Abstract
Myocardial dysfunction is an important manifestation of sepsis. Previous studies suggest that melatonin is protective against sepsis. In addition, activation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway has been reported to be beneficial in sepsis. However, the role of PI3K/Akt signaling in the protective effect of melatonin against sepsis-induced myocardial dysfunction remains unclear. Here, LY294002, a PI3K inhibitor, was used to investigate the role of PI3K/Akt signaling in mediating the effects of melatonin on sepsis-induced myocardial injury. Cecal ligation and puncture (CLP) surgery was used to establish a rat model of sepsis. Melatonin was administrated to rats intraperitoneally (30 mg/kg). The survival rate, measures of myocardial injury and cardiac performance, serum lactate dehydrogenase level, inflammatory cytokine levels, oxidative stress level, and the extent of myocardial apoptosis were assessed. The results suggest that melatonin administration after CLP surgery improved survival rates and cardiac function, attenuated myocardial injury and apoptosis, and decreased the serum lactate dehydrogenase level. Melatonin decreased the production of the inflammatory cytokines TNF-α, IL-1β, and HMGB1, increased anti-oxidant enzyme activity, and decreased the expression of markers of oxidative damage. Levels of phosphorylated Akt (p-Akt), unphosphorylated Akt (Akt), Bcl-2, and Bax were measured by Western blot. Melatonin increased p-Akt levels, which suggests Akt pathway activation. Melatonin induced higher Bcl-2 expression and lower Bax expression, suggesting inhibition of apoptosis. All protective effects of melatonin were abolished by LY294002, the PI3K inhibitor. In conclusion, our results demonstrate that melatonin mitigates myocardial injury in sepsis via PI3K/Akt signaling activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis, which is characterized by a systemic inflammatory response to severe infection and progressive organ damage, is the leading cause of death in hospitalized patients worldwide [6, 76]. Sepsis can trigger damage to various organs; for instance, it can cause brain injury, cardiac dysfunction, kidney injury, liver injury, and lung injury [7, 24, 38, 44, 63, 67, 87, 94, 106, 107, 127]. Of the complications of sepsis, cardiac dysfunction is a typical manifestation and is closely associated with increased mortality [80, 94, 105]. Sepsis patients experiencing cardiac dysfunction have a 70–90 % mortality rate, while patients without cardiac dysfunction experience only a 20 % mortality rate [80, 86]. Therefore, it is important to develop a novel therapeutic agent against sepsis-induced cardiac depression.
Melatonin, a hormone mainly secreted by the pineal gland, is known to exert various biological effects, such as cardioprotection [4, 82, 108], neuroprotection [3, 117], anti-tumor activity [42, 43], anti-inflammatory activity [2, 78], and anti-oxidant activity [35, 124]. Recently, it has been suggested that melatonin exhibits protective effects against cardiac dysfunction induced by ischemia/reperfusion [25, 36, 68, 119, 121]. In addition, there is some evidence that melatonin protects against sepsis-induced cardiac dysfunction, which may be related to melatonin’s ability to attenuate mitochondrial dysfunction, disrupt apoptosis, decrease inflammation, and prevent oxidative damage [64, 85, 123], but the mechanisms of these actions are unclear.
The phosphatidylinositol-3-kinase (PI3K)/Akt pathway, a well-conserved family of signal transduction molecules, coordinates a variety of intracellular signals, controls cell response to extrinsic stimuli, and regulates cell proliferation and survival [16, 30, 81, 101]. The PI3Ks and the downstream serine/threonine kinase Akt (also known as protein kinase B or PKB) regulate cellular activation, inflammatory responses, chemotaxis, and apoptosis [16]. It has been demonstrated that PI3K/Akt pathway activation is protective against myocardial ischemia–reperfusion injury [31, 41, 53, 88, 99]. Furthermore, of critical significance to the present study, activation of the PI3K/Akt signaling pathway has been suggested to improve cardiac dysfunction and mortality during sepsis [18, 33, 51, 55, 61, 65, 75, 104, 120, 129]. However, the precise role of the PI3K/Akt signaling pathway in melatonin’s protection against sepsis-induced myocardial injury remains unclear.
Therefore, we hypothesized that melatonin protects against sepsis-induced myocardial injury via a PI3K-dependent mechanism. In order to investigate the underlying mechanism of melatonin’s cardioprotective effects, we established an animal septic model using the cecal ligation and puncture (CLP) method and then evaluated cardiac function in the presence and absence of LY294002 (LY), a PI3K inhibitor.
Materials and methods
Animals
All experiments were performed on healthy adult male Sprague–Dawley rats that weighed between 220 and 250 g. The rats were obtained from the animal center of the Fourth Military Medical University. Rats were kept under pathogen-free conditions at about 22 °C on a 12 h light–dark cycle with free access to food and water. This study was performed according to the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (National Institutes of Health Publication No. 85-23, revised 1996) and was approved by the Ethics Committee of the Fourth Military Medical University.
Reagents
Melatonin (Mel), LY294002 (LY), and 4′,6-diamino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The lactate dehydrogenase (LDH) ELISA kit was purchased from Jiancheng Bioengineering Institute (Nanjing, China). Rat TNF-α and IL-1β ELISA kits were purchased from Thermo Fisher Scientific (MA, USA). The HMGB1 ELISA kit was purchased from IBL International (Germany). Superoxide dismutase (SOD) and malondialdehyde (MDA) kits were purchased from Sigma-Aldrich (St. Louis, MO, USA). The catalase (CAT) kit was purchased from Beyotime (Shanghai, China). Antibodies against Akt, phospho-Akt (Ser473), Bcl-2, Bax, and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). The rabbit anti-goat, goat anti-rabbit, and goat anti-mouse secondary antibodies were purchased from Beyotime (Shanghai, China). Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) kits were purchased from Roche (Mannheim, Germany).
