Abstract
Purpose
To compare refractive outcomes calculated using intraocular lens (IOL) power calculation formulas loaded onto the IOLMaster 700 with the employment of anterior keratometry (K) and total keratometry (TK).
Methods
A total of 225 eyes of 225 patients underwent uneventful cataract surgery and implantation of a single model of nontoric monofocal IOL by a single surgeon. All eyes underwent preoperative ocular biometric measurements with the IOLMaster 700. Refractive outcomes, including the mean numerical prediction error (MNE); standard deviation (SD); adjusted mean absolute prediction error (MAE); adjusted median absolute prediction error (MedAE); percentages of eyes with adjusted prediction error (PE) within ± 0.25, ± 0.50, ± 0.75, and ± 1.00 diopter; and IOL Formula Performance Index (FPI), were compared between the K-based formula and the TK-based formula of Barrett Universal II (BUII), Haigis, SRK/T, Holladay 2, and Hoffer Q. Axial length (short, medium, and long) subgroup analyses and anterior and posterior keratometry (flat, medium, and steep) subgroup analyses were conducted.
Results
The K-based formula performed better than the TK-based formula in the accuracy of refractive prediction of each IOL calculation formula: BUII-K (FPI 0.690), BUII-TK (0.677), Haigis-K (0.617), Haigis-TK (0.584), SRK/T-K (0.608), SRK/T-TK (0.595), Holladay 2-K (0.419), Holladay 2-TK (0.406), Hoffer Q-K (0.364), and Hoffer Q-TK (0.356). The subgroup analyses of refractive prediction outcomes showed that TK influenced the refractive outcomes in eyes with relatively normal ranges of axial length and anterior keratometry.
Conclusions
Using TK instead of K leads to lower refractive prediction accuracy of the IOL power calculation formulas loaded on the IOLMaster 700.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
Cataract surgery has increasingly become a refractive surgery in developed countries due to the improvement of surgical machines, examination apparatuses, surgical devices and techniques; the advent of premium intraocular lenses (IOLs); and the higher expectations of patients for quality of vision and life [1]. Against this background, IOL power calculation formulas have been updated to minimize the postoperative refractive error. IOL formulas are now categorized into historical/refraction-based, regression, vergence, artificial intelligence, and ray tracing [2]. In recent years, there has been a transition in the vergence formula from SRK/T [3] and Hoffer Q [4] to Haigis [5], Holladay 2 (unpublished), and Barrett Universal II (BUII) (unpublished). Among them, the BUII formula is reported to perform better than others by many articles [6,7,8,9] and in widespread use to determine the IOL power.
In addition to refining the IOL power calculation formula, swept-source optical coherence tomography (OCT) technology has revolutionized the measurement of ocular biometry with toric IOLs, multifocal IOLs, and laser vision correction (LVC), leading to the development of sophisticated devices such as the IOLMaster 700 (Carl Zeiss Meditec) [10, 11]. This device can measure total keratometry (TK) by combining anterior and posterior corneal surfaces, in addition to anterior keratometry (K) [12]. It has been reported that the measurement of posterior corneal astigmatism has the potential to reduce residual astigmatic error in toric IOL implantation [13, 14]. In fact, toric calculators with posterior corneal astigmatism measurement achieve comparable or better results in the prediction of postoperative astigmatic outcome [15, 16] and IOL power calculation in eyes that have undergone LVC [17, 18]. However, a considerable number of reports have shown that directly measured total corneal astigmatism (TCA) for toric IOL calculation is not superior to the calculated TCA, suggesting that the optimization of corneal astigmatism measurements is necessary [19,20,21]. Reports on the usefulness of TK for refractive outcomes in normal eyes implanted with nontoric IOLs are scarce. Fabian and Wehner reported that in comparison to standard K, a higher prediction accuracy can be expected by using TK values in relatively high astigmatic eyes (0.75 D or greater) [22]. Srivannaboon and Chirapapaisan reported that conventional K and TK for IOL calculation showed strong agreement with a nonsignificant trend toward better refractive outcomes using TK [23]. If the TK-based formula should be superior to the K-based formula in the prediction of postoperative refractive errors in normal eyes, then the K-based formula will be of no use.
