Abstract
Purpose
To compare the refractive outcomes following cataract surgery using conventional keratometry (K) and total keratometry (TK) for intraocular lens (IOL) calculation in the SRK/T, HofferQ, Haigis, and Holladay 1 and 2, as well as Barrett and Barrett TK Universal II formulas.
Methods
Sixty eyes of 60 patients from Siriraj Hospital, Thailand, were prospectively enrolled in this comparative study. Eyes were assessed using a swept-source optical biometer (IOLMaster 700; Carl Zeiss Meditec, Jena, Germany). Posterior keratometry, K, TK, central corneal thickness, anterior chamber depth, lens thickness, axial length, and white-to-white corneal diameter were recorded. Emmetropic IOL power was calculated using K and TK in all formulas. Selected IOL power and predicted refractive outcomes were recorded. Postoperative manifest refraction was measured 3 months postoperatively. Mean absolute errors (MAEs), median absolute errors (MedAEs), and percentage of eyes within ± 0.25, ± 0.50, and ± 1.00 D of predicted refraction were calculated for all formulas in both groups.
Results
Mean difference between K and TK was 0.03 D (44.56 ± 1.18 vs. 44.59 ± 1.22 D), showing excellent agreement (ICC = 0.99, all p < 0.001). Emmetropic IOL powers in all formulas for both groups were very similar, with a trend toward lower MAEs and MedAEs for TK when compared with K. The Barrett TK Universal II formula demonstrated the lowest MAEs. Proportion of eyes within ± 0.25, ± 0.50, and ± 1.00 D of predicted refraction were slightly higher in the TK group.
Conclusions
Conventional K and TK for IOL calculation showed strong agreement with a trend toward better refractive outcomes using TK. The same IOL constant can be used for both K and TK.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Modern techniques in cataract surgery have continued to improve the ability to fine tune refractive outcomes with increasing accuracy. Precise measurements of ocular parameters and ideal formula selection are key to predicting and achieving optimal refractive results. The addition of posterior corneal data to intraocular lens (IOL) calculation formulae has become a point of debate in cataract surgery. Several publications have reported better astigmatic outcomes for toric IOL calculation using total corneal astigmatism, which include posterior corneal data [1,2,3]. As well, several studies have evaluated conventional monofocal IOL using posterior corneal data in the calculation, with varied methodology and results [4,5,6].
Posterior corneal measurements are not routinely used or available to most cataract surgeons. These measurements require a selective function of a corneal topography or tomography unit in order to detect the posterior corneal surface and further; the data then need to be transferred to an optical biometer for IOL calculation.
Recently, a new optical biometer (IOLMaster 700; Carl Zeiss Meditec, Jena, Germany) that integrates swept-source OCT (SS-OCT) technology for ocular biometry has been developed. The platform can assess all the parameters that are needed for IOL power calculation including conventional keratometry (K), central corneal thickness (CCT), anterior chamber depth (ACD), lens thickness (LT), horizontal white-to-white (WTW) corneal diameter, and axial length (AL). Assessment of the posterior corneal surface can be done by combining the data from the anterior corneal surface, obtained by telecentric keratometry, with the pachymetry data obtained by the SS-OCT.
The concept of posterior corneal measurement is not novel. It has been employed in an earlier device (Visante Omni; Carl Zeiss Meditec, Jena, Germany) with good repeatability and reproducibility [7]. Up to present, there has been a huge variety of instruments that can effectively measure posterior corneal curvature and assist in broad range of clinical applications such as Galilei® (Ziemer Group, Switzerland), Pentacam® (Oculus Optikgeräte GmbH, Germany), Sirius® (CSO, Italy), TMS-5® (Tomey corporation, Japan), and Casia 2® (Tomey corporation, Japan) [4, 8, 9]. Regarding this, total keratometry (TK) values adopting this concept are calculated using data from both the anterior and posterior cornea, as well as corneal thickness, combined using the thick lens formula. Being that TK is derived by combining two separate measurements, the confidence of its application to current IOL formulas requires validation. Furthermore, the new Barrett TK Universal II formula has been developed to be used with this new TK methodology; however, the results require further evaluation.