Cecal ligation and puncture (CLP) model
Fasting was performed for 8 h for all rats but water was allowed ad libitum before the experiments. The CLP model was established as previously reported with some modifications [113]. In brief, after rats were anesthetized with intraperitoneal injection of chloral hydrate (350 mg/kg), they were immobilized on an aseptic operating table. In a sterile operation environment, a 2–3 cm abdominal midline incision was made to expose the cecum, which was ligated below the ileocecal valve and punctured once with an 18-gauge needle. A small amount of stool was squeezed through the puncture site. The bowel was then situated back in the abdomen and the incision was sutured with a sterile 5–0 silk. The rats in sham-operated group underwent a similar operation without cecal ligation and puncture. All animals received fluid resuscitation with 0.9 % saline solution (subcutaneously, 40 mL/kg of body weight) immediately after the surgery.
Experimental protocol
Three-hundred rats were randomly assigned to five groups: the Sham group received the sham operation and no drug treatments; the CLP group received the cecal ligation and puncture (CLP) surgery; the CLP + Mel group received the CLP surgery and melatonin; the CLP + Mel + LY group received the CLP surgery and both melatonin and LY treatments; and the CLP + LY group received the CLP surgery and LY treatment. All rats had free access to food and water. Twenty rats from each group were used to evaluate survival rates, and forty rats from each group were used for other experiments. The survival rate was evaluated 7 days after the sham or CLP operation. Melatonin dissolved in 1 % ethanol (dissolved in normal saline) was administered intraperitoneally at a dose of 30 mg/kg per injection per rat, at 3, 6, 12, 18, and 24 h after surgery. LY or the same volume of vehicle was intraperitoneally injected at a dose of 10 mg/kg per rat every 2 days for a total of four times before CLP surgery. The dose and administration routes of melatonin and LY were chosen based on previous reports [69, 123].
Evaluation of survival rate
The rats in each group had free access to food and water and were kept under pathogen-free conditions. The survival rate was evaluated within 7 days after the sham or CLP operation.
Evaluation of cardiac function by echocardiography and invasive hemodynamic assessment
Transthoracic echocardiographic examinations were established under isoflurane anesthesia (2 %) of rats in each group 48 h after CLP. Echocardiographic images were obtained using an ACUSON echocardiography instrument equipped with a 13 MHz phased-array transducer (Siemens, USA). The M-mode images of left ventricular (LV) dimensions were obtained. The left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) were recorded.
After the echocardiography, a high-fidelity pressure-transducing catheter was inserted via the right carotid artery into the left ventricle to measure the left ventricular pressure (LVP). When the rats returned to stable conditions, left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), and their first derivative with respect to time (±dp/dt max) were continuously measured.
Evaluation of morphological changes of myocardial tissues
Rats were killed at 48 h after surgery, and left ventricular myocardial tissues were collected. Tissue sections of the myocardium were stained with hematoxylin–eosin (H&E) staining, and morphological changes were evaluated using light microscopy at a magnification of 400×.
TUNEL staining
Myocardial apoptosis was analyzed using a terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay. The paraffin-embedded tissue was cut into sections 4–5 μm thick. Then, 50 μL of TUNEL reaction mixture was added to each sample, and the slides were incubated in humidified atmosphere for 60 min at 37 °C in the dark and then rinsed with PBS (pH 7.4) three times, for 5 min each time. To detect the nuclei, the slides were incubated with DAPI for 5 min at room temperature in the dark, rinsed with PBS three times, for 5 min each time, and observed using fluorescence microscopy. The TUNEL-positive cells produced green fluorescence and the nuclei produced with blue fluorescence. The apoptotic index was calculated as the ratio of the number of TUNEL-positive neurons to the total number of nuclei.
Measurement of LDH release
The activity of lactate dehydrogenase in the serum was detected using a commercially available ELISA kit, according to the manufacturer’s instructions. The LDH activity was expressed as U/L.
Evaluation of inflammatory cytokines
Inflammatory cytokines in the serum and myocardial tissue were measured 48 h after surgery by using commercially available TNF-α, IL-1β, and HMGB1 ELISA kits, according to the manufacturer’s instructions. Data were analyzed using a microplate reader (Multiskan Spectrum, Thermo Scientific, USA).
Measurement of CAT, SOD, and MDA
The serum and myocardial tissue were collected 48 h after surgery to measure CAT and SOD activities and MDA content using commercially available kits, according to the manufacturer’s instructions. Data were analyzed using a microplate reader (Multiskan Spectrum, Thermo Scientific, USA).