Thus, this study aimed to determine whether the TK-based IOL power calculation formula performs better than the K-based formula in terms of refractive prediction accuracy. To this end, we compared the postoperative refractive errors calculated by BUII, Haigis, SRK/T, Holladay 2, and Hoffer Q (which are built-in formulas of the IOLMaster 700) with use of K and TK, and we conducted subgroup analyses according to axial length and keratometry.
Methods
This retrospective study was approved by the institutional review board of Osaka Minato Central Hospital and adhered to the tenets of the Declaration of Helsinki.
The enrolled cataract eyes were measured preoperatively with the IOLMaster 700 to assess the following ocular biometric variables: axial length (AL), K, TK, posterior keratometry (PK), anterior chamber depth (ACD), lens thickness (LT), and white-to-white (WTW). All operations were performed by a single skilled surgeon (Y.D.) at Osaka Minato Central Hospital from September 1, 2019 to August 31, 2021. The eyes underwent uneventful phacoemulsification and implantation of a single IOL model (ZCBOOV, Johnson and Johnson Vision) through a temporal 2.4-mm clear corneal incision and were followed up for at least 1 month postoperatively. They achieved a corrected distance visual acuity (CDVA) of 20/32 or better. If both eyes of a patient were operated on, only the first eye was enrolled since the second eye may have been adjusted for IOL power calculation and surgical procedure based on the outcome of the first eye [24]. Eyes with previous ocular surgery, corneal irregular astigmatism, corneal epithelial damage, and significant vitreous opacity were excluded.
Preoperative examinations included CDVA, anterior autokeratometry (AK) measured with an autokeratometer (TONOREF III, NIDEK), corneal topography (TMS-4 N and CASIA2, TOMEY), and the measurement of AL, K, TK, PK, ACD, LT, and WTW with the IOLMaster 700 (version 1.90.12.05). Manifest CDVA and AK with the autokeratometer were measured at 1 month postoperatively. The CDVA was measured using a Landolt ring at 5 m, and the decimal acuity was converted to the Snellen equivalent of the visual acuity conversion chart [25]. To assess the surgically induced astigmatism (SIA), the AK and its astigmatic axis measured pre- and postoperatively with the autokeratometer were input into the computer program (http://links.lww.com/JRS/A281, accessed February 1, 2022) created by Næser [26]. The IOL power for implantation was determined by entering a target refraction into the TK-based BUII (BUII-TK) formula of IOLMaster 700. The postoperative predicted refractions were back-calculated from the implanted IOL power using BUII-K and -TK, Haigis-K and -TK, SRK/T-K and -TK, Holladay 2-K and -TK, and Hoffer Q-K and -TK, all of which are the built-in formulas of IOLMaster 700. Both the BUII-K and the BUII-TK used all the variables, including K and TK, respectively, AL, ACD, LT, and WTW, to calculate IOL power. The lens factor for the BUII was + 2.15, the a0, a1, and a2 for the Haigis − 0.329, + 0.332, and + 0.203, respectively, the A-constant for the SRK/T 119.3, the ACD for the Holladay 2 + 5.951, and the pseudophakic ACD for the Hoffer Q + 5.98.
The postoperative manifest refraction at 1 month after surgery was subtracted by the formula-predicted refraction calculated using the respective IOL formulas to obtain the postoperative prediction error (PE). The absolute value of PE was defined as the absolute prediction error (APE). The mean numerical prediction error (MNE) for the mean of the PE, the standard deviation (SD) of the PE, the mean absolute prediction error (MAE) for the mean of the APE, and the median absolute prediction error (MedAE) for the median of the APE were calculated. Since the MNE should ideally be zero, the adjusted PE was computed by subtracting the MNE from the PE to obtain the adjusted MNE (equal to zero), adjusted MAE, and adjusted MedAE. The percentages of eyes with adjusted PEs within ± 0.25, ± 0.50, ± 0.75, and ± 1.00 diopter (D) calculated by the individual formulas were obtained. The IOL Formula Performance Index (FPI) proposed by Wolfgang Haigis, which is available in the On Demand section at www.escrs.org (accessed January 26, 2022) and introduced by Hoffer and Savini [27], was calculated to rank the accuracy of the formula.
