Abstract
Purpose
To investigate the effect of posterior keratometry (PK) on the accuracy of 10 intraocular lens (IOL) power calculation formulas using standard keratometry (K) and total keratometry (TK).
Methods
This is a retrospective consecutive case-series study. The IOL power was calculated using K and TK measured by IOLMaster 700 in 6 new-generation formulas (Barrett Universal II, Emmetropia Verifying Optical (EVO) 2.0, RBF Calculator 3.0, Hoffer QST, Kane, and Ladas Super Formula) and 4 traditional formulas (Haigis, Hoffer Q, Holladay 1, and SRK/T). The arithmetic prediction error (PE) and mean absolute PE (MAE) were evaluated. The locally-weighted scatterplot smoothing was performed to assess the relationship between PE and PK.
Results
A total of 576 patients (576 eyes) who underwent cataract surgery were included. Compared with using K, all formulas using TK showed a hyperopic shift in the whole group. Specifically, for eyes with PK exceeding -5.90 D, all formulas using TK exhibited a hyperopic shift (all P < 0.001), while eyes with PK less than -5.90 D showed a myopic shift (all P < 0.001). The MAE of new-generation formulas calculated with TK and K showed no statistical differences, while the MAE of traditional formulas with TK was larger (TK: 0.34 ~ 0.43 D; K: 0.33 ~ 0.42 D, all P < 0.05).
Conclusions
The prediction bias of formulas with TK increased as PK deviated from -5.90 D. TK did not improve the prediction accuracy of new-generation formulas, and even performed worse in traditional formulas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
Precise optical biometry and ideal formula selection are crucial factors in achieving a satisfactory refractive outcome after cataract surgery [1]. The refractive errors resulting from inaccurate keratometry measurement remained [2]. Traditional biometers are unable to measure directly the posterior corneal surface; therefore, the standard keratometry (K), calculated from the anterior corneal curvature using a standard keratometric index (usually 1.3375), has been widely used for intraocular lens (IOL) power calculation [3,4,5]. However, the cornea is not a single refractive surface, but a thick lens with anterior and posterior surfaces and thickness. Ignoring the true posterior corneal surface and the corneal thickness can lead to an inaccurate evaluation of keratometry.
Theoretically, by taking into account the true refractive power of the posterior corneal surface, the total keratometry (TK) could potentially improve the prediction accuracy of certain IOL power calculation formulas [6, 7]. Until now, only a few studies investigated the application of TK obtained by the IOLMaster 700 in the IOL power calculation for monofocal IOLs. Srivannaboon S et al. reported that K and TK for IOL calculation yielded consistent results, with a trend toward better refractive outcomes using TK [1]. However, recent papers increasingly show that the usefulness of TK comes into question. [1, 2, 8,9,10,11] Given that PK is the key to distinguish TK from K, further investigations into the effect of PK on the accuracy of IOL calculation formulas for monofocal IOLs are warranted.
Therefore, this study was designed to evaluate how PK affects the prediction accuracy of 6 new-generation formulas (Barrett Universal II [BUII], Emmetropia Verifying Optical [EVO] 2.0, RBF Calculator 3.0, Hoffer QST, Kane, and Ladas Super Formula [LSF]) and 4 traditional formulas (Haigis, Hoffer Q, Holladay 1, and SRK/T) calculated with K and TK.
Methods
This retrospective, consecutive case-series study was performed at the Zhongshan Ophthalmic Center (ZOC) of Sun Yat-sen University, Guangzhou, China. All procedures of this study conformed to the Declaration of Helsinki and were conducted with the approval of the Institutional Review Board/Ethics Committee of ZOC (2019KYPJ033). Informed consents were waived because only the medical records were involved without identifiable private information.