Western blot
Left ventricular myocardial tissues were collected and lysed with lysis buffer. After sonication, the lysates were centrifuged, and the proteins were separated using SDS–PAGE and then transferred to Immobilon-NC membranes (Millipore, Boston, MA, USA). After being blocked with 5 % skim milk in Tris-buffered saline at room temperature for 2 h, the membrane was incubated with primary antibodies against p-Akt, Akt, Bcl-2, Bax, and β-actin (1:1000) overnight at 4 °C, washed three times with TBST, and then incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at 37 °C. The blots were imaged using a Bio-Rad imaging system (Bio-Rad, Hercules, CA, USA) and quantified using the Quantity One software package (West Berkeley, CA, USA). The value for the sham group was defined as 100 %.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). SPSS 18.0 was used to analyze data in this study. Survival rates were calculated using Fisher’s exact test. Comparisons among multiple groups were assessed by one-way analysis of variance. The LSD t test was used to make intergroup comparisons. Probabilities of 0.05 or less were considered statistically significant.
Results
Melatonin improved survival rate in septic rats
The survival rate is shown in Fig. 1. The 7-day survival rate in the sham group was almost 100 %. However, 7 days after CLP surgery, there was a dramatic decrease in the survival rate for the CLP group (17.8 %) (versus sham group, P < 0.05). With the administration of melatonin, the survival rate in the CLP + Mel group increased significantly to nearly 50 % (versus CLP group, P < 0.05). However, LY294002 treatment abolished the protective effect of melatonin; the survival in the CLP + Mel + LY group (28.6 %) was much lower than that in the CLP + Mel group (versus CLP + Mel group, P < 0.05).
Effect of melatonin on the 7-day survival rate after CLP surgery. Rats were treated with melatonin after CLP, and melatonin was given at 3, 6, 12, 18, and 24 h after CLP surgery. LY294002 was intraperitoneally injected at a dose of 10 mg/kg or the same volume of vehicle every 2 days for a total of four times before CLP surgery. Values are expressed as survival percentage (n = 20 for each group). **P < 0.05 in comparison to the sham group, ## P < 0.05 in comparison to the CLP group, and $$ P < 0.05 in comparison to the CLP + Mel group. CLP cecal ligation and puncture, Mel melatonin, LY LY294002
Melatonin improved cardiac function in septic rats
We assessed cardiac function with echocardiography. As shown in Fig. 2, melatonin significantly decreased myocardial injury induced by sepsis in the CLP + Mel group (versus CLP group, P < 0.05), as evidenced by improved cardiac function. To investigate whether PI3K plays a critical role in melatonin’s cardioprotective effect, LY294002 was used to inhibit the PI3K/Akt pathway. As expected, the cardiac function of rats in the CLP + Mel + LY group was lower than that in the CLP + Mel group, indicating that PI3K plays a key role in the protective effect of melatonin against sepsis-induced myocardial injury.
Echocardiography evaluation suggests that cardiac dysfunction is attenuated by melatonin. a The evaluation of cardiac function by echocardiography. Representative M-mode images are shown. b Left ventricle ejection fraction. The results are expressed as the mean ± SEM (n = 8 for each group). **P < 0.05 in comparison to the sham group, ## P < 0.05 in comparison to the CLP group, and $$ P < 0.05 in comparison to the CLP + Mel group. Mel melatonin, CLP cecal ligation and puncture, LVEF left ventricle ejection fraction, LY LY294002
In addition, we used invasive hemodynamic evaluation methods to assess cardiac function. As shown in Fig. 3, melatonin treatment significantly caused an increase in LVSP and LV ± dP/dt max and a dramatic decrease in LVEDP in the CLP + Mel group (versus CLP group, P < 0.05). Consistent with electrocardiography results, LY294002 markedly suppressed cardiac function in the CLP + Mel + LY group (versus CLP + Mel group, P < 0.05), suggesting again that the PI3K pathway plays a role in protection against sepsis-induced myocardial injury by melatonin.
Invasive hemodynamic evaluation suggests that melatonin improves cardiac function. The results are expressed as the mean ± SEM (n = 8 for each group). **P < 0.05 in comparison to the sham group, ## P < 0.05 in comparison to the CLP group, and $$ P < 0.05 in comparison to the CLP + Mel group. Mel melatonin, CLP cecal ligation and puncture, LY LY294002, LVSP left ventricular systolic pressure, LVEDP left ventricular end-diastolic pressure, LV ± dP/dt max the instantaneous first derivation of left ventricle pressure
Melatonin attenuated myocardial injury as indicated by HE staining
As shown in Fig. 4, the myocardial sections were stained with hematoxylin and eosin to evaluate damage to the myocardium. In the sham group, the cardiomyocytes were intact and there was no evidence of necrosis or inflammatory cell infiltration. The cardiac muscle cross striations were clearly visible. In the CLP group, necrosis and inflammatory cell infiltration were evident and the cardiac muscle cross striations were no longer visible. Melatonin administration attenuated the injury due to CLP surgery. However, co-treatment with melatonin and LY abolished melatonin’s protection against sepsis-induced myocardial injury, indicating that PI3K activation is involved in melatonin’s protective effect.
Melatonin alleviated myocardial apoptosis in septic rats
To evaluate apoptosis induced by sepsis, TUNEL was performed. As shown in Fig. 5, melatonin treatment significantly decreased the apoptotic index in the CLP + Mel group (versus CLP group, P < 0.05). However, LY abolished the protective effect of melatonin in the CLP + Mel + LY group (versus CLP + Mel group, P < 0.05), revealing that PI3K activation is associated with melatonin’s protection against sepsis-induced myocardial apoptosis.
