Abstract
Downbeat nystagmus (DBN) is caused by an impairment of Purkinje cells in the flocculus. The decreased cerebellar inhibitory input affects otolith pathways. Since ocular and cervical vestibular evoked myogenic potentials (o-/cVEMP) test the otoliths, the VEMP were measured in DBN patients and in controls. Sixteen patients with DBN, 14 cerebellar oculomotor disorder patients without DBN (COMD), and 16 healthy controls were examined with o-/cVEMP. Computational modeling was used to predict VEMP differences between groups. DBN patients had significantly higher oVEMP peak-to-peak (PP) amplitudes than COMD patients without DBN and controls. Cervical VEMP did not differ. The computational model of DBN predicted a twofold oVEMP increase for DBN patients. These findings suggest an enhancement of the utriculo-ocular response. The unchanged cVEMP indicate no effect on the otolith-cervical reflex in DBN. Computational modeling suggests that the utriculo-ocular enhancement is caused by an impaired vertical neural integrator resulting in the increased influence of utricular signals. This also explains the gravitational dependence of DBN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Downbeat nystagmus (DBN) is a frequent type of acquired nystagmus, which is proposed to be most often caused by an impaired function of the flocculus/paraflocculus [1–3]. It consists of slow upward drifts of the eye and compensatory downward fast phase eye movements. Vertical upward drift has two parts: vertical gaze-evoked drift attributed to leakiness of the vertical gaze-holding mechanism and an upwardly directed spontaneous or bias drift that is present with gaze straight ahead. The spontaneous upward drift can be subdivided into a gravity-independent component, which is present in gaze straight ahead, and a gravity-dependent component, which is maximal in prone and minimal in supine positions [4]. Gaze-evoked drift follows Alexander’s law, i.e. vertical drift velocity increases with gaze in the direction of the fast phase, i.e. downward. The vertical drifts in DBN patients, including the gravity-dependent otolith-mediated drift, have been mainly attributed to a reduction of floccular inhibitory influence on the floccular target neurons in the superior vestibular nucleus in the brainstem due to floccular [5, 6] or, in rare cases, paramedian tract impairment [7, 8, 9].
Clinically, the function of the otolith organs can be tested by vestibular evoked myogenic potentials (VEMP). Tap stimuli to the forehead stimulate utricular hair cells and give rise to ocular VEMP. The utricular signals are transmitted to the extraocular muscles, in particular the inferior oblique muscle [10, 11], via the superior part of the vestibular nerve to the superior and medial vestibular nuclei in the brainstem reaching the contralateral oculomotor nucleus [12, 13]. Saccular afferent fibers are stimulated via high-amplitude tone bursts causing cervical VEMP. The respective pathway projects through the inferior vestibular nerve, the vestibulospinal tract, the accessory nucleus and via the accessory nerve to the ipsilateral sternocleidomastoid muscle [14].
The increase of the gravity-dependent component of vertical drift in DBN described above suggests a central enhancement of utriculo-ocular responses in DBN patients. In the current study, we therefore investigated whether oVEMP amplitudes are higher in patients with DBN than in controls. We hypothesized that cVEMP amplitudes in these patients do not change because the floccular damage underlying DBN affects the vestibulo-ocular but not the vestibulo-collic reflex and because the enhancement of otolith-ocular responses is supposed to be of central origin, thus not affecting the sacculus. We also performed oVEMP examination in patients with cerebellar ocular motor disorders but without DBN to test whether cerebellar impairment without vertical ocular motor symptoms would affect oVEMP responses.
Subjects and methods
This is a prospective study conducted in the tertiary outpatient center. All subjects underwent a complete neurological, neuro-ophthalmological, and neuro-otological examination. Patients were divided into two groups on the basis of the neuro-ophthalmological findings: first, patients with DBN and additional cerebellar ocular motor disorders; second, patients with cerebellar ocular motor disorders but no DBN.