Patients
The medical records of 20,113 cataract patients undergoing phacoemulsification and IOL implantation surgery from April 28, 2021 to March 1, 2022 at ZOC, involving various types of IOLs, were reviewed. The inclusion criteria were as follows: (1) age ≥ 18 years; (2) with preoperative ocular biometry measured successfully by the IOLMaster 700 (v 1.88.1.64861, Carl Zeiss Meditec AG, Jena, Germany); (3) eyes undergoing uneventful cataract phacoemulsification with in-the-bag IOL implantation of the Tecnis ZCB00 IOL (Johnson & Johnson Vision Care Incorporated, USA); and (4) postoperative best-corrected distance visual acuity at least 1 month after surgery better than 20/40. If both eyes of a patient meet the criteria, the right eye was selected for inclusion. The exclusion criteria included: (1) history of ocular surgery or trauma; (2) incomplete follow-up information; or (3) severe eye diseases such as corneal scar, glaucoma and retinal diseases.
Data Collection
The following patient data was collected: age, gender, history of ocular diseases and surgeries, preoperative ocular biometric parameters obtained by IOLMaster 700 (axial length [AL], anterior chamber depth [ACD, measured as the distance from the corneal epithelial to the anterior surface of crystalline lens], lens thickness [LT], white-to-white [WTW], central corneal thickness [CCT], K, TK, PK), surgical procedures, type and power of IOL implanted, and subjective refraction at least 1 month postoperatively.
Formula Calculations
We evaluated formulas including BUII (https://calc.apacrs.org/barrett_universal2105/; accessed 08 Mar 2022), EVO 2.0 (https://www.evoiolcalculator.com/; accessed 10 Jan 2023), RBF 3.0 (https://rbfcalculator.com/; accessed 18 Mar 2021), Hoffer QST (https://hofferqst.com/;accessed 18 Feb 2023), Kane (https://www.iolformula.com/; accessed 24 Jul 2022), LSF,[12] Haigis [13], Hoffer Q [14], Holladay 1 [15], and SRK/T [16]. K and TK were applied respectively for IOL power calculation. The ULIB constant was used and the mean prediction error (ME) was zeroed out in the whole group for all IOL formulas to eliminate any systematic error arising from the clinical environment. Subsequently, analyses were performed, including locally-weighted scatterplot smoothing (LOWESS) regression[17], in the whole group and subgroups to further evaluate the absolute prediction error for each formula [18].
Formula Evaluation
The prediction accuracy of the formula was evaluated by the following parameters. First, each prediction error (PE) was back-calculated as the difference between the actual postoperative and predicted spherical equivalent (SE). The ME was the mean of all the PEs for each formula evaluated, which reflects the systemic bias of the formula. The positive and negative ME suggest the hyperopic and myopic bias, respectively. The standard deviation (SD) of PE was also reported. Second, the absolute PE (AE) was the absolute value of each PE. The mean and median of AE were expressed as the mean AE (MAE) and median AE (MedAE), respectively. Third, the percentages of eyes with PE within ± 0.25 diopter (D), ± 0.50 D, ± 0.75 D and ± 1.00 D were reported. The MAE, MedAE and the percentages of eyes within certain range of PE were calculated after ME was zeroed out in the whole group. The MAE was set to be the main parameter when comparing the prediction accuracy of different formulas [19]. To investigate the effect of PK on prediction accuracy for each formula calculated with K and TK, the LOWESS regression was used and an intersection point was found at PK = -5.90 D. Further subgroups were performed for eyes with PK ≤ -5.90 D (Group 1) and PK > -5.90 D (Group 2). To assess the association between PEs and biometric parameters, Spearman correlation analyses were conducted.
Statistical Analysis
Statistical analyses were performed according to the published protocols using the SPSS 26.0 software (IBM, Chicago, Illinois, USA) and Excel software (Office 2019, Microsoft Corp., Redmond, WA, USA) [19]. A sample size calculation was performed using the PASS 2023 software, which indicated that 320 subjects would be required to detect a difference in prediction accuracy of the IOL calculation formulas calculated with K and TK in two-sided test with a type I error rate (alpha) of 0.15 and 80% power. A P value less than 0.05 was considered statistically significant. The normality of data was evaluated by the Kolmogorov–Smirnov test. The independent t-test or Wilcoxon rank sum test (for continuous variables) and chi-squared test (for categorical variables) were used to compare the differences in participant characteristics between Group 1 and Group 2. The Wilcoxon signed-rank test was used as the data was not normally distributed. The heteroscedastic method was used for comparisons of the SD of the PE among the formulas [20]. The MAEs of all formulas using either the K or TK method were compared using the Friedman test with Bonferroni-Dunn’s post hoc correction. The paired McNemar’s Chi-squared test was used to compare the percentages of eyes within ± 0.25 D, ± 0.50 D, ± 0.75 D and ± 1.00 D of PE between the K and TK methods, while Cochran’s Q test was used to compare the performances of all formulas. We used the Stata (Stata v. 14.0, StataCorp LP, USA) "LOWESS" command to generate a LOWESS curve to graphically depict the relationship between PE and biometric parameters (AL, ACD, CCT, K, LT, PK and WTW) in all the patients. The Spearman correlation analyses were also conducted with Stata. The violin plots were performed with R package v.4.2.1.