Melatonin attenuation of sepsis-induced myocardial apoptosis is abolished by LY294002. Representative images of apoptosis are shown. The apoptotic cells were detected by TUNEL (green), and the nuclei were detected by DAPI (blue). The scale bar 20 μm. The results are expressed as the mean ± SEM (n = 8 for each group). **P < 0.05 in comparison to the sham group, ## P < 0.05 in comparison to the CLP group, and $$ P < 0.05 in comparison to the CLP + Mel group. Mel melatonin, CLP cecal ligation and puncture, LY LY294002
Melatonin mitigated LDH leakage in septic rats
LDH release is an indicator of myocardial injury, so we measured LDH levels in the serum. As shown in Fig. 6, CLP surgery triggered a dramatic increase in serum LDH release. Melatonin administration significantly decreased the LDH release after surgery in the CLP + Mel group (versus CLP group, P < 0.05). As expected, LDH release was markedly higher for the CLP + Mel + LY group (versus CLP + Mel group, P < 0.05), indicating that PI3K participates in melatonin’s protection against sepsis-induced myocardial injury.
Effects of melatonin and LY294002 on sepsis-induced LDH release. The LDH release was determined using an ELISA kit according to the manufacturer’s instructions. The results are expressed as the mean ± SEM (n = 8 for each group). **P < 0.05 in comparison to the sham group, ## P < 0.05 in comparison to the CLP group, and $$ P < 0.05 in comparison to the CLP + Mel group. Mel melatonin, CLP cecal ligation and puncture, LY LY294002
Melatonin lowered inflammatory cytokines production in serum and myocardial tissue of septic rats
Subsequently, the effects of melatonin on inflammatory cytokines in serum and myocardial tissue of septic rats were assessed as another measure of myocardial injury. As shown in Fig. 7, the levels of the inflammatory cytokines TNF-α, IL-1β and HMGB1 in the serum and myocardial tissue were markedly lower in the CLP + Mel group than those in the CLP group (P < 0.05); LY abolished the effect of melatonin.
Melatonin decreased the levels of inflammatory cytokines in the serum and myocardial tissues. a Serum TNF-α level. b Myocardial TNF-α level. c Serum IL-1β level. d Myocardial IL-1β level. e Serum HMGB1 level. f Myocardial HMGB1 level. The values are expressed as the mean ± SEM (n = 8 for each group). **P < 0.05 in comparison to the sham group, ## P < 0.05 in comparison to the CLP group, and $$ P < 0.05 in comparison to the CLP + Mel group. Mel melatonin, CLP cecal ligation and puncture, LY LY294002, HMGB1 high-mobility group box 1
Melatonin decreased oxidative stress in serum and myocardial tissue of septic rats
As shown in Fig. 8, the effects of melatonin on sepsis-induced oxidative stress in the serum and myocardial tissues were evaluated. In the CLP + Mel group, the levels of SOD and CAT in both the serum and myocardial tissues were markedly higher than those in the CLP group (P < 0.05); LY abolished the effect of melatonin. MDA level, which is a marker of oxidative damage, was lower in the CLP + Mel group than that in the CLP group; LY abolished the effect of melatonin.
Melatonin increased the levels of SOD and CAT and decreased MDA content in the serum and myocardial tissues. a Serum SOD level. b Myocardial SOD level. c Serum CAT level. d Myocardial CAT level. e Serum MDA level. f Myocardial MDA level. The values are expressed as the mean ± SEM (n = 8 for each group). **P < 0.05 in comparison to the sham group, ## P < 0.05 in comparison to the CLP group, and $$ P < 0.05 in comparison to the CLP + Mel group. Mel melatonin, CLP cecal ligation and puncture, LY LY294002, SOD superoxide dismutase, CAT catalase, MDA malondialdehyde
The role of Akt, Bcl-2, and Bax in the protective effects of melatonin
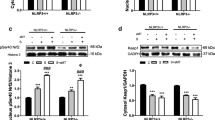
To further investigate the molecular mechanism underlying melatonin-mediated cardioprotection against sepsis, we detected p-Akt/Akt, Bcl-2, and Bax protein levels by Western blot. As shown in Fig. 9, CLP surgery significantly increased Akt phosphorylation relative to the sham group. Akt phosphorylation was further enhanced with melatonin administration. However, LY abolished the increase in Akt phosphorylation when co-administered with melatonin. As shown in Fig. 9, CLP surgery induced a dramatic increase in Bax expression relative to the sham group. Melatonin administration decreased the expression of Bax, but this decrease was significantly abolished by co-administration with LY. In contrast, CLP dramatically decreased Bcl-2 expression. Melatonin administration increased the expression of Bcl-2, but this increase was significantly abolished by co-administration with LY.
Effect of melatonin on the expression of phosphorylation-Akt, Bcl-2, and Bax following sepsis. Representative images of the Western blot results are shown. Melatonin increased the ratio of p-Akt/Akt, which was significantly abolished by Akt-inhibitor LY294002. Melatonin increased Bcl-2 expression and decreases Bax expression, an effect that is reversed by the by Akt-inhibitor LY294002. The values are expressed as the mean ± SEM (n = 8 for each group). **P < 0.05 in comparison to the sham group, ## P < 0.05 in comparison to the CLP group, and $$ P < 0.05 in comparison to the CLP + Mel group. Mel melatonin, CLP cecal ligation and puncture, LY LY294002
Discussion
In this study, we found that melatonin attenuated myocardial dysfunction induced by sepsis. Melatonin improved cardiac function, mitigated myocardial apoptosis, and decreased inflammation and oxidative stress associated with sepsis. The protective effect of melatonin was closely associated with the activation of the PI3K/Akt signaling pathway. Melatonin administration dramatically increased the 7-day survival rate of rats that underwent CLP surgery and attenuated cardiac dysfunction and myocardial apoptosis observed 48 h after CLP surgery. Moreover, melatonin lowered the release of inflammatory cytokines such as TNF-α, IL-1β, and HMGB1 as well as the production of MDA, which is an oxidative lipid product. It also increased the activity of anti-oxidant enzymes, such as SOD and CAT. However, these protective effects of melatonin were abolished by treatment with LY294002, a PI3K signaling inhibitor, indicating that melatonin acts via the activation of PI3K/Akt signaling.