Subjects
Sixteen patients with cerebellar ocular motor disorder and DBN (8 F, age: mean ± SD 73.4 ± 10.8 years), 14 patients with cerebellar ocular motor disorders without DBN (8 F, 72.1 ± 9.1 years), and 16 age-matched healthy subjects (4 F, 72.6 ± 4.8 years) with no evidence of cerebellar, vestibular, or ocular motor disorders were included.
The etiologies of patients with cerebellar ocular motor disorders with DBN comprised Arnold-Chiari malformation type I (n = 1, F), cerebellar ischemia (n = 1, F), hereditary spinocerebellar ataxia (n = 1, M), cerebellar degeneration after lymphomatous meningitis (n = 1, M), and “idiopathic cases” (n = 12, 5 F), with neither cerebellar nor brainstem pathological MRI signs. Three of the “idiopathic causes” were “pure DBN” (3 F), i.e. no other cerebellar ocular motor disorders were present, and nine were “cerebellar DBN” (4 F), (for details see Table 1A).
In three patients of the cerebellar group without DBN, the MRI revealed a microangiopathy of the cerebellum. None of these patients had high-grade cerebellar atrophy on MRI as evaluated by the experienced neuroradiologist (for details see Table 1B).
The study was performed in accordance with the Helsinki II Declaration and approved by the Ethics Committee of the Medical Faculty of the Munich University Hospital. Written informed consent was obtained from all participants in the study (consent for research).
Recording of vestibular evoked myogenic potentials
The VEMP examination was performed as published elsewhere [15]. Briefly, oVEMP were recorded with recording electrode placed over the inferior oblique muscle bilaterally, approximately 3 mm below the eye and centered beneath the pupil, a reference electrode on the chin; and a ground electrode placed under the chin. “Mini taps” were delivered with a Bruel and Kjaer Mini-Shaker Type 4810 at the midline of the hairline, 30 % of the distance between the inion and nasion. The responses to 50–100 stimuli were averaged. The first negative and positive peaks of the oVEMP response that occurred between 10 and 20 ms after stimulus onset were designated n10 and p15, respectively [16, 17]. For cVEMP, supine-positioned subjects were instructed to lift their heads to actively flex their neck against gravity during stimulation and recording to provide tonic background muscle activity. Air-conducted 500-Hz, 100-dB SPL tone bursts were delivered monaurally via intra-auricular headphones. Cervical VEMP were recorded from an electrode montage consisting of a recording electrode placed at the midpoint of the ipsilateral sternocleidomastoid muscle belly, a reference electrode placed on the manubrium sterni, and a ground electrode placed on the forehead.
EMG activity was recorded (Nicolet Biomedical Inc, Madison WI, USA), amplified and bandpass filtered and the responses to 50–100 stimuli were averaged. The first positive and negative peaks that occurred between 13 and 23 ms after stimulus onset were designated as p13 and n23, respectively.
Statistical analysis
The statistical analysis compared the sizes of averaged oVEMP n10, PP amplitudes and n10 latencies, as well as of the averaged cVEMP p13 and PP amplitudes between the cerebellar patients with/without DBN and the same-aged group of control subjects. The oVEMP amplitudes were analyzed by repeated-measures ANOVA with one within-subject factor (SIDE: right/left) and two between-subject factors (SEX: m/f; GROUP: DBN/control/cerebellar only). For post hoc tests, the Scheffe test was used. Statistical testing was performed using Statistica 6.1 (StatSoft Inc., Tulsa, USA). The significance level was set to p = 0.05.
