imaging and SLN detection
ICG fluorescence was visualized with a fluorescence imaging system (IC-View, Pulsion Medical Systems, Munich, Germany) consisting of a digital video camera with an integrated near-infrared (NIR) light source (energy 0.16 W, wavelength 780 nm). The objective of the camera was covered with a filter (835 nm) to collect NIR radiation and reject visible light. Intraoperatively, a mean of 2.0 ml (range, 1–4 ml) ICG solution (5 mg/ml) was injected during the operation by another surgeon for in situ visualization (16 patients) or intraoperatively around the tumor (ten patients). After a short latency (approximately 3–10 min), the lymphatic transport of ICG to the lymphonodal compartments was observed in real time with fluorescence imaging. The end of the lymphatic vessel was explored for SLN detection. The bright nodes were dissected from the surrounding fatty tissue and re-examined to confirm the internal fluorescence. Fluorescence lymphography including SLN identification and SLN biopsy was performed by two experienced surgeons.
Histopathology
First, the SLNs were analyzed with a conventional staining. All SLN negative for metastatic involvement underwent specific evaluation using a standardized protocol for immunohistochemistry. The detailed technique has been described by Meyer [24]. Briefly, SLN tissue was sliced at 2-mm intervals for fixation and paraffin embedding. Microsections were prepared repeatedly at intervals of 250 μm and examined by routine hematoxylin–eosin (HE) staining. Negative SLN were re-examined by serial sectioning at intervals of 5 μm, and one microsection of each level was analyzed by HE staining and a second by cytokeratin antibody for immunohistochemistry (MNF 116, Dako, Hamburg, Germany). All non-SLNs were examined by standard pathologic measures including HE staining. The extent of lymph node dissection for colon cancer was not modified depending on the sentinel lymph node status.
Results
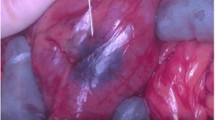
None of the 26 patients experienced adverse reactions related to the injection of ICG. The mean number of lymph nodes excised was 32.9 (range, 10–143) for colon cancer including the SLNs. Identification of the SLN was facilitated by transmesenterial visualization of lymphatic vessels and lymph node basin exploration by fluorescence navigation to identify the bright node (Fig. 1). Lymph nodes taking up ICG appeared as round spots emitting a clearly differentiated fluorescence for identification of the SLN after lymphography and were excised for approval by fluorescence (Fig. 1). ICG fluorescence detection revealed a mean number of 1.7 SLNs (range, 0–5 SLN) in 25 out of 26 patients (detection rate, 96%), although none of the lymph nodes showed green staining. The case with an undetected SLN was a patient treated with colon cancer; the histopathological dissection resulted in a lymphonodal negative stage. The clinicopathological data of all patients are summarized in Table 1.
SLN detection in right colon cancer: After mobilization of the colon and the lymph node regions (a), a fluorescence image for real-time lymphography is obtained. The bright node at the end of the lymphatic vessels (arrows) is identified as the SLN (b) and excised under room light conditions (c). A final fluorescence image approves excised SLN with fluorescence retention (d)
Eleven out of 26 patients had metastatic lymph node involvement after dissection of the basin. ICG fluorescence imaging identified metastatic SLNs in nine out of 11 patients after histopathological analysis according to the SLN protocol (sensitivity, 82%). There were two cases with both a negative SLN after right hemicolectomy for colon cancer with positive non-SLN (NSLN) resulting in a false-negative rate of 18%. Immunohistochemistry revealed three out of nine cases with isolated tumor cells (ITC) in the SLN which would have not been detected by conventional histological methods providing upstaging in 3 cases (33%). Altogether, five out of nine cases with positive SLN showed only lymphonodal involvement of the SLN with negative NSLN.
All patient with positive isolated SLN or lymphonodal positive patients received adjuvant chemotherapy for colon cancer. The detailed results of SLN fluorescence navigation are displayed in Table 2.
Discussion
Sentinel lymph node biopsy in colon carcinoma aims at an accurately tailored lymphonodal approach by upstaging and identification of aberrant lymph nodes, but validity, clinical usefulness, and the optimal technique of SLN mapping for colon cancer remain unclear and are still a major focus of investigations [16].
In this study evaluating the SLNB for colon carcinoma by ICG guidance, the identification rate of 96% demonstrates the feasibility of fluorescence navigation for SLNB in colon cancer patients providing accurate detection of the bright SLN. The lymphatic drainage and the nodes were clearly visualized with fluorescence of the nodes. None of the SLNs were visible in green color by room light. The accuracy of ICG imaging was not due to an increased number of analyzed SLNs, only a mean of 1.7 SLNs was detected and processed.
Most studies on SLNB for colon cancer have predominantly used a radiocolloid, a blue dye, or combination of both, but some drawbacks remain with the application of both methods. For colon cancer, conventional methods showed detection rates ranging between 81% and 98% with a sensitivity in larger series of up to 90% [25–27]. ICG fluorescence guidance enables an alternative technical approach focusing on the drawbacks of the conventional methods with promising accuracy for further investigations. ICG guidance has been shown not to be influenced by the body mass index (BMI) or lymphatic invasion as shown for the conventional methods [12]. Fluorescence navigation in colon cancer aims at ultrastaging and allows detection of aberrant lymphatic drainage outside the planned resection. Fluorescence guidance as demonstrated with ICG appears as an interesting tool for investigations on laparoscopic SLN biopsy with an endoscopic camera [28].