Sepsis, the systemic inflammatory response to infection, causes high mortality among the critically ill [6, 45], primarily as a result of multiple organ damage. Myocardial dysfunction is regarded as a critical manifestation of this syndrome [21, 32, 58, 71, 89, 95, 110]. The underlying mechanisms of myocardial dysfunction during sepsis include circulatory changes [46, 93, 94], autonomic dysregulation [96, 97], metabolic changes [102], mitochondrial dysfunction [15, 22], cell death (necrosis and apoptosis) [47, 84], inflammation, and oxidative stress [8, 23, 59]. However, the pathogenesis of sepsis-induced myocardial dysfunction is complex and involves a multitude of molecular players. Studies have suggested that there is a reduction in the levels of cardiac dihydropyridine receptors, such as L-type calcium channels, during sepsis [60, 128]. A recent study suggested that mitochondrial nitric oxide (NO) could be involved in myocardial depression [115]. As for the inflammatory cytokines, TNF-α and IL-1 might be involved [8].
Previous studies have demonstrated the protective role of melatonin against sepsis. It has been reported that 5-hydroxy-2′-isobutyl-streptochlorin (HIS), a novel derivative of melatonin, inhibits inflammation via regulation of TRIF-dependent signaling and inflammasome activation [98]. It has been reported that melatonin is protective against sepsis-induced kidney injury [19]. In addition, with respect to sepsis-induced myocardial injury, melatonin attenuated mitochondrial impairment and improved survival rates [85, 123]. Consistent with previous results, we found that melatonin improved the 7-day survival rate after CLP surgery. The cardiac dysfunction induced by sepsis is mitigated by melatonin as evidenced by the echocardiography, hemodynamic evaluation, and morphology. Garcia et al. demonstrated the NF-κB/NLRP3 inflammasome connection during sepsis, leading to a disproportionate inflammatory response to sepsis [34]. Melatonin administration blunts NF-κB transcriptional activity via a sirtuin1-dependent NF-κB deacetylation in septic mice. In addition, melatonin decreased NF-κB-dependent proinflammatory response and restored redox balance and mitochondrial homeostasis, thus inhibiting the NLRP3 inflammasome. The study heralds a promising therapeutic application for melatonin in the treatment of sepsis. In our present study, we found that melatonin protects the heart from sepsis via a PI3K/Akt-dependent mechanism and the protective effects of melatonin can be abolished by LY294002, a specific PI3K antagonist. Therefore, melatonin, as a promising therapeutic application in the treatment of sepsis, may exert its protection via PI3K, which provides another therapeutic target. Recently, Lorente et al. described higher mortality rates for sepsis patients with high melatonin levels [70], which appears inconsistent with our results. However, some problems should be considered: (1) differences may exist between the treatment of sepsis in a CLP-induced rat model treatment and in human beings. Patients with sepsis are treated with certain kinds of antibodies and medications, which are not used in a rat model. (2) Melatonin in human originates endogenously mainly from the pineal gland in humans. In this study, increased melatonin levels in septic patients might have resulted from sepsis-induced pineal damage. However, we considered melatonin as a treatment reagent that was obtained from an exogenous source. Furthermore, melatonin has a protective effect against sepsis-induced myocardial injury. (3) Melatonin, as an endogenous hormone, is expressed at different levels at different times. Moreover, melatonin levels are associated with the circadian rhythm and may differ among individuals.