Results
Demographic and Clinical Characteristics of Patients
A total of 576 eyes from 576 recruited patients were analyzed in this study. There were 296 and 280 eyes in Group 1 (PK ≤ -5.90 D) and Group 2 (PK > -5.90 D), respectively. The detailed preoperative characteristics of the included patients are summarized in Table 1. Overall, the mean age of the patients was 63.88 ± 11.66 years (range 22 to 93 years). Of these patients, 332 were females (57.64%), and 309 operated eyes were right eyes (53.65%). The PK was normally distributed, with a mean and median of -5.91D. Patients in the Group 2 had longer AL, deeper ACD, wider WTW, thicker CCT, and flatter K, TK and PK, compared with those of Group 1 (all P < 0.05). The mean IOL power inserted was significantly different between Group 1 (20.87 ± 4.56 D) and Group 2 (18.56 ± 4.40 D) (P < 0.001). There were no significant differences in age, lens thickness, and the follow-up period between the 2 groups (all P > 0.05).
Prediction Accuracy in All Patients
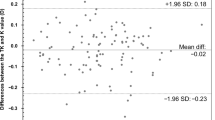
The prediction outcomes of 10 IOL calculation formulas in patients were shown in Table 2, Fig. 1 and Supplementary Fig. 1 (A). The formulas were ranked first by generation order and then in descending order of MAE. Before ME was zeroed, all formulas using TK showed more hyperopic bias compared with those using K (TK: -0.02 ~ 0.15 D; K: -0.06 ~ 0.11 D, all P < 0.001). All formulas using TK had significantly higher SD than using K (all P < 0.05), with the exceptions of EVO, RBF and BUII (all P > 0.05). The prediction accuracy of new-generation formulas calculated with TK and K showed no statistical differences in various parameters, including AE and percentages of cases within ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D of PE (all P > 0.05). The only exception to this trend was observed when comparing the percentage of cases within ± 0.50 D for Hoffer QST (TK: 78.82%; K: 80.21%, P = 0.04). However, the traditional formulas calculated with TK had significantly higher MAE than those with K (TK: 0.34 ~ 0.43 D; K: 0.33 ~ 0.42 D, all P < 0.05). Overall, EVO showed the lowest MAE (0.29 D), whereas the prediction accuracy of EVO, RBF, BUII, and Kane formulas calculated with TK and K showed no statistical difference in MAE (0.29 ~ 0.31 D, all P > 0.05). Hoffer QST, LSF and 4 traditional formulas using K and TK exhibited significantly higher MAE (0.32 ~ 0.43 D) compared to EVO, RBF, BUII, and Kane formulas (0.29 ~ 0.31 D) (all P < 0.05). The predictive outcomes without zeroing ME out yielded similar results (see Supplementary Table 1). However, it was worth noting that all formulas (except for Kane) calculated with TK had significantly higher MAEs than those with K (TK: 0.31 ~ 0.46 D; K: 0.30 ~ 0.44 D, all P < 0.05).