Computational modeling
The mathematical model of the vertical ocular motor system published previously [18] was extended to include oVEMP stimulation. Briefly, the model consists of system-level differential equations describing the function of various cortical and subcortical areas involved in eye movement generation. Emphasis is placed on the role of brainstem and cerebellar areas for slow eye movements, i.e. the vestibulo-ocular reflex and smooth pursuit. For the present work, the oVEMP stimulation was done in the model as a 2-ms pulse of acceleration to the otoliths with the model’s simulated head position at 60° pitch backward and gaze direction 45° upward. No other changes were made to the original model [18]. Two simulations were performed: one for a healthy subject and another one for a DBN patient. The DBN patient simulation was performed as described previously by changing the input–output relationship of the floccular Purkinje cells [18]. To quantify the simulated oVEMP response, the effect of the acceleration pulse on the slow phase velocity was extracted. The computational simulation was verified analytically (see “Appendix”).
Results
The patients’ clinical characteristics are given in Table 1A, B. In patients with DBN the oVEMP n10 amplitudes were 16.6 ± 7.2 µV and the PP amplitudes, 32.6 ± 11.5 µV. In patients with cerebellar ocular motor disorders without DBN the oVEMP n10 amplitudes were 8.8 ± 4.2 µV and the PP 19.1 ± 8.8 µV. In controls, the oVEMP amplitudes were 10 ± 2.7 µV and 23 ± 6.1 µV (Table 2).
The oVEMP PP amplitudes showed a significant main effect for GROUP [F(2,40) = 9.22, p = 0.0005], but no dependence on SIDE or SEX and no significant interactions. The main effect of GROUP was caused by a difference between oVEMP amplitudes in DBN and control subjects (post hoc Scheffe p = 0.019) and a difference between DBN and cerebellar subjects (p = 0.001), but not between control and cerebellar subjects (Fig. 1). The n10 amplitude showed a similar result with a main effect of GROUP [F(2,40) = 10.9, p = 0.0002], again caused by differences between DBN and the other two groups. The three-way interaction (SIDExSEXxGROUP) also became significant [F(2,40) = 3.63, p = 0.035] due to a slight side difference between male and female participants in the DBN group.
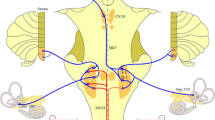
For the cVEMP, there were no significant differences of p13 and PP amplitudes between the three groups (p > 0.05, Table 3). The correlation between the amplitude and the latency of n10 oVEMP in all enrolled subjects was not statistically notable. OVEMP n10 latencies did not significantly differ between groups. Age had no significant influence on the oVEMP and cVEMP amplitudes. If other pathological vestibular findings in DBN patients are taken into account, seven DBN patients had a pathological head-impulse test. No statistically significant relation between these abnormal findings and the oVEMP testing was observed. The pathophysiologically relevant brainstem pathways are depicted in Fig. 2a.
a Simplified scheme of the proposed neural pathways for oVEMP. Solid red line depicts the proposed main otolith-ocular reflex (oVEMP pathway) from utricle to the vestibular nuclei (VN), through the medial longitudinal fascicle (MLF) to the interstitial nucleus of Cajal (INC), to the oculomotor nuclei (OMN), and from there to the extraocular muscles (especially inferior oblique muscle—IOM). The direct VOR pathway (dashed-dotted line) connects the VN and the oculomotor nuclei and may also carry utricular information. A parallel feedback loop (red dashed lines) via the paramedian tract (PMT) and the cerebellar floccular lobe (FL) controls, among other functions, VOR adaptation and gaze holding (integrator time constant). In DBN patients, the loop VN-PMT-FL is disturbed, e.g., due to damage of floccular Purkinje cells, which leads to a disinhibition of floccular target neurons in the VN. This, in turn, leads to an increased size of oVEMP. For details, see “Appendix”. b The simulated oVEMP response to a impulse-like head tap transmitted via the utricles and the pathways in Fig. 2a to the extraocular eye muscles during backward tilt of the head and upward gaze for a model generating downbeat nystagmus (black line, DBN) and a ‘healthy’ model (gray line, healthy). In the DBN simulation, the disturbance of slow phase velocity by otolith input due to oVEMP stimulation is higher than in the healthy model
To evaluate the present results in the framework of the previously published mathematical model of DBN [18], the oVEMP stimulation was simulated computationally. The 2-ms acceleration pulses to the utricle, which were simulated during 60° backward tilt of the head and 45° upward gaze direction, caused brief deviations in the slow phase velocity (Fig. 2b). The simulated response amplitude for a patient with DBN (slow phase velocity 3.0 °/s in upright position with gaze straight ahead) was 140 % of the simulated response of a healthy test subject. The computational simulation was verified analytically (see “Appendix”), confirming that responses in simulated DBN are larger than in the healthy system.