Sepsis induces the release of enormous endotoxins, such as lipopolysaccharide (LPS), triggering a cascade of proinflammatory cytokines (TNF-α, IL-1, etc.) [122]. Among the cytokines, TNF-α induces apoptosis in rat heart [83, 125]. In addition, high-mobility group box 1 protein (HMGB1) has been identified as an important late-acting mediator of inflammation in sepsis [5, 109]. HMGB1 is a nuclear non-histone DNA-binding protein that is produced extracellularly during inflammation [62]. Previous studies have demonstrated that HMGB1 can activate the immune system and induce cell proliferation, adhesion, migration, and cytokine release [40, 52, 56, 72]. In the present study, melatonin treatment significantly attenuated inflammation by suppressing the levels of TNF-α, IL-1β, and HMGB1 in the serum and myocardial tissue. Additionally, melatonin treatment also significantly decreased myocardial apoptosis, as evidenced by the lowered apoptotic index, the increased level of Bcl-2, and decreased level of Bax. However, Gerd et al. demonstrated that the release of endotoxins is beneficial under certain conditions [9]. Preconditioned hearts (Ischemic/LPS pretreated) demonstrated increased tolerance against myocardial ischemia that was associated with a reduced TNF-α concentration and an increased TNF-α inhibitory plasma activity. Their study illustrated the important role for TNF-α as well as the TNF-α inhibitory serum activity in the progress of myocardial ischemia with respect to mortality, hemodynamics and regional myocardial blood flow, and infarct size. Consequently, TNF-α inhibitory serum activity is expected to provide new insights into cardioprotection against ischemia–reperfusion afforded by ischemic preconditioning and early exposure to LPS. Dipeptidyl peptidase (DPP)-4, which is responsible for a degradation of the hormone glucagon-like peptide-1 (GLP-1), plays a key role in glucose metabolism and in the control of glycemic status. Sebastian et al. demonstrated the DPP-4 inhibitor linagliptin improves survival and suppresses LPS-induced inflammatory pathways, improving vascular dysfunction and reducing oxidative stress in endotoxemic rats. These protective effects are associated with GLP-1-mediated decrease of iNOS expression as well as activation of the AMPK signaling pathway. And this study heralds a promising therapeutic application for linagliptin in the treatment of sepsis [103]. Another study investigated whether phosphorylation of RISK (reperfusion injury salvage kinases, proposed to be protective by previous reports in mostly rodent models) is causal for the protection of ischemia postconditioning (IPoC) [100]. In pig model of IPoC, pharmacological RISK inhibitors were used to block increases in RISK phosphorylation during reperfusion. However, differences in the infarct size were not significant, which may be attributed to species differences between rodents and larger mammals. Additionally, maintenance of acidosis and subsequent inhibition of mitochondrial permeability rather than phosphorylation of RISK mediates postconditioning in pigs [20]. To further elucidate the underlying mechanisms, Langendorff apparatus can be used to establish the global ischemia model to diminish the latent errors in sampling tissues. For now, few studies have analyzed the RISK in different species and further research is warrant.
Oxidative stress during sepsis is also another critical factor contributing to the myocardial dysfunction [57, 66]. During sepsis, excessive reactive oxygen species (ROS) production activates lipid peroxidation, leading to cell and mitochondrial membrane damage, which triggers cell apoptosis and necrosis [17, 49, 50]. In addition, ROS can modify the inner mitochondrial membrane potential and induce the release of cytochrome c into the cytosol, eventually leading to cell apoptosis [37, 111, 118]. SOD and CAT are anti-oxidant enzymes that serve as ROS scavengers [130], and MDA is an indicator of lipid peroxidation [13, 116]. In our study, the results suggest that melatonin treatment alleviates oxidative stress via increasing the activity of CAT and SOD and decreasing the production of MDA.
PI3K/Akt signaling has been reported to play a protective role in sepsis [12, 51, 65, 112, 126]. Moreover, transgenic overexpression of Akt protects against sepsis in the mice infected with the Gram-negative bacteria [90]. Once activated, PI3K leads to the phosphorylation of Akt, leading to the phosphorylation of diverse target molecules (such as Bcl-2 family) that act to preserve mitochondrial integrity and promote cell survival [69]. Melatonin has been reported to be protective against hemorrhagic shock-induced liver injury in rats through an Akt-dependent HO-1 pathway [48]. Our results suggested that melatonin treatment significantly increased Akt phosphorylation. Taken together, our results suggest that PI3K/Akt signaling pathway is involved in the protective effect of melatonin. We hypothesize that melatonin protects against sepsis-induced myocardial dysfunction via the PI3K/Akt pathway.
MT1 and MT2, both G-protein coupled receptors, are involved in the transduction of melatonin signaling [92]. These receptors are expressed in several different organs and tissues; therefore melatonin modulates multiple aspects of human physiology, and melatonin dysfunction and its receptors are associated with sleep and circadian dysfunction [39], diabetes [14, 73], and Alzheimer’s and Parkinson’s diseases [1, 114]. Melatonin as a free-radical scavenger is receptor-independent; however, its indirect anti-oxidative action may be mediated by receptors [54, 91]. In addition, melatonin interacts with intracellular proteins such as calmodulin [11], calreticulin [74], or tubulin [79], and antagonizes the binding of Ca2+ to calmodulin [10]. Furthermore, melatonin receptors have been identified in the cardiovascular system [26, 121]. Animal studies have indicated that melatonin has dual effects on the vasculature with vasoconstriction being observed through MT1 receptor activation, and vasodilatation through MT2 receptor activation [27, 77]. Additionally, melatonin has been shown to activate PI3K/Akt pathway via its receptors [28]. As for sepsis, melatonin receptors have been suggested to mediate improvements with respect to survival in a septic model [29]. The results showed that MT1 and MT2 mediate at least a part of the effects induced by melatonin to improve survival. Therefore, we hypothesize that the activation of PI3K/Akt signaling by melatonin in septic rats is likely mediated by melatonin receptors. This, however, requires further validation using melatonin receptor antagonists.
Melatonin has not only been widely used in experimental animal research but also in clinic for human beings. It shows a relatively high adaptation for most people with different pathologies, and can be safely used in numerous disorders. The toxic dose, however, remains unknown owing to a lack of research. Our results (150 mg/kg for sepsis rats) suggest that the possible melatonin dose that can be administered for myocardial dysfunction in sepsis patients was 3 mg/kg according to dosage conversion ratio between species (human:rat is nearly 1:25–50). However, further research is needed in clinical scenarios.
In summary, our study suggests that melatonin treatment confers a significant protective effect against myocardial dysfunction induced by sepsis in a PI3K/Akt-dependent manner. PI3K/Akt activation augments anti-oxidant activity, inhibits inflammation, and suppresses apoptosis, contributing to the attenuation of myocardial depression. Our work warrants a more thorough examination of the clinical use of melatonin in the treatment of sepsis-induced myocardial injury.