The purpose of this study was to compare the measurement of K and TK in eyes undergoing simple cataract surgery. Additionally, this study evaluated the refractive outcomes of the cataract surgery using the K and TK in all current standard formulas including SRK/T, HofferQ, Haigis, Holladay 1, Holladay 2, Barrett Universal II, and the new Barrett TK Universal II formulas.
Materials and methods
This prospective, comparative study was conducted in adherence to the tenets of the Declaration of Helsinki and approved by the local institutional review board of Mahidol University. Informed consent was obtained from 60 individual patients, included as participants in the study that presented to the Department of Ophthalmology at Siriraj Hospital for cataract surgery between July 2017 and December 2017. Patients were not eligible for inclusion if they had undergone previous ocular surgery, experienced trauma, or had other significant ocular diseases. For patients undergoing bilateral cataract surgery, one eye from each patient was randomly selected for inclusion in analysis as the “study eye.”
Each patient underwent routine pre-operative assessment for cataract removal and IOL placement with IOLMaster 700. All optical biometric parameters including conventional K, TK, posterior keratometry (PK), CCT, WTW corneal diameter, ACD, LT, and AL were measured. To ensure repeatability of measurements, parameters were evaluated twice by an experienced technician. An intra-rater repeatability test was performed using intraclass correlation coefficient (ICC). An ICC of more than 0.8 was considered excellent repeatability of the data measurement [10]. The IOL power was then calculated using optimized constants from User Group for Laser Interference Biometry (ULIB) website. However, the constant of the Holladay 2 is not provided by the ULIB website. Therefore, we used personalized IOL constant of the surgeon that was further optimized from the standard constant provided by the device (IOL Master 700). Moreover, the Lens Factor of the Barrett formula was derived from Barrett Universal II Formula calculator provided by APACRS website (Table 5).
K (K group) and TK (TK group) were used for IOL power calculation in all current standard formulas including SRK/T, HofferQ, Haigis, Holladay 1, Holladay 2, Barrett Universal II, and Barrett TK Universal II [11, 12]. Only TK was used for the Barrett TK Universal II formula, as this formula is designed for use only with the TK value. The IOL power was selected and recorded according to surgeon preferences. The predicted postoperative spherical equivalent refractions for all formulas were also recorded.
Following completion of pre-operative assessment and measurements, all patients underwent scheduled cataract surgery, performed by a single, experienced surgeon. All surgeries were performed using standard phacoemulsification techniques, implanting 601P/PY model (Carl Zeiss Meditec, Jena, Germany) IOL for all cases. No intraoperative complications occurred.
Postoperative manifest refraction was measured at 3 months after surgery. Refractive outcomes were adjusted using systematic error of the mean as suggested by Koch et al. before evaluating each parameter in both groups [13]. The evaluation was done using the mean absolute errors (MAEs), the median absolute errors (MedAEs), and proportion of eyes within ± 0.25 D, ± 0.50 D, and ± 1.00 D of predicted postoperative spherical equivalent refraction. The MAEs were defined as the mean “absolute” difference of postoperative spherical equivalent refraction and the predicted postoperative spherical equivalent refraction while the MedAEs were defined as the median “absolute” value of the difference between postoperative spherical equivalent refraction and the predicted postoperative spherical equivalent refraction [14]. All data were tested for normality. The intraclass correlation (ICC) with 95% confidence interval was applied for comparing the ocular parameters obtained between 2 measurements, and the IOL power across all formulas between the two groups (K group vs. TK group). Based on percentage of eyes within ± 0.25 diopter of predicted postoperative spherical equivalent refraction (SE), a paired McNemar’s chi-square test for sample size required a minimum of 52 eyes to reach statistical significance [15]. Paired t tests were also performed to evaluate the difference of IOL powers between both groups. The MAEs and MedAEs from each group of all formulas were compared using analysis of the variance (ANOVA) test and the signed ranks test with Bonferroni adjustment for multiple comparisons. A p value less than 0.05 was considered statistically significant difference for each comparison. Statistical analysis was performed using SPSS software (version 18.0, SPSS Inc., Chicago, IL, USA) and Microsoft Excel 2008 (Microsoft Corp, USA).