Eyes were divided into 3 subgroups of short (AL < 22.5 mm), medium (AL ≧ 22.5 mm and < 25.5 mm), and long (AL ≧ 25.5 mm) based on the AL values; 3 subgroups of flat (AK < 42 D), medium (AK ≧ 42 D and < 46 D), and steep (AK ≧ 46 D) based on the preoperative mean autokeratometric values; and 3 subgroups of flat (PK ≧ − 5.74 D), medium (PK < − 5.74 D and ≧ − 6.08 D), and steep (PK < − 6.08 D) based on the interquartile range (IQR) of the preoperative mean PK: the value of − 6.08 D for the 1st quartile and − 5.74 D for the 3rd quartile.
Comparison of the MNE and the adjusted MAE between the K-based formula and the TK-based formula was conducted using the Wilcoxon signed rank test. Comparison of the SD between the IOL formulas was conducted using the heteroscedastic statistical method [28]. K and TK values of the subgroups were also compared using the Wilcoxon signed rank test. All analyses except for the heteroscedastic method were processed using BellCurve for Excel version 3.23 (Social Survey Research Information Co., Ltd.). The heteroscedastic method was performed by downloading the R Project Computing at https://www.r-project.org/ and the files at https://osf.io/nvd59/quickfiles (accessed May 27, 2022). A P value < 0.05 was considered statistically significant.
Results
A total of 225 eyes of 225 patients (109 right eyes, 126 females) were included in the study. The demographics and characteristics of the study population are shown in Table 1.
Comparison of postoperative refractive outcomes between the K-based and TK-based formulas
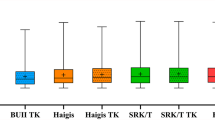
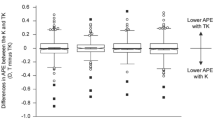
Comparisons of the postoperative refractive prediction outcomes derived from the individual IOL power calculation formulas using K and TK are shown in Table 2. Except for the BUII (P = 0.420), the TK-based formula had a significantly larger MNE, i.e., hyperopic shift, than the K-based formula for the Haigis (P = 0.014), SRK/T (P = 0.004), Holladay 2 (P = 0.007), and Hoffer Q (P = 0.013) formulas. The SD of the MNE was larger in the TK-based formula than in the K-based formula for each IOL power calculation formula. The heteroscedastic statistical method shows that there is a statistical significance in the SD between the BUII-K and the Holladay 2-TK, the Hoffer Q-K, and the Hoffer Q-TK, respectively, and between the BUII-TK and the Hoffer Q-K and the Hoffer Q-TK, respectively (Table 3). The adjusted MAE was significantly larger in the TK-based formula than in the K-based formula for the Haigis (P = 0.043) and the SRK/T (P = 0.043) and tended to be larger in the TK-based formula for the BUII (P = 0.050), the Holladay 2 (P = 0.063), and the Hoffer Q (P = 0.072). The adjusted MedAE was larger in the TK-based formula than in the K-based formula for each IOL power calculation formula. The BUII-K had higher percentages of eyes than the other formulas in all the brackets within ± 0.25, ± 0.50, ± 0.75, and ± 1.00 D of the adjusted PE, except that those of the BUII-K and BUII-TK were equal in the bracket within ± 0.75 D (also shown in Figure 1). The accuracy of the IOL power calculation formulas was ranked by using the FPI. The BUII-K held the first place in the ranking of accuracy. Moreover, the K-based formula had a better FPI than the TK-based formula for each IOL power calculation formula.
Axial length and keratometry subgroup analyses of refractive prediction outcomes between K- and TK-based formulas
Table 4 shows the refractive prediction outcomes in 3 subgroups of short, medium, and long AL. In the short AL group, the SD was larger in each TK-based formula than in the corresponding K-based formula, and the adjusted MAE was significantly larger in the BUII-TK (P = 0.030) and the Holladay 2-TK (P = 0.006) than in the BUII-K and the Holladay 2-K, respectively. In the medium AL group, the MNE was significantly larger, i.e., hyperopic shift, in each TK-based formula than in the K-based formula except for the BUII: the BUII (P = 0.123), the Haigis (P = 0.008), the SRK/T (P = 0.002), the Holladay 2 (P = 0.005), and the Hoffer Q (P = 0.008). The SD was larger in each TK-based formula than in the K-based formula except for SRK/T. The adjusted MAE of the medium AL group was larger in each TK-based formula than in the K-based formula, but the difference was not statistically significant. In the long AL group, there was no significant difference in the MNE and the adjusted MAE between the K-based and TK-based formulas for each IOL power calculation formula. However, the SD was larger in each TK-based formula than in the K-based formula.