Discussion
In this study, we found significantly higher oVEMP amplitudes in patients with DBN compared to age-matched controls and patients with cerebellar ocular motor disorders without DBN; this suggests that the effect was associated with DBN and thus with impaired pathways for vertical eye movements rather than with cerebellar disorders in general. The increased oVEMP amplitudes indicate an enhanced pass-through of otolith-ocular responses in patients suffering from DBN. The pitch-dependent hyperactivity of otolith circuits in DBN was previously reported in humans [4, 5, 7] as well as in a mouse model of DBN [19]. Our computational simulation of DBN also supports this interpretation.
Based on these findings, we propose the following mechanisms:
-
1.
Impairment of vertical ocular motor pathways involving the floccular lobe leads to DBN [5] and to leakiness of the vertical oculomotor integrator, which results in vertical gaze-dependent nystagmus [20].
-
2.
The vertical integrator receives signals from the utricle via an indirect otolith-ocular pathway, which is responsible for static otolith-driven changes in eye position [21, 22].
-
3.
The leaky integrator causes increased pass-through of the utricular signal.
The same cerebellar dysfunction that causes the increase in utricular responses also explains the well-known strong dependence of DBN on head position relative to gravity: in supine position the upward drift and intensity of DBN are smallest and in prone position the upward drift and DBN are largest [23]. In line with our theory, cVEMP amplitudes in DBN did not increase suggesting that vestibulo-collic reflexes remain unchanged, probably caused by different pathways conveying the ascending utricular and descending saccular projections.
However, the upward deviation of the head described in the mouse model, which indicates hyperactivity also in vestibulo-collic otolith circuits, is in contrast to our findings of the absent effect on the saccular responses measured by cVEMP. There may be two reasons for this: first, due to inter-species differences, the floccular impairment in mouse may lead to the hyperactivity of the vestibulo-collic reflex, whereas in humans, it is not the case. Lack of symptoms in head posture or head movements in DBN patients supports this hypothesis. Second, the sensitivity of oVEMP and cVEMP measurements might not be comparable, since oVEMP act in an excitatory fashion, while cVEMP are inhibitory in nature. In addition, the vestibulo-collic reflex could be weakened in our elderly study group, since the cVEMP strongly depend on muscle tone, which decreases with age [24, 25]. In contrast to air-conducted cVEMP, the bone-conducted oVEMP elicited by tap stimuli to the forehead do not significantly decrease with age [26, 27]. Moreover, the air-conducted cVEMP might be reduced in patients with mild to moderate conductive hearing loss due to attenuation in the level of sound transmitted to the inner ear [28, 29]. Therefore, the higher reliability of bone-conducted oVEMP may reflect the otolith function better than cVEMP.
In a preceding study investigating the effect of alcohol and gaze-evoked nystagmus on oVEMP and cVEMP reflexes, a significant decrease of oVEMP but not cVEMP amplitudes was found [30]. The authors suggested that there might be a specific central effect of alcohol, such as modulation of vestibulo-ocular processes. This is in line with our theory that central deficits underlying the DBN pathophysiology are responsible for the increase in oVEMP but not cVEMP reflex. The result of the head-impulse test in DBN patients was not related to oVEMP amplitudes. This is expected considering that DBN is caused by a central rather than a peripheral deficit and that central processing of horizontal canal and utricular signals for eye movements is different.