Results
Ocular biometry, including K, TK, PK, CCT, ACD, LT, AL, and WTW, is shown in Table 1. The mean difference between the two consecutive measurements of all parameters was very low. The repeatability of all parameters was also high (ICC > 0.90). There was no statistically significant difference between the two consecutive measurements.
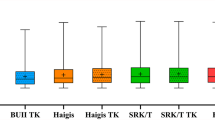
The mean difference between K and TK was 0.03 D (K 44.56 ± 1.18, TK 44.59 ± 1.22 D), demonstrating very good agreement (ICC = 0.99) as shown in Fig. 1. The emmetropic IOL powers in all formulas for both groups were very similar (all p values > 0.2), demonstrated in Fig. 2. The IOL power derived by the K group was slightly higher than the IOL power derived by the TK group; however, the difference was small and the agreement between groups was very high (Table 2). There was no difference between the IOL powers selected by the surgeon using either K or TK.
Agreement of IOL power derived by conventional keratometry (K) and total keratometry (TK) across six IOL formulas (a Bland Altman Plot of SRK/T formula, b Bland Altman Plot of Hoffer Q formula, c Bland Altman Plot of Haigis formula, d Bland Altman Plot of Holladay 1 formula, e Bland Altman Plot of Holladay 2 formula, f Bland Altman Plot of Barrett Universal II formula)
After adjusting the systematic error of the mean arithmetic prediction of refractive outcomes, MAEs and MedAEs are shown in Table 3. The TK values were slightly lower in both MAEs and MedAEs than the K group in all formulas but there were no statistically significant differences. The new Barrett TK formula showed the lowest MAEs, compared with other formulas. The proportion of eyes within ± 0.25 D, ± 0.50 D, and ± 1.00 D of predicted postoperative spherical equivalent refraction (SE) across all formulas are shown in Table 4. The TK group showed slightly higher proportion of eyes within all predicted postoperative SE ranges than those in the K group in the majority of formulas applied.
Discussion
This study demonstrated that the repeatability of K, TK, PK, and all other parameters was high. The IOL power derived by K and TK was similar in all formulas. The prediction errors in all formulas were slightly lower in the TK group than the K group.
The concept of using total corneal power has been well accepted for the IOL calculation following refractive surgery [16,17,18]. However, in uncomplicated cataract surgery, the benefit to using total corneal power remains uncertain. Some studies have shown the improvement of refractive outcomes of total corneal power over conventional keratometry [5, 6]. Alternatively, other studies did not show any benefit [4, 19]. Notably, the total corneal power in previously published studies was measured using Scheimpflug camera system for corneal topography. They showed a lower value of total corneal power when compared with conventional K. Each topography system applies its own algorithm to calculate for the total corneal power, which may yield a different value. Some topography systems use a thick lens formula, some use ray tracing analysis. Therefore, the benefit of using the total corneal power for the IOL calculation may be variable. We aimed to evaluate benefit of using the new total corneal power, TK, derived by the SS-OCT-type optical biometer for the IOL calculation. As described above, TK was derived by the combination of the anterior corneal surface and corneal thickness. Due to the movement artifacts that generally influence the thickness measurements, less so on OCT than the surface curvature image, this technique is expected to be more reliable than the direct measurement of the posterior corneal surface [20]. In the current study, TK showed very high repeatability. Although, the value was slightly higher when compared with K, the difference between them was very low (0.03 D). The emmetropic IOL power derived by the TK and K was similar in all formulas and the chosen IOL powers were not statistically different. Therefore, the same IOL constant should be able to be applied for both K and TK with no clinically significant difference.