Table 5 shows the refractive prediction outcomes in 3 subgroups of flat, medium, and steep AK. In the flat AK group, we found no significant difference in the MNE or the adjusted MAE between the K-based and TK-based formulas. In the medium AK group, the MNE was statistically significantly larger, i.e., hyperopic shift, in each TK-based formula than the K-based formula except for the BUII: the BUII (P = 0.379), the Haigis (P = 0.021), the SRK/T (P = 0.013), the Holladay 2 (P = 0.011), and the Hoffer Q (P = 0.025), while the adjusted MAE was larger in each TK-based formula, with statistical significance in the BUII formula (P = 0.024) but not in the other IOL power calculation formulas, namely, the Haigis (P = 0.068), the SRK/T (P = 0.103), the Holladay 2 (P = 0.055), and the Hoffer Q (P = 0.073). The SD was larger in each TK-based formula than in the K-based formula. In the steep AK group, there was no significant difference in the MNE and the adjusted MAE between the K-based and TK-based formulas, although the SD was larger in each TK-based formula than in the K-based formula.
Table 6 shows the refractive prediction outcomes in 3 subgroups of flat, medium, and steep PK. In the flat PK group, the MNE was significantly larger, i.e., hyperopic shift, in each TK-based formula than in the K-based formula (P < 0.001), while the adjusted MAE was significantly larger in each TK-based formula, except for the Haigis formula. The SD was larger in each TK-based formula than in the K-based formula except for the SRK/T. In the medium PK group, we found no significant difference in the MNE or the adjusted MAE between the K-based and TK-based formulas. In the steep PK group, the MNE was statistically significantly smaller, i.e., myopic shift, in each TK-based formula than in the K-based formula, while the adjusted MAE was smaller in the TK-based formula of the BUII, the Haigis, and the Hoffer Q and larger in that of the SRK/T and the Holladay 2, with no statistical significance.
Axial length and keratometry subgroup analyses of K and TK
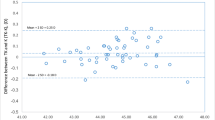
The above results from the AL and keratometry subgroup analyses imply that TK influences refractive prediction outcomes in eyes with relatively normal ranges of axial length and anterior keratometry. In a total of 225 eyes, the mean K and the mean TK were 44.26 ± 1.53 D and 44.28 ± 1.53 D (Table 1), respectively; there was a statistically significant difference between them (P = 0.006). Therefore, K and TK were compared in the AL and keratometry subgroups (Table 7). The TK was statistically significantly larger than the K in both the medium AL group (P = 0.003) and the medium AK group (P = 0.009) but not in the other categories of AL and AK. On the other hand, in the PK subgroups, the TK was statistically significantly larger than the K in the flat PK group (P < 0.001), not different in the medium PK group, and statistically significantly smaller in the steep PK group (P = 0.004).
Discussion
This study showed that the BUII-K formula, but not the BUII-TK, is the best nontoric monofocal IOL power calculation formula among the built-in IOLMaster 700 formulas in terms of its accuracy of refractive prediction outcomes for normal eyes. The better performance of the K-based formula is true for the other IOL power calculation formulas. The use of TK degraded the formula performance substantially. The subgroup analysis of the refractive prediction outcomes suggests that TK affects the refractive outcomes in eyes with relatively normal ranges of axial length and anterior keratometry. We confirmed that this resulted from the significant differences between K and TK in the medium ranges of axial length and anterior keratometry. Our study demonstrated that use of TK instead of K leads to a lower refractive prediction accuracy of the IOL power calculation formula.