The present study is relevant for understanding the pathogenesis of DBN because it confirms again the previously found effect on otolith-ocular pathways, which leads to the strong dependence of DBN on postural orientation with respect to gravity. Apart from this, it is highly relevant for the interpretation of abnormal oVEMP in patients. The present example shows that abnormal oVEMP results do not necessarily reflect a utricular dysfunction but may be caused by impairment of central pathways carrying completely normal utricular signals to the eye muscles. In the present case, we hypothesize that the abnormal oVEMP responses reflect a specific involvement of otolith-ocular pathways through the vertical oculomotor integrator, which is impaired due to malfunction of the floccular lobe, but do not indicate otolith dysfunction in general.
References
Kalla R, Deutschlander A, Hufner K, Stephan T, Jahn K, Glasauer S, Brandt T, Strupp M (2006) Detection of floccular hypometabolism in downbeat nystagmus by fMRI. Neurology 66:281–283
Hüfner K, Stephan T, Kalla R, Deutschländer A, Wagner J, Holtmannspötter M, Schulte-Altedorneburg G, Strupp M, Brandt T, Glasauer S (2007) Structural and functional MRIs disclose cerebellar pathologies in idiopathic downbeat nystagmus. Neurology 69:1128–1235
Wagner JN, Glaser M, Brandt T, Strupp M (2008) Downbeat nystagmus: aetiology and comorbidity in 117 patients. J Neurol Neurosurg Psychiatry 79:672–677
Marti S, Palla A, Straumann D (2002) Gravity dependence of ocular drift in patients with cerebellar downbeat nystagmus. Ann Neurol 52:712–721
Zee DS, Yamazaki A, Butler PH, Gücer G (1981) Effects of ablation of flocculus and paraflocculus on eye movements in primate. J Neurophysiol 46:878–899
Glasauer S, Strupp M, Kalla R, Büttner U, Brandt T (2005) Effect of 4-aminopyridine on upbeat and downbeat nystagmus elucidates the mechanism of downbeat nystagmus. Ann NY Acad Sci 1039:528–531
Wagner J, Lehnen N, Glasauer S, Rettinger N, Büttner U, Brandt T, Strupp M (2009) Downbeat nystagmus caused by a paramedian ponto-medullary lesion. J Neurol 256:1572–1574
Nakamagoe K, Fujizuka N, Koganezawa T, Yamaguchi T, Tamaoka A (2013) Downbeat nystagmus associated with damage to the medial longitudinal fasciculus of the pons: a vestibular balance control mechanism via the lower brainstem paramedian tract neurons. J Neurol Sci 328:98–101
Helmchen C, Glasauer S, Sprenger A (2013) Inverse eye position dependency of downbeat nystagmus in midline medullary lesion. J Neurol 260:2908–2910
Curthoys IS, Burgess AM, MacDougall HG, McGarvie LA, Halmagyi GM, Smulders YE, Iwasaki S (2009) Testing human otolith function using bone-conducted vibration. Ann NY Acad Sci 1164:344–346
Curthoys IS, Iwasaki S, Chihara Y, Ushio M, McGarvie LA, Burgess AM (2011) The ocular vestibular-evoked myogenic potential to air-conducted sound; probable superior vestibular nerve origin. Clin Neurophysiol 122:611–616
Colebatch JG, Halmagyi GM (2000) Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology 42:1635–1636
Curthoys I, Manzari L (2013) Otolithic disease: clinical features and the role of vestibular evoked myogenic potentials. Semin Neurol 33:231–237
Colebatch JG, Halmagyi GM, Skuse NF (1994) Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatr 57:190–197
Agrawal Y, Bremova T, Kremmyda O, Strupp M (2013) Semicircular canal, saccular and utricular function in patients with bilateral vestibulopathy: analysis based on etiology. J Neurol 260:876–883