References
Adi N, Mash DC, Ali Y, Singer C, Shehadeh L, Papapetropoulos S (2010) Melatonin MT1 and MT2 receptor expression in Parkinson’s disease. Med Sci Monit 16:BR61–BR67
Agil A, Reiter RJ, Jimenez-Aranda A, Iban-Arias R, Navarro-Alarcon M, Marchal JA, Adem A, Fernandez-Vazquez G (2013) Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J Pineal Res 54:381–388. doi:10.1111/jpi.12012
Ali T, Kim MO (2015) Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3beta pathway in the mouse hippocampus. J Pineal Res. doi:10.1111/jpi.12238
Amin AH, El-Missiry MA, Othman AI (2015) Melatonin ameliorates metabolic risk factors, modulates apoptotic proteins, and protects the rat heart against diabetes-induced apoptosis. Eur J Pharmacol 747:166–173. doi:10.1016/j.ejphar.2014.12.002
Andersson U, Tracey KJ (2003) HMGB1 in sepsis. Scand J Infect Dis 35:577–584
Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365:63–78. doi:10.1016/S0140-6736(04)17667-8
Annane D, Sharshar T (2015) Cognitive decline after sepsis. Lancet Respir Med 3:61–69. doi:10.1016/S2213-2600(14)70246-2
Antonucci E, Fiaccadori E, Donadello K, Taccone FS, Franchi F, Scolletta S (2014) Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care 29:500–511. doi:10.1016/j.jcrc.2014.03.028
Belosjorow S, Schulz R, Dorge H, Schade FU, Heusch G (1999) Endotoxin and ischemic preconditioning: TNF-alpha concentration and myocardial infarct development in rabbits. Am J Physiol 277:H2470–H2475
Benitez-King G (2006) Melatonin as a cytoskeletal modulator: implications for cell physiology and disease. J Pineal Res 40:1–9. doi:10.1111/j.1600-079X.2005.00282.x
Benitez-King G, Anton-Tay F (1993) Calmodulin mediates melatonin cytoskeletal effects. Experientia 49:635–641
Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS (2004) Akt decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol 172:7583–7591
Bondor CI, Potra AR, Moldovan D, Rusu CC, Ciorba Pop M, Muresan A, Vladutiu DS, Kacso IM (2015) Relationship of adiponectin to markers of oxidative stress in type 2 diabetic patients: influence of incipient diabetes-associated kidney disease. Int Urol Nephrol. doi:10.1007/s11255-015-1004-2
Bonnefond A, Clement N, Fawcett K, Yengo L, Vaillant E, Guillaume JL, Dechaume A, Payne F, Roussel R, Czernichow S, Hercberg S, Hadjadj S, Balkau B, Marre M, Lantieri O, Langenberg C, Bouatia-Naji N, Meta-Analysis of G, Insulin-Related Traits C, Charpentier G, Vaxillaire M, Rocheleau G, Wareham NJ, Sladek R, McCarthy MI, Dina C, Barroso I, Jockers R, Froguel P (2012) Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet 44:297–301. doi:10.1038/ng.1053
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M (2002) Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360:219–223. doi:10.1016/S0140-6736(02)09459-X
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296:1655–1657. doi:10.1126/science.296.5573.1655
Chandra J, Samali A, Orrenius S (2000) Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 29:323–333
Chen G, Zhao J, Yin Y, Wang B, Liu Q, Li P, Zhao L, Zhou H (2014) C-type natriuretic peptide attenuates LPS-induced endothelial activation: involvement of p38, Akt, and NF-kappaB pathways. Amino Acids 46:2653–2663. doi:10.1007/s00726-014-1816-x
Chen HH, Lin KC, Wallace CG, Chen YT, Yang CC, Leu S, Chen YC, Sun CK, Tsai TH, Chen YL, Chung SY, Chang CL, Yip HK (2014) Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. J Pineal Res 57:16–32. doi:10.1111/jpi.12140
Cohen MV, Yang XM, Downey JM (2007) The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation 115:1895–1903. doi:10.1161/CIRCULATIONAHA.106.675710
Court O, Kumar A, Parrillo JE, Kumar A (2002) Clinical review: myocardial depression in sepsis and septic shock. Crit Care 6:500–508
Crouser ED (2004) Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion 4:729–741. doi:10.1016/j.mito.2004.07.023
Cuello F, Shankar-Hari M, Mayr U, Yin X, Marshall M, Suna G, Willeit P, Langley SR, Jayawardhana T, Zeller T, Terblanche M, Shah AM, Mayr M (2014) Redox state of pentraxin 3 as a novel biomarker for resolution of inflammation and survival in sepsis. Mol Cell Proteomics 13:2545–2557. doi:10.1074/mcp.M114.039446
Dai X, Zeng Z, Fu C, Zhang S, Cai Y, Chen Z (2015) Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit Care 19:223. doi:10.1186/s13054-015-0941-6
Ekelof SV, Halladin NL, Jensen SE, Zaremba T, Aaroe J, Kjaergaard B, Simonsen CW, Rosenberg J, Gogenur I (2014) Effects of intracoronary melatonin on ischemia-reperfusion injury in ST-elevation myocardial infarction. Heart Vessels. doi:10.1007/s00380-014-0589-1