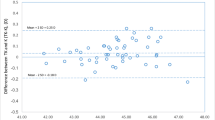
The relationship between ME and PK was shown in the Fig. 2. The LOWESS curves of all formulas exhibited increasing or decreasing against PK and intersecting near the mean value of PK (-5.91 D). Additionally, larger PEs were observed as PK deviated from the intersection point. After ME was zeroed out in the whole group, compared with formulas calculated with K, all formulas calculated with TK showed a myopic bias in eyes with PK ≤ -5.90 D (all P < 0.001) (Table 3), but a hyperopic bias in eyes with PK > -5.90 D (all P < 0.001) (Table 4). The PEs of formulas calculated with TK or K changed similarly with other biometric parameters (AL, ACD, K, LT, and WTW). While the difference between PEs calculated with K and TK gradually increased as CCT deviated from its median value, the divergence was notably smaller than that observed in PK (Supplementary Fig. 2). The PE of traditional formulas displayed larger deviation with biometric parameters (AL, ACD, CCT, K, LT, PK and WTW) compared with new formulas. The Spearman correlations were statistically significant between the biometric parameters and the difference in PEs of formulas with K and TK. Notably, PK exhibited much stronger correlations (rho: 0.397 ~ 0.422, P < 0.05) with the PEs difference (calculated using K and TK) than the other factors (AL: 0.088 ~ 0.120, P < 0.05; WTW: 0.192 ~ 0.216, P < 0.05; CCT: -0.187 ~ -0.174, P < 0.05) (Supplementary Table 2).
The smoothed line graph showing the prediction error (in diopters [D]) versus posterior keratometry (PK, in [D]). (A) Line graph of the traditional formulas. (B) Line graph of the new-generation formulas. K = standard keratometry; TK = total keratometry; Myopic → = myopic bias; Hyperopic → = hyperopic bias; BUII = Barrett Universal II; EVO = Emmetropia Verifying Optical formula 2.0; LSF = Ladas Super Formula; RBF = RBF Calculator3.0
Prediction Accuracy in Subgroups
The prediction outcomes of 10 IOL calculation formulas in Group 1 were shown in Table 3, Supplementary Fig. 1 (B) and Supplementary Fig. 3 (A). After ME was zeroed out in the whole group, all formulas using TK showed more myopic bias than those using K in group 1 (TK: -0.22 ~ 0.02 D; K: -0.19 ~ 0.05 D, all P < 0.001). No significant difference was observed in parameters including SD, AE and percentages of cases within ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D of PE of all formulas between using TK and K. The only exception to this trend was observed when comparing the SD for Haigis (TK: 0.48; K: 0.47, P = 0.023). Among all formulas, the EVO displayed the lowest MAE (0.32 D), but was not statistically different from the prediction accuracy of RBF, BUII, Kane, and Hoffer QST formulas using K and TK (0.34 to 0.36 D, all P > 0.05). LSF and 4 traditional formulas using K and TK showed statistically significant higher MAE (0.36 ~ 0.48 D, all P < 0.05) compared with the above new-generation formulas. The predictive outcomes without zeroing ME out was showed in Supplementary Table 3. New-generation formulas (except EVO and Hoffer QST) calculated with TK showed more hyperopic bias (all P < 0.05). The Haigis calculated with TK had a significantly higher MAE than that with K (0.39 D to 0.37 D, all P < 0.05). The trends in other outcomes were similar to those observed when ME was zeroed out.
The prediction outcomes of 10 IOL calculation formulas in Group 2 are shown in Table 4, Supplementary Fig. 1 (C) and Supplementary Fig. 3 (B). After ME was zeroed out in the whole group, all formulas using TK showed more hyperopic bias compared with those using K in group 2 (TK: -0.02 ~ 0.23 D; K: -0.06 ~ 0.21 D, all P < 0.001). For new-generation formulas, there were no statistically significant differences in the parameters including SD, AE and percentages of cases within ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D of PE between using TK and K. For traditional formulas, Haigis and Hoffer Q using TK had significantly higher SD than using K (both P < 0.05). All the traditional formulas (except for Holladay 1) using TK had significantly higher AE than those using K (all P < 0.05). SRK/T, Hoffer Q and Holladay 1 formulas calculated with K and TK showed significantly higher MAE (0.35 ~ 0.41 D) compared with Haigis and all new-generation formulas (0.26 ~ 0.30 D, all P < 0.05). The predictive outcomes, when not zeroing ME out, produced comparable results (see Supplementary Table 4). It's important to highlight that all formulas calculated with TK exhibited significantly higher MAEs compared to those with K (TK: 0.27 ~ 0.46 D; K: 0.25 ~ 0.43 D, all P < 0.05).