As proofed in this study, ex vivo dye injection was an effective alternative to in vivo injection for identifying SLNs as demonstrated before [26, 29]. An upstaging by ITC detection was seen in 33% of all patients. These patients underwent adjuvant chemotherapy without evidence for lymph node metastases according to conventional staining. In another study, the upstaging for colon cancer patients was achieved in up to 33% of cases as well [30]. Nevertheless, the prognostic significance and optimal management according to stage migration after ITC identification and ultrastaging remain unclear [31]. In a recent study, Wiese et al. analyzed whether ultrastaging of initially negative non-SLNs would increase nodal positivity in colon cancer and demonstrated only little benefit by ultrastaging only 1% of patients [32]. With regard to current literature, SLNB for colon cancer should only be approached according to research protocols [33].
However, some drawbacks of this study remain; there is only a reduced number of cases which has been included into this study with limited validity. Nevertheless, the method has been shown as feasible and safe which justifies further analyses in larger series to determine the definitive role of ICG-guided SLNB in colon cancer patients. Despite the accurate preoperative selection of patients without advanced tumor stage, the study population included a few patients with advanced or metastatic disease due to histopathological specimen, after all, that may have affected the results of this study but without influence on the detection rate. There was a high detection rate by fluorescence navigation, but the false-negative rate needs to be optimized furthermore in larger series. The study lacks an intraindividually applied, direct comparison to other methods. Bearing in mind a methodical drawback, there were some concerns on possible interactions between both blue dye and ICG with decreased detection rate. A direct comparison analyzing possible interactions with an increased number of patients would help to provide further validation of the ICG technique.
The ICG-guided method was shown to conjoin advantages of both the dye and radiocolloid methods and allowed real-time lymphography. At this time, we recommend the procedure for ultrastaging of colon cancer patients as a basis for adjuvant therapeutic regimes in ambiguous cases and to reduce the rate of potentially under-staged patients using study protocols [34]. Altogether, ICG navigation enables a broad application spectrum for SLNB in solid tumors; thus, investment in equipment appears reasonable with limited level of evidence for use under study conditions.
Abbreviations
- SLN:
-
Sentinel lymph node
- ICG:
-
Indocyanine green
References
Zavagno G, De Salvo GL, Scalco G et al (2008) A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg 247:207–213
Sabel MS, Griffith KA, Arora A et al (2007) Inguinal node dissection for melanoma in the era of sentinel lymph node biopsy. Surgery 141:728–735
Mulsow J, Winter DC, O’Keane JC, O’Connell PR (2003) Sentinel lymph node mapping in colorectal cancer. Br J Surg 90:659–667
Kelder W, Braat AE, Karrenbeld A et al (2007) The sentinel node procedure in colon carcinoma: a multi-centre study in The Netherlands. Int J Colorectal Dis 22:1509–1514
Hermanek P (1995) pTNM and residual tumor classifications: problems of assessment and prognostic significance. World J Surg 19:184–190
Kitagawa Y, Fujii H, Mukai M, Kubota T, Otani Y, Kitajima M (2002) Radio-guided sentinel node detection for gastric cancer. Br J Surg 89:604–608
Trocha SD, Nora DT, Saha SS, Morton DL, Wiese D, Bilchik AJ (2003) Combination probe and dye-directed lymphatic mapping detects micrometastases in early colorectal cancer. J Gastrointest Surg 7:340–345
Bembenek A, Schneider U, Gretschel S, Fischer J, Schlag PM (2005) Detection of lymph node micrometastases and isolated tumor cells in sentinel and nonsentinel lymph nodes of colon cancer patients. World J Surg 29:1172–1175
Iddings D, Bilchik A (2007) The biologic significance of micrometastatic disease and sentinel lymph node technology on colorectal cancer. J Surg Oncol 96:671–677
Joseph NE, Sigurdson ER, Hanlon AL et al (2003) Accuracy of determining nodal negativity in colorectal cancer on the basis of the number of nodes retrieved on resection. Ann Surg Oncol 10:213–218
Bell SW, Mourra N, Flejou JF, Parc R, Tiret E (2005) Ex vivo sentinel lymph node mapping in colorectal cancer. Dis Colon Rectum 48:74–79
Bembenek AE, Rosenberg R, Wagler E et al (2007) Sentinel lymph node biopsy in colon cancer: a prospective multicenter trial. Ann Surg 245:858–863