There are many factors influencing postoperative refractive errors following cataract surgery [29]. These include individual ocular characteristics; accuracy of ocular biometric measurement [30]; labeling of the IOL, including the material and configuration [31]; surgeon’s skill; IOL power calculation formula, etc. Regarding ocular characteristics, irregular astigmatism such as keratoconus, long or short axial length, fragile ciliary zonules represented by pseudoexfoliation syndrome, eyes after LVC, etc. The accuracy of ocular biometric measurement has been improved by the recent OCT technology. Although the detailed labeling of IOLs is rarely publicized by the manufacturer, the production and quality control of IOLs are rigorous and under the regulation of the American National Standards Institute (ANSI). The skill of experienced surgeons is much the same in terms of surgical complications and SIA. With respect to the IOL power calculation formula, the newer vergence formulas of BUII, Haigis, and Holladay 2 have been replacing the SRK/T and Hoffer Q, yet formulas based on new concepts are in the developmental stage. Aside from the individual ocular characteristics, numerous predecessors have made considerable effort in every field to minimize the postoperative refractive error. Nevertheless, not only can we not make this error zero, but we also (infrequently) encounter a refractive surprise.
The significance of TK is that direct measurement of total keratometry negates the systematic error related to the mathematical estimation of the posterior corneal curve based on the anterior corneal curve measurement. Savini et al. [32] showed that the mean K and the mean TK measured on 69 unoperated cataract eyes with IOLMaster 700 were 43.14 ± 1.37 D and 43.18 ± 1.37 D, respectively, with statistical significance (P = 0.0006). These results were consistent with our findings in the current study, although the researchers explained that this difference was not clinically significant without evaluating refractive prediction outcomes. However, they also mentioned that using the measured TCA is less accurate than using the predicted TCA for toric IOL calculation [21]. In fact, direct measurement of TCA is not superior to calculation of TCA in terms of obtaining better refractive astigmatism for toric IOL [19, 20]. The same was true for total keratometry or TK, as shown in the present study. Using TK is not accurate enough to achieve better refractive outcomes than using K. Recently, Ryu et al. [33] reported that MAE tended to be lower for TK-based than for K-based formulas in eyes undergoing femtosecond laser-assisted cataract surgery, and Chung et al. [34] showed a lower prediction accuracy of TK-based formulas than that of K-based formulas for multifocal IOLs. Our study reinforces this reality in terms of refractive prediction outcomes for nontoric monofocal IOLs. We demonstrated in the subgroup analysis of K and TK that, especially in the relatively normal ranges of axial length and anterior keratometry, there were significant differences between T and TK, which must have led to the lower refractive prediction accuracy of the TK-based formulas. This may require IOL formula adjustments, or there is a possibility that there is a gap between the accuracy of the anterior and posterior corneal refraction values in the measurement equipment. In the latter case, it is highly possible that the measurement error of TK, which is the sum of the two values, is greater than that of K, which is a single value. Our PK subgroup analysis may be a clue to this issue. In the flat PK group, the adjusted MAEs of the TK-based formulas were significantly larger than those of the K-based formulas except for Haigis. In contrast, in the steep PK group, all the adjusted MAEs of the TK-based formulas were not significantly different from those of the K-based formulas, suggesting that using TK has no advantage over using K. Given no knowledge of the normal range of PK, we refrain from further discussions on this issue. Instead, we additionally show the values of PK corresponding to the subgroups in Table 7. Whether the issue of TK is because of a problem with the IOL formulas or because of a measurement error inherent in the IOLMaster 700 remains to be determined.
The strength of this study is that a single IOL model was implanted by a single skillful surgeon, which obviates the constant optimization and adjustment of the surgeon’s factor. On the other hand, the weakness is the small sample size. However, this study should be exempt from such a criticism because of its elaborate design conforming to Hoffer and Savini’s protocol [27]. Nonetheless, it is possible that the conclusions may change with continued data accumulation. It remains to be studied whether the TK-based formula performs better for eyes with toric and/or multifocal IOL implantation in reducing postoperative refractive error as well as postoperative astigmatism.
In conclusion, either the TK-based IOL power calculation formulas or the measurement of TK with the IOLMaster 700, or both, may need to be optimized.