Discussion
By exploring the effect of PK on the accuracy of the new-generation and traditional formulas calculated with K and TK based on a large sample size, we found that the difference in the prediction accuracy of IOL calculation formulas calculated with K and TK was mainly related to PK. Compared with formulas calculated with K, all formulas with TK showed a myopic bias in eyes with PK ≤ -5.90 D but a hyperopic bias in eyes with PK > -5.90 D. In addition, TK was not superior to K for prediction accuracy, and even resulted in poorer performance in traditional formulas, indicating that further corrections were needed.
The LOWESS and Spearman correlation analysis showed that the impact of AL, ACD, CCT, Km, LT, and WTW on the difference between PEs of formulas calculated with K and TK was minimal. It was observed that the LOWESS curves of all formulas calculated with K and TK intersected at the mean value of PK, and TK increased the hyperopic or myopic bias of formulas as PK deviated from -5.90 D. In our study, the mean PK was -5.91 D and this distribution was comparable to previous reports done in Israel by Wasser LM et al. (mean = -5.87 D) and in Japan by Danjo Y et al. (mean = -5.93 D).[10, 21] Danjo Y et al. evaluated BUII and traditional formulas and reported that in the subgroup with PK ≥ − 5.74 D, TK gave more hyperopic prediction (0.07 D to 0.09 D, P < 0.001), but more myopic prediction (0.03 D to 0.06 D, P < 0.001) in the subgroup with PK < − 6.08 D.[10] These results were similar to our subgroup results, however, the overall trends of PE with PK were not analyzed. In this study, LOWESS curve describing a parameter-free smoothing was performed to visualize the overall trend and showed that the PE was larger when PK deviated from -5.90 D. The variations might be explained by the trends of TK and K values. Overall, we observed that TK value was larger than K value, but as PK was less than -5.90 D, the proportion of K larger than TK gradually increased. Therefore, the intersection point near -5.90 D might indicate a node to divide the samples into subgroups and to adjust the IOL calculation formulas in future studies.
Compared with deduced PK, measured PK could better evaluate the true corneal power [6,7,8, 22]. However, we found TK did not improve the prediction accuracy of the IOL power calculation formulas and even increased the prediction error of traditional formulas. Previous studies reported inconsistent findings of the effect of TK on the accuracy of IOL calculation formulas. Tsessler et al. found that TK did not provide a significant improvement to the postoperative SE prediction accuracy [8]. Danjo et al. and Savini et al. reported that using TK instead of K led to a lower refractive prediction accuracy in traditional and BUII formulas for IOLMaster 700 or Sirius (CSO, Florence, Italy) [10, 23]. Other studies reported that the TK dataset showed a better trend of refractive outcomes mainly in the special study populations (highly myopic eyes, flat and steep keratometry eyes) [2, 9]. However, most previous studies consisted of small sample sizes (60 ~ 231), used multiple IOL models, and evaluated different biometers such as IOLMaster 700, Lenstar LS900 and Sirius, which could be the reasons attributed to the inconsistent results [1, 2, 8,9,10, 23, 24]. In this study, the sample size (N = 576) was the largest to date and only a single type of IOL was included, so the conclusions were more reliable. To sum up, our study suggested that unless internal corrections for TK were made in formulas, there is no need to be calculated with TK for monofocal IOL power calculation.
Moreover, TK obtained with IOLMaster 700 has been designed to be compatible with K data and the existing optimized IOL constants such as ULIB constants [7, 24]. However, we found that before ME was zeroed, formulas calculated with TK showed more hyperopic bias of 0.03 D to 0.04 D in all patients and 0.06 D to 0.08 D in subgroups than with K. Therefore, to apply TK more accurately, either the IOL power calculation formula needs to be adjusted accordingly, or the internal correction method for the measured TK with IOLMaster 700 needs to be further optimized.