Cimmino VM, Brown AC, Szocik JF et al (2001) Allergic reactions to isosulfan blue during sentinel node biopsy—a common event. Surgery 130:439–442
Saha S, Sehgal R, Patel M et al (2006) A multicenter trial of sentinel lymph node mapping in colorectal cancer: prognostic implications for nodal staging and recurrence. Am J Surg 191:305–310
Read TE, Fleshman JW, Caushaj PF (2005) Sentinel lymph node mapping for adenocarcinoma of the colon does not improve staging accuracy. Dis Colon Rectum 48:80–85
Bembenek A, String A, Gretschel S, Schlag PM (2008) Technique and clinical consequences of sentinel lymph node biopsy in colorectal cancer. Surg Oncol 17:183–193
Stratmann SL, McCarty TM, Kuhn JA (1999) Radiation safety with breast sentinel node biopsy. Am J Surg 178:454–457
Fujiwara M, Mizukami T, Suzuki A, Fukamizu H (2009) Sentinel lymph node detection in skin cancer patients using real-time fluorescence navigation with indocyanine green: preliminary experience. J Plast Reconstr Aesthet Surg 62:e373–e378
Hirche C, Murawa D, Mohr Z, Kneif S, Hunerbein M (2010) ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat 121:373–378
Hirche C, Dresel S, Krempien R, Hunerbein M (2010) Sentinel node biopsy by indocyanine green retention fluorescence detection for inguinal lymph node staging of anal cancer: preliminary experience. Ann Surg Oncol 17:2357–2362
Ogasawara Y, Ikeda H, Takahashi M, Kawasaki K, Doihara H (2008) Evaluation of breast lymphatic pathways with indocyanine green fluorescence imaging in patients with breast cancer. World J Surg 32:1924–1929
Tajima Y, Murakami M, Yamazaki K, Kato T, Kusano M (2009) Sentinel node mapping guided by indocyanine green fluorescence imaging in gastric cancer. Ann Surg 249:58–62
Kusano M, Tajima Y, Yamazaki K, Kato M, Watanabe M, Miwa M (2008) Sentinel node mapping guided by indocyanine green fluorescence imaging: a new method for sentinel node navigation surgery in gastrointestinal cancer. Dig Surg 25:103–108
Meyer JS (1998) Sentinel lymph node biopsy: strategies for pathologic examination of the specimen. J Surg Oncol 69:212–218
Cahill RA, Bembenek A, Sirop S et al (2009) Sentinel node biopsy for the individualization of surgical strategy for cure of early-stage colon cancer. Ann Surg Oncol 16:2170–2180
Park JS, Chang IT, Park SJ et al (2009) Comparison of ex vivo and in vivo injection of blue dye in sentinel lymph node mapping for colorectal cancer. World J Surg 33:539–546
Joosten JJ, Strobbe LJ, Wauters CA, Pruszczynski M, Wobbes T, Ruers TJ (1999) Intraoperative lymphatic mapping and the sentinel node concept in colorectal carcinoma. Br J Surg 86:482–486
Ishikawa K, Yasuda K, Shiromizu A, Etoh T, Shiraishi N, Kitano S (2007) Laparoscopic sentinel node navigation achieved by infrared ray electronic endoscopy system in patients with gastric cancer. Surg Endosc 21:1131–1134
Rivet EB, Mutch MG, Ritter JH et al (2010) Ex vivo sentinel lymph node mapping in laparoscopic resection of colon cancer. Colorectal Dis. doi:10.1111/j.1463-1318.2010.02450.x
Saha S, Dan AG, Viehl CT, Zuber M, Wiese D (2005) Sentinel lymph node mapping in colon and rectal cancer: its impact on staging, limitations, and pitfalls. Cancer Treat Res 127:105–122
Soni M, Wiese D, Korant A et al (2011) Comparison of nodal positivity between SLNM vs conventional surgery in colon cancer patients with <12 and ≥12 lymph nodes harvested. Am J Surg 202:207–2013
Wiese D, Sirop S, Yestrepsky B et al (2010) Ultrastaging of sentinel lymph nodes (SLNs) vs. non-SLNs in colorectal cancer—do we need both? Am J Surg 199:354–358
Madbouly KM, Senagore AJ, Mukerjee A, Delaney CP, Connor J, Fazio VW (2007) Does immunostaining effectively upstage colorectal cancer by identifying micrometastatic nodal disease? Int J Colorectal Dis 22:39–48
Des Guetz G, Uzzan B, Nicolas P et al (2007) Is sentinel lymph node mapping in colorectal cancer a future prognostic factor? A meta-analysis. World J Surg 31:1304–1312
Acknowledgments
This study was supported by grants of the HELIOS Research Center (HRC), Berlin, Germany and the Charite-Universitätsmedizin Berlin, Germany.
Conflict of interest statement
Mr. C. Hirche, Mrs. Z. Mohr, Mr. S. Kneif, Mr. S. Doniga, Mr. D. Murawa, Mr. M. Strik, and Mr. M. Hünerbein did not receive any support from industry which may be linked to this study, and no conflict of interest in participating in and publishing this study is declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirche, C., Mohr, Z., Kneif, S. et al. Ultrastaging of colon cancer by sentinel node biopsy using fluorescence navigation with indocyanine green. Int J Colorectal Dis 27, 319–324 (2012). https://doi.org/10.1007/s00384-011-1306-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-011-1306-5