Smulders YE, Welgampola MS, Burgess AM, McGarvie LA, Halmagyi GM, Curthoys IS (2009) The n10 component of the ocular vestibular-evoked myogenic potential (oVEMP) is distinct from the R1 component of the blink reflex. Clin Neurophysiol 120:1567–1576
Nguyen KD, Welgampola MS, Carey JP (2010) Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol 31:793–802
Marti S, Straumann D, Büttner U, Glasauer S (2008) A model-based theory on the origin of downbeat nystagmus. Exp Brain Res 188:613–631
Stahl JS, Thumser ZC, Oommen BS (2012) The ataxic mouse as a model for studying downbeat nystagmus. J Vestib Res 22:221–241
Glasauer S, Hoshi M, Kempermann U, Eggert T, Büttner U (2003) Three-dimensional eye position and slow phase velocity in humans with downbeat nystagmus. J Neurophysiol 89:338–354
Crawford JD, Tweed DB, Vilis T (2003) Static ocular counterroll is implemented through the 3-D neural integrator. J Neurophysiol 90:2777–2784
Glasauer S, Dieterich M, Brandt T (2001) Central positional nystagmus simulated by a mathematical ocular motor model of otolith-dependent modification of Listing’s plane. J Neurophysiol 86:1546–1554
Spiegel R, Kalla R, Rettinger N, Schneider E, Straumann D, Marti S, Glasauer S, Brandt T, Strupp M (2010) Head position during resting modifies spontaneous daytime decrease of downbeat nystagmus. Neurology 75:1928–1932
Akin FW, Murnane OD, Tampas JW, Clinard CG (2011) The effect of age on the vestibular evoked myogenic potential and sternocleidomastoid muscle tonic electromyogram level. Ear Hear 32:617–622
Singh NK, Kashyap RS, Supreetha L, Sahana V (2014) Characterization of age-related changes in sacculocolic response parameters assessed by cervical vestibular evoked myogenic potentials. Eur Arch Otorhinolaryngol 271:1869–1877
Rosengren SM, Govender S, Colebatch JG (2011) Ocular and cervical vestibular evoked myogenic potentials produced by air- and bone-conducted stimuli: comparative properties and effects of age. Clin Neurophysiol 122:2282–2289
Colebatch JG, Govender S, Rosengren SM (2013) Two distinct patterns of VEMP changes with age. Clin Neurophysiol 124:2066–2068
Bath AP, Harris N, McEwan J, Yardley MP (1999) Effect of conductive hearing loss on the vestibulo-collic reflex. Clin Otolaryngol Allied Sci 24:181–183
Wang MC, Lee GS (2007) Vestibular evoked myogenic potentials in middle ear effusion. Acta Otolaryngol 127:700–704
Rosengren SM, Weber KP, Hegemann SC, Roth TN (2014) The effect of alcohol on cervical and ocular vestibular evoked myogenic potentials in healthy volunteers. Clin Neurophysiol 125:1700–1708
Acknowledgments
This study was supported by the German Federal Ministry of Education and Research (BMBF) to the German Center for Vertigo and Balance Disorders (grant code 01 EO 0901 and 01 EO 1401) and the Bernstein Center for Computational Neuroscience (grant code 01 GQ 0440).
Conflict of interest
T. Bremova received speaker’s honoraria from Actelion. S. Glasauer received funding from the DFG and the BMBF, serves as expert reviewer for the European Commission, and holds shares in EyeSeeTec GmbH. M. Strupp is Joint Editor-in-Chief of the Journal of Neurology, Editor-in-Chief of Frontiers of Neuro-otology and Section Editor of F1000. He received speaker’s honoraria from Abbott, UCB, GSK, TEVA, Biogen Idec, Pierre-Fabre, Eisai, and HennigPharma.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The previously published model ([18], see their Figure 1) can be slightly simplified for the present simulations by setting several inputs to zero.