Our study compared the performance of TK and K in 6 new-generation formulas (BUII, EVO, Hoffer QST, Kane, LSF, and RBF) and 4 traditional formulas based on a larger sample size. Overall, new-generation formulas showed higher prediction accuracy than traditional formulas (MAE: 0.29 ~ 0.35 D vs 0.34 D ~ 0.43 D), of which the EVO formulas exhibited a slightly superior performance in all patients and subgroups. This trend was similar to the findings of Qin Y et al. [2]. In addition, Tsessler et al. reported that there was no statistical differences in standard deviation of PE among RBF 3.0, RBF 2.0, BUII and EVO formulas [8]. Moreover, we found that the PE of new-generation formulas appeared to be less affected by K and PK than the traditional formulas (Fig. 2 and Supplementary Fig. 2), perhaps due to the internal correction factors incorporated for TK in new-generation formulas.
There are some limitations in this study. Firstly, we did not assess other modern formulas like Holladay 2 and Olsen formulas. Secondly, only 1 type of IOL was included in this study. Considering that different IOL types could affect the performance and prediction errors of different formulas, the generalization of our conclusion will be limited to some extent. Thirdly, a total of 5 surgeons contributed cases, which might introduce bias due to differences in surgical style. However, all surgeons underwent standardized professional training, minimizing the bias to a certain extent. Additionally, this is a single center retrospective study. Multicenter and prospective studies with a larger sample size are needed for further evaluation in the future.
In summary, the TK dataset increased the hyperopic or myopic bias of formulas as PK deviated from -5.90 D. TK did not provide an improvement to the prediction accuracy of new-generation formulas, and even led to lower prediction accuracy of traditional formulas, indicating that TK obtained by IOLMaster 700 may require further improvement.
Funding Support
This study was funded by the National Natural Science Foundation of China (No. 82070940) and the Construction Project of High-Level Hospitals in Guangdong Province (No.303020102). The funding organization had no role in the design or conduct of this research.
Change history
27 January 2024
The corresponding emails of the two co-corresponding authors have been interchanged. This has been corrected.
References
Srivannaboon S, Chirapapaisan C (2019) Comparison of refractive outcomes using conventional keratometry or total keratometry for IOL power calculation in cataract surgery. Graefes Arch Clin Exp Ophthalmol 257:2677–2682. https://doi.org/10.1007/s00417-019-04443-7
Qin Y, Liu L, Mao Y, Ding Y, Ye S, Sun A, Wu M (2022) Accuracy of intraocular lens power calculation based on total keratometry in patients with flat and steep corneas. Am J Ophthalmol. https://doi.org/10.1016/j.ajo.2022.11.011
Ueno Y, Hiraoka T, Miyazaki M, Ito M, Oshika T (2015) Corneal thickness profile and posterior corneal astigmatism in normal corneas. Ophthalmology 122:1072–1078. https://doi.org/10.1016/j.ophtha.2015.01.021
Koch DD (2015) The posterior cornea: hiding in plain sight. Ophthalmology 122:1070–1071. https://doi.org/10.1016/j.ophtha.2015.01.022
Holladay JT (1997) Standardizing constants for ultrasonic biometry, keratometry, and intraocular lens power calculations. J Cataract Refract Surg 23:1356–1370. https://doi.org/10.1016/s0886-3350(97)80115-0
Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L (2012) Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg 38:2080–2087. https://doi.org/10.1016/j.jcrs.2012.08.036
Wang L, Spektor T, de Souza RG, Koch DD (2019) Evaluation of total keratometry and its accuracy for intraocular lens power calculation in eyes after corneal refractive surgery. J Cataract Refract Surg 45:1416–1421. https://doi.org/10.1016/j.jcrs.2019.05.020
Tsessler M, Cohen S, Wang L, Koch DD, Zadok D, Abulafia A (2022) Evaluating the prediction accuracy of the Hill-RBF 3.0 formula using a heteroscedastic statistical method. J Cataract Refract Surg 48:37–43. https://doi.org/10.1097/j.jcrs.0000000000000702