Furthermore, mean K and PK in our study were 44.56 ± 1.18 D and − 5.95 ± 0.19 D, respectively. By using a Gaussian optics formula and the refractive index of air (1.000), cornea (1.376), aqueous (1.336), and keratometric index being used in the device (1.3375), the radius of curvature for anterior cornea (ranterior) and posterior cornea (rposterior) can be calculated. In our study, the mean ranterior was 7.58 ± 0.20 mm and the mean rposterior was 6.73 ± 0.20 mm. Interestingly, the ratio of the back to the front was 0.887 (6.73/7.58) which was quite similar to the ratio of the Gullstrand’s exact schematic eye (0.883) [21, 22]. Using the back calculation for the equivalent keratometric index for the population, as described by Olsen et al., a value of 1.3320 is calculated [18]. The calculated keratometric index of 1.3315 in the current study was notably similar to the value proposed by Olsen et al. [23].
This study followed the protocol for studies on IOL formula accuracy, proposed by Hoffer et al. [24]. A single surgeon performed all surgeries and a single IOL model (601P/PY model [Zeiss]) with an optimized IOL constant was used to avoid variability of results. We randomly used one eye from each patient for the analysis. Moreover, all the results were adjusted using systematic error of the mean before the comparison (Table 5), providing an improved method of evaluation of the accuracy of the IOL formula [13].
The refractive outcomes of the TK group appeared to be slightly better than the K group in all formulas, which was not unexpected as the true posterior corneal data was included in the calculation. There was no assumption made by using keratometric index. Although most of the IOL formulas were developed for the keratometric index values, the proprietary algorithm of the TK appears to align and compensate for any variances. This may be the reason we did not find any significant difference value between the K and the TK, as well as the IOL powers derived in our study.
The new Barrett TK Universal II formula was designed to be used with the TK; therefore, it was not surprising that it showed the lowest MAEs when compared with the others. Also, a very high proportion of eyes achieved refractive outcomes within ± 0.25, ± 0.50, and ± 1.00 D using the formula. Therefore, the Barrett TK Universal II formula can be used with TK quite effectively and it should be generally recommended over the Barrett Universal II for the use with a TK value. To our knowledge, this is the first study of refractive outcomes applying the Barrett TK Universal II formula.
It is surprising that the percentage of eyes with a prediction of ± 0.5 D for the Barrett formula in our study was just 59.6% when using conventional K. It might probably be from general optimized constants we used in the study. Although it has been proposed by Aristodemou et al. [25] that optimized IOL constants could substantially improve refractive outcomes, far exceeding personalizing IOL constants, some error might occur in specific group of population. Further larger number of eyes included in the study may lead to better results. The limitation of this study was that it included a relatively small sample size and all cases were confined within normal range of all parameters. The results might not be applicable to eyes in extreme parameters related to very long or short axial length. A larger sample size may be required to confirm the results.
In conclusion, our preliminary results suggest that TK is comparable with K and can be used to determine IOL calculation in simple cataract surgery using the same optimized IOL constant. The refractive outcomes of TK were slightly better when compared with K and the new Barrett TK Universal II formula can be used with TK for the IOL calculation with very good refractive outcomes.