In particular, for the oVEMP simulation we can neglect saccadic eye movements, there is no semicircular canal input, the visual input can be neglected due to the open-loop character of oVEMP, and the eye plant equation describing mainly the dynamics of the eyeball is not required, since oVEMP are measured at the level of the extraocular eye muscles. Consequently, the remaining equations aredepends on the PC gain
-
1.
the utricular input \(u = g_{u} \cdot \sin \alpha + \delta_{\text{tap}}\) with α being the pitch angle of the head and δ tap the oVEMP stimulus (“otoliths” in [18] Figure 1),
-
2.
the brainstem integrator (“INC” in [18] Figure 1) in Laplace notation (s is the complex frequency) can be written as \(e_{i} = \left( { - p + c_{\text{ft}} - u} \right) \cdot \frac{{\tau_{\text{b}} - \tau_{\text{e}} }}{{1 + \tau_{\text{b}} s}}\) with τ b and τ e being the time constants of brainstem integrator and eye plant, p being the PC output, and c ft a bias term compensating for the PC resting discharge,
-
3.
the motor command \(m = \left( { - p + c_{\text{ft}} } \right) \cdot \tau_{\text{e}} + e_{i}\) sent to the eye muscles (input to “eye plant” in [18] Figure 1),
-
4.
the floccular loop yielding the PC output \(p = g \cdot v_{\text{e}} + p_{\text{rest}}\) (“FL-Purkinje-cells” in [18] Figure 1) with g being the PC gain factor, v e being the internal estimate of eye velocity \(v_{\text{e}} = \frac{s}{{1 + \tau_{\text{e}} s}} \cdot m\), and p rest being the PC resting discharge (normally p rest = c ft).
Given these equations, we can now solve for the motor command following an oVEMP tap δ tap. First, we set \(p = g \cdot \frac{s}{{1 + \tau_{\text{e}} s}}m + p_{\text{rest}}\) and plug (2) into (3) as \(m = ( - p + c_{\text{ft}} ) \cdot \tau_{\text{e}} + ( - p + c_{\text{ft}} - u)\frac{{\tau_{\text{b}} - \tau_{\text{e}} }}{{1 + \tau_{\text{b}} s}}\). Ignoring the bias terms (assuming they cancel out) we can simplify and get \(m = - p \cdot \frac{{(1 + \tau_{\text{e}} s)\tau_{\text{b}} }}{{1 + \tau_{\text{e}} s}} - u\frac{{\tau_{\text{b}} - \tau_{\text{e}} }}{{1 - \tau_{\text{b}} s}}\). Now we can insert p and solve for m: \(m = - \frac{{\tau_{\text{b}} - \tau_{\text{e}} }}{{1 + \tau_{\text{b}} (1 + g)s}}u\). The oVEMP input to the utricle can be approximated as impulse with amplitude d resulting in a muscle response of \(m = - \frac{{\tau_{\text{b}} - \tau_{\text{e}} }}{{\tau_{\text{b}} (1 + g)}}d\), which depends on the PC gain g. As in [18], to simulate healthy subjects, the gain is set to g = 10, while for an average DBN patient the gain can be set to about g = 5. If the oVEMP amplitude d is the same in both healthy subjects and patients, independently of the other constants we thus get \(m_{\text{DBN}} = \frac{{1 + g_{\text{healthy}} }}{{1 + g_{\text{DBN}} }} \cdot m_{\text{healthy}} = 1.83 \cdot m_{\text{healthy}}\), i.e. a motor command to the eye muscles which is about 180 % of the amplitude in DBN patients. Note that this analytical derivation assumes upright position and gaze straight ahead. Due to the nonlinear Purkinje cell activation function, the relation changes to lower values with upward gaze and pitch-back position.
Rights and permissions
About this article
Cite this article
Bremova, T., Glasauer, S. & Strupp, M. Downbeat nystagmus: evidence for enhancement of utriculo-ocular pathways by ocular vestibular evoked myogenic potentials?. Eur Arch Otorhinolaryngol 272, 3575–3583 (2015). https://doi.org/10.1007/s00405-015-3653-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-015-3653-2