Wei L, Cheng K, He W, Zhu X, Lu Y (2022) Application of total keratometry in ten intraocular lens power calculation formulas in highly myopic eyes. Eye Vis (Lond) 9:21. https://doi.org/10.1186/s40662-022-00293-3
Danjo Y, Ohji R, Maeno S (2022) Lower refractive prediction accuracy of total keratometry using intraocular lens formulas loaded onto a swept-source optical biometer. Graefes Arch Clin Exp Ophthalmol. https://doi.org/10.1007/s00417-022-05777-5
Zhao H, Chen X, Liu B, Liu X, Liu Y (2023) Accuracy of refractive outcomes using standard or total keratometry for intraocular lens power formulas in conventional cataract surgery. BMC Ophthalmol 23:346. https://doi.org/10.1186/s12886-023-03094-x
Ladas JG, Siddiqui AA, Devgan U, Jun AS (2015) A 3-D “Super Surface” Combining Modern Intraocular Lens Formulas to Generate a “Super Formula” and Maximize Accuracy. JAMA Ophthalmol 133:1431–1436. https://doi.org/10.1001/jamaophthalmol.2015.3832
Haigis W, Lege B, Miller N, Schneider B (2000) Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol 238:765–773. https://doi.org/10.1007/s004170000188
Hoffer KJ (1993) The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg 19:700–712. https://doi.org/10.1016/s0886-3350(13)80338-0
Holladay JT, Prager TC, Chandler TY, Musgrove KH, Lewis JW, Ruiz RS (1988) A three-part system for refining intraocular lens power calculations. J Cataract Refract Surg 14:17–24. https://doi.org/10.1016/s0886-3350(88)80059-2
Retzlaff JA, Sanders DR, Kraff MC (1990) Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refract Surg 16:333–340. https://doi.org/10.1016/s0886-3350(13)80705-5
Cleveland WS, Devlin SJ (1988) Locally Weighted Regression: An Approach to Regression Analysis by Local Fitting. J Am Stat Ass 83:596–610. https://doi.org/10.1080/01621459.1988.10478639
Wang L, Koch DD, Hill W, Abulafia A (2017) Pursuing perfection in intraocular lens calculations: III. Criteria for analyzing outcomes. J Cataract Refract Surg 43:999–1002. https://doi.org/10.1016/j.jcrs.2017.08.003
Hoffer KJ, Savini G (2021) Update on Intraocular Lens Power Calculation Study Protocols: The Better Way to Design and Report Clinical Trials. Ophthalmology 128:e115–e120. https://doi.org/10.1016/j.ophtha.2020.07.005
Holladay JT, Wilcox RR, Koch DD, Wang L (2021) Review and recommendations for univariate statistical analysis of spherical equivalent prediction error for IOL power calculations. J Cataract Refract Surg 47:65–77. https://doi.org/10.1097/j.jcrs.0000000000000370
Wasser LM, Tsessler M, Weill Y, Zadok D, Abulafia A (2022) Ocular Biometric Characteristics Measured by Swept-Source Optical Coherence Tomography in Individuals Undergoing Cataract Surgery. Am J Ophthalmol 233:38–47. https://doi.org/10.1016/j.ajo.2021.06.032
Zheng T, Xu J, Lu Y (2020) Comparison of the accuracy of four Pentacam corneal astigmatism values in non-toric pseudophakic eyes. Graefes Arch Clin Exp Ophthalmol 258:795–803. https://doi.org/10.1007/s00417-019-04585-8
Savini G, Hoffer KJ, Lomoriello DS, Ducoli P (2017) Simulated Keratometry Versus Total Corneal Power by Ray Tracing: A Comparison in Prediction Accuracy of Intraocular Lens Power. Cornea 36:1368–1372. https://doi.org/10.1097/ico.0000000000001343
Fabian E, Wehner W (2019) Prediction Accuracy of Total Keratometry Compared to Standard Keratometry Using Different Intraocular Lens Power Formulas. J Refract Surg 35:362–368. https://doi.org/10.3928/1081597x-20190422-02
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Ethics Committee of Zhongshan Ophthalmic Center, Sun Yat-sen University (ID: 2023KYPJ230) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consents were waived because only the medical records were involved without identifiable private information.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aixia Jin, Jiaqing Zhang and Xuhua Tan are Co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, A., Zhang, J., Tan, X. et al. Effect of Posterior Keratometry on the Accuracy of 10 Intraocular Lens Calculation Formulas: Standard Keratometry versus Total Keratometry. Graefes Arch Clin Exp Ophthalmol 262, 1829–1838 (2024). https://doi.org/10.1007/s00417-023-06367-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06367-9