References
Sano M, Hiraoka T, Ueno Y (2016) Influence of posterior corneal astigmatism on postoperative refractive astigmatism in pseudophakic eyes after cataract surgery. BMC Ophthalmol 16:212
Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI (2016) Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: comparison of methodologies. J Cataract Refract Surg 42:217–225
Savini G, Næser K (2015) An analysis of the factors influencing the residual refractive astigmatism after cataract surgery with toric intraocular lenses. Invest Ophthalmol Vis Sci 13:827–835
Savini G, Hoffer KJ, Lomoriello DS, Ducoli P (2017) Simulated keratometry versus total corneal power by ray tracing: a comparison in prediction accuracy of intraocular lens power. Cornea 36:1368–1372
Kirgiz A, Atalay K, Kaldirim H, Cabuk KS, Akdemir MO, Taskapili M (2017) Scheimpflug camera combined with placido-disk corneal topography and optical biometry for intraocular lens power calculation. Int Ophthalmol 37:781–786
Saad E, Shammas MC, Shammas HJ (2013) Scheimpflug corneal power measurements for intraocular lens power calculation in cataract surgery. Am J Ophthalmol 156:460–467
Srivannaboon S, Chotikavanich S, Chirapapaisan C, Kasemson S, Po-ngam W (2012) Precision analysis of posterior corneal topography measured by Visante Omni: repeatability, reproducibility, and agreement with Orbscan II. J Refract Surg 28:133–138
Aramberri J, Araiz L, Garcia A, Illarramendi I, Olmos J, Oyanarte I, Romay A, Vigara I (2012) Dual versus single Scheimpflug camera for anterior segment analysis: precision and agreement. J Cataract Refract Surg 38:1934–1949
Chan TCY, Biswas S, Yu M, Jhanji V (2017) Comparison of corneal measurements in keratoconus using swept-source optical coherence tomography and combined Placido-Scheimpflug imaging. Acta Ophthalmol 95:486–e494. https://doi.org/10.1111/aos.13298
Rosner B (2000) Fundamental of biostatistic. Duxbury, California
Shajari M, Kolb CM, Petermann K, Böhm M, Herzog M, de’Lorenzo N, Schönbrunn S, Kohnen T (2018) Comparison of 9 modern intraocular lens power calculation formulas for a quadrifocal intraocular lens. J Cataract Refract Surg 44:942–948
Fabian E, Wehner W (2019) Prediction accuracy of total keratometry compared to standard keratometry using different intraocular lens power formulas. J Refract Surg 35:362–368
Wang L, Koch DD, Hill W, Abulafia A (2017) Pursuing perfection in intraocular lens calculations: III. Criteria for analyzing outcomes. J Cataract Refract Surg 43:999–1002
Aristodemou P, Cartwright NK, Sparrow JM, Johnston R (2011) Intraocular lens calculations. Ophthalmology 118:1221
Lachin JM (1992) Power and sample size evaluation for the McNemar test with application to matched case-control studies. Stat Med 11:1239–1251
Tang M, Wang L, Koch DD, Li Y, Huang D (2012) Intraocular lens power calculation after previous myopic laser vision correction based on corneal power measured by Fourier-domain optical coherence tomography. J Cataract Refract Surg 238:589–594
Qazi MA, Cua IY, Roberts CJ, Pepose JS (2007) Determining corneal power using Orbscan II videokeratography for intraocular lens calculation after excimer laser surgery for myopia. J Cataract Refract Surg 33:21–30
Kwitko S, Marinho DR, Rymer S, Severo N, Arce CG (2012) Orbscan II and double-K method for IOL calculation after refractive surgery. Graefes Arch Clin Exp Ophthalmol 2250:1029–1034
Shammas HJ, Hoffer KJ, Shammas MC (2009) Scheimpflug photography keratometry readings for routine intraocular lens power calculation. J Cataract Refract Surg 35:330–334
Ayala M, Strandås R (2015) Accuracy of optical coherence tomography (OCT) in pachymetry for glaucoma patients. BMC Ophthalmol 15:124
Gullstrand A (1911) Einfihrung in die Methoden der Dioptrik des Auges des Menschen. Hirzel, Leipzig
Stenstrom S (1964) Optics and the eye. Goteborg, Akademiforlaget
Olsen T (1986) On the calculation of power from curvature of the cornea. Br J Ophthalmol 70:152–154
Hoffer KJ, Aramberri J, Haigis W, Olsen T, Savini G, Shammas HJ, Bentow S (2015) Protocols for studies of intraocular lens formula accuracy. Am J Ophthalmol 160:403–405
Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL (2011) Intraocular lens formula constant optimization and partial coherence interferometry biometry: refractive outcomes in 8108 eyes after cataract surgery. J Cataract Refract Surg 37:50–62
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The first author has received a speaker honorarium from Company Carl Zeiss; the second author declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Faculty of Medicine Siriraj Hospital, Mahidol University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Srivannaboon, S., Chirapapaisan, C. Comparison of refractive outcomes using conventional keratometry or total keratometry for IOL power calculation in cataract surgery. Graefes Arch Clin Exp Ophthalmol 257, 2677–2682 (2019). https://doi.org/10.1007/s00417-019-04443-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04443-7