References
Olsen T (2007) Calculation of intraocular lens power: a review. Acta Ophthalmol Scand 85:472–485. https://doi.org/10.1111/j.1600-0420.2007.00879.x
Koch DD, Hill W, Abulafia A, Wang L (2017) Pursuing perfection in intraocular lens calculations: I. Logical approach for classifying IOL calculation formulas. J Cataract Refract Surg 43:717–718. https://doi.org/10.1016/j.jcrs.2017.06.006
Retzlaff JA, Sanders DR, Kraff MC (1990) Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refract Surg 16:333–340. https://doi.org/10.1016/s0886-3350(13)80705-5 (Erratum.In:JCataractRefractSurg(1990)16:528)
Hoffer KJ (1993) The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg 19:700–712. https://doi.org/10.1016/s0886-3350(13)80338-0 (errata, (1994) 20(677), pp. 33, 2007.2-3)
Haigis W, Lege B, Miller N, Schneider B (2000) Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol 238:765–773. https://doi.org/10.1007/s004170000188
Cooke DL, Cooke TL (2016) Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg 42:1157–1164. https://doi.org/10.1016/j.jcrs.2016.06.029
Kane JX, Van Heerden A, Atik A, Petsoglou C (2016) Intraocular lens power formula accuracy: comparison of 7 formulas. J Cataract Refract Surg 42:1490–1500. https://doi.org/10.1016/j.jcrs.2016.07.021
Kane JX, Van Heerden A, Atik A, Petsoglou C (2017) Accuracy of 3 new methods for intraocular lens power selection. J Cataract Refract Surg 43:333–339. https://doi.org/10.1016/j.jcrs.2016.12.021
Melles RB, Holladay JT, Chang WJ (2018) Accuracy of intraocular lens calculation formulas. Ophthalmology 125:169–178. https://doi.org/10.1016/j.ophtha.2017.08.027
Srivannaboon S, Chirapapaisan C, Chonpimai P, Loket S (2015) Clinical comparison of a new swept-source optical coherence tomography-based optical biometer and a time-domain optical coherence tomography-based optical biometer. J Cataract Refract Surg 41:2224–2232. https://doi.org/10.1016/j.jcrs.2015.03.019
Montés-Micó R, Pastor-Pascual F, Ruiz-Mesa R, Tañá-Rivero P (2021) Ocular biometry with swept-source optical coherence tomography. J Cataract Refract Surg 47:802–814. https://doi.org/10.1097/j.jcrs.0000000000000551
LaHood BR, Goggin M (2018) Measurement of posterior corneal astigmatism by the IOLMaster 700. J Refract Surg 34:331–336. https://doi.org/10.3928/1081597X-20180214-02
Savini G, Næser K (2015) An analysis of the factors influencing the residual refractive astigmatism after cataract surgery with toric intraocular lenses. Invest Ophthalmol Vis Sci 56:827–835. https://doi.org/10.1167/iovs.14-15903
Klijn S, Reus NJ, van der Sommen CM, Sicam VA (2016) Accuracy of total corneal astigmatism measurements with a Scheimpflug imager and a color light-emitting diode corneal topographer. Am J Ophthalmol 167:72–78. https://doi.org/10.1016/j.ajo.2016.04.011
Abulafia A, Hill WE, Franchina M, Barrett GD (2015) Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg 31:699–707. https://doi.org/10.3928/1081597X-20150928-03
Yang S, Byun YS, Kim HS, Chung SH (2021) Comparative accuracy of Barrett toric calculator with and without posterior corneal astigmatism measurements and the Kane toric formula. Am J Ophthalmol 231:48–57. https://doi.org/10.1016/j.ajo.2021.05.028
Lawless M, Jiang JY, Hodge C, Sutton G, Roberts TV, Barrett G (2020) Total keratometry in intraocular lens power calculations in eyes with previous laser refractive surgery. Clin Exp Ophthalmol 48:749–756. https://doi.org/10.1111/ceo.13760
Yeo TK, Heng WJ, Pek D, Wong J, Fam HB (2021) Accuracy of intraocular lens formulas using total keratometry in eyes with previous myopic laser refractive surgery. Eye (Lond) 35:1705–1711. https://doi.org/10.1038/s41433-020-01159-5
Savini G, Næser K, Schiano-Lomoriello D, Ducoli P (2017) Optimized keratometry and total corneal astigmatism for toric intraocular lens calculation. J Cataract Refract Surg 43:1140–1148. https://doi.org/10.1016/j.jcrs.2017.06.040
Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG (2017) Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg 33:794–800. https://doi.org/10.3928/1081597X-20171004-03
Savini G, Taroni L, Hoffer KJ (2020) Recent developments in intraocular lens power calculation methods—update 2020. Ann Transl Med 8:1553. https://doi.org/10.21037/atm-20-2290
Fabian E, Wehner W (2019) Prediction accuracy of total keratometry compared to standard keratometry using different intraocular lens power formulas. J Refract Surg 35:362–368. https://doi.org/10.3928/1081597X-20190422-02
Srivannaboon S, Chirapapaisan C (2019) Comparison of refractive outcomes using conventional keratometry or total keratometry for IOL power calculation in cataract surgery. Graefes Arch Clin Exp Ophthalmol 257:2677–2682. https://doi.org/10.1007/s00417-019-04443-7
Turnbull AMJ, Barrett GD (2019) Using the first-eye prediction error in cataract surgery to refine the refractive outcome of the second eye. J Cataract Refract Surg 45:1239–1245. https://doi.org/10.1016/j.jcrs.2019.04.008
Holladay JT (2004) Visual acuity measurements. J Cataract Refract Surg 30:287–290. https://doi.org/10.1016/j.jcrs.2004.01.014
Næser K (2021) Surgically induced astigmatism made easy: calculating the surgically induced change in sphere and cylinder for corneal incisional, corneal laser, and intraocular lens-based surgery. J Cataract Refract Surg 47:118–122. https://doi.org/10.1097/j.jcrs.0000000000000518
Hoffer KJ, Savini G (2021) Update on intraocular lens power calculation study protocols: the better way to design and report clinical trials. Ophthalmology 128:e115–e120. https://doi.org/10.1016/j.ophtha.2020.07.005
Holladay JT, Wilcox RR, Koch DD, Wang L (2021) Review and recommendations for univariate statistical analysis of spherical equivalent prediction error for IOL power calculations. J Cataract Refract Surg 47:65–77. https://doi.org/10.1097/j.jcrs.0000000000000370
Kieval JZ, Al-Hashimi S, Davidson RS et al (2020) Prevention and management of refractive prediction errors following cataract surgery. J Cataract Refract Surg 46:1189–1197. https://doi.org/10.1097/j.jcrs.0000000000000269
Olsen T (1992) Sources of error in intraocular lens power calculation. J Cataract Refract Surg 18:125–129. https://doi.org/10.1016/s0886-3350(13)80917-0
Kohnen S (2000) Postoperative refractive error resulting from incorrectly labeled intraocular lens power. J Cataract Refract Surg 26:777–778. https://doi.org/10.1016/s0886-3350(00)00373-4
Savini G, Taroni L, Schiano-Lomoriello D, Hoffer KJ (2021) Repeatability of total keratometry and standard keratometry by the IOLMaster 700 and comparison to total corneal astigmatism by Scheimpflug imaging. Eye (Lond) 35:307–315. https://doi.org/10.1038/s41433-020-01245-8
Ryu S, Jun I, Kim TI, Seo KY, Kim EK (2021) Prediction accuracy of conventional and total keratometry for intraocular lens power calculation in femtosecond laser-assisted cataract surgery. Sci Rep 11:12869. https://doi.org/10.1038/s41598-021-92354-1
Chung HS, Chung JL, Kim YJ, Lee H, Kim JY, Tchah H (2021) Comparing prediction accuracy between total keratometry and conventional keratometry in cataract surgery with refractive multifocal intraocular lens implantation. Sci Rep 11:19234. https://doi.org/10.1038/s41598-021-98491-x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This retrospective study was approved by the institutional review board of Osaka Minato Central Hospital and adhered to the tenets of the Declaration of Helsinki.
Consent to participate
Not applicable.
Consent for publication
No informed consent was required in accordance with the institutional review board of Osaka Minato Central Hospital.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Danjo, Y., Ohji, R. & Maeno, S. Lower refractive prediction accuracy of total keratometry using intraocular lens formulas loaded onto a swept-source optical biometer. Graefes Arch Clin Exp Ophthalmol 261, 137–146 (2023). https://doi.org/10.1007/s00417-022-05777-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05777-5