Ekmekcioglu C, Haslmayer P, Philipp C, Mehrabi MR, Glogar HD, Grimm M, Leibetseder VJ, Thalhammer T, Marktl W (2001) Expression of the MT1 melatonin receptor subtype in human coronary arteries. J Recept Signal Transduct Res 21:85–91. doi:10.1081/RRS-100107144
Ersahin C, Masana MI, Dubocovich ML (2002) Constitutively active melatonin MT(1) receptors in male rat caudal arteries. Eur J Pharmacol 439:171–172
Faria JA, Kinote A, Ignacio-Souza LM, de Araujo TM, Razolli DS, Doneda DL, Paschoal LB, Lellis-Santos C, Bertolini GL, Velloso LA, Bordin S, Anhe GF (2013) Melatonin acts through MT1/MT2 receptors to activate hypothalamic Akt and suppress hepatic gluconeogenesis in rats. Am J Physiol Endocrinol Metab 305:E230–E242. doi:10.1152/ajpendo.00094.2013
Fink T, Glas M, Wolf A, Kleber A, Reus E, Wolff M, Kiefer D, Wolf B, Rensing H, Volk T, Mathes AM (2014) Melatonin receptors mediate improvements of survival in a model of polymicrobial sepsis. Crit Care Med 42:e22–e31. doi:10.1097/CCM.0b013e3182a63e2b
Fruman DA, Cantley LC (2002) Phosphoinositide 3-kinase in immunological systems. Semin Immunol 14:7–18. doi:10.1006/smim.2001.0337
Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K (2000) Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101:660–667
Gamkrelidze M, Intskirveli N, Vardosanidze K, Goliadze L, Chikhladze K, Ratiani L (2014) Myocardial dysfunction during septic shock (review). Georgian Med News 237:40–46
Gao M, Ha T, Zhang X, Wang X, Liu L, Kalbfleisch J, Singh K, Williams D, Li C (2013) The Toll-like receptor 9 ligand, CpG oligodeoxynucleotide, attenuates cardiac dysfunction in polymicrobial sepsis, involving activation of both phosphoinositide 3 kinase/Akt and extracellular-signal-related kinase signaling. J Infect Dis 207:1471–1479. doi:10.1093/infdis/jit036
Garcia JA, Volt H, Venegas C, Doerrier C, Escames G, Lopez LC, Acuna-Castroviejo D (2015) Disruption of the NF-kappaB/NLRP3 connection by melatonin requires retinoid-related orphan receptor-alpha and blocks the septic response in mice. FASEB J. doi:10.1096/fj.15-273656
Garcia JJ, Lopez-Pingarron L, Almeida-Souza P, Tres A, Escudero P, Garcia-Gil FA, Tan DX, Reiter RJ, Ramirez JM, Bernal-Perez M (2014) Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J Pineal Res 56:225–237. doi:10.1111/jpi.12128
Gogenur I, Kucukakin B, Panduro Jensen L, Reiter RJ, Rosenberg J (2014) Melatonin reduces cardiac morbidity and markers of myocardial ischemia after elective abdominal aortic aneurism repair: a randomized, placebo-controlled, clinical trial. J Pineal Res 57:10–15. doi:10.1111/jpi.12138
Gottlieb RA (2003) Mitochondrial signaling in apoptosis: mitochondrial daggers to the breaking heart. Basic Res Cardiol 98:242–249. doi:10.1007/s00395-003-0404-0
Gyurkovska V, Ivanovska N (2015) Tyrosine kinase inhibitor tyrphostin AG490 reduces liver injury in LPS-induced shock. Eur J Pharmacol 751:118–126. doi:10.1016/j.ejphar.2015.01.045
Hardeland R (2009) Melatonin: signaling mechanisms of a pleiotropic agent. BioFactors 35:183–192. doi:10.1002/biof.23
Harris HE, Andersson U, Pisetsky DS (2012) HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol 8:195–202. doi:10.1038/nrrheum.2011.222
Hernandez-Resendiz S, Palma-Flores C, De Los Santos S, Roman-Anguiano NG, Flores M, de la Pena A, Flores PL, Fernandez GJ, Coral-Vazquez RM, Zazueta C (2015) Reduction of no-reflow and reperfusion injury with the synthetic 17beta-aminoestrogen compound Prolame is associated with PI3K/Akt/eNOS signaling cascade. Basic Res Cardiol 110:1. doi:10.1007/s00395-015-0464-y
Hevia D, Gonzalez-Menendez P, Quiros-Gonzalez I, Miar A, Rodriguez-Garcia A, Tan DX, Reiter RJ, Mayo JC, Sainz RM (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J Pineal Res 58:234–250. doi:10.1111/jpi.12210
Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L, Hauch A, Lundberg P, Summers W, Yuan L, Frasch T, Blask DE (2015) Melatonin: an inhibitor of breast cancer. Endocr Relat Cancer. doi:10.1530/ERC-15-0030
Hirano Y, Aziz M, Yang WL, Wang Z, Zhou M, Ochani M, Khader A, Wang P (2015) Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury. Crit Care 19:53. doi:10.1186/s13054-015-0782-3
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150. doi:10.1056/NEJMra021333
Hotchkiss RS, Karl IE (1992) Reevaluation of the role of cellular hypoxia and bioenergetic failure in sepsis. JAMA 267:1503–1510
Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE (1999) Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 27:1230–1251
Hsu JT, Kuo CJ, Chen TH, Wang F, Lin CJ, Yeh TS, Hwang TL, Jan YY (2012) Melatonin prevents hemorrhagic shock-induced liver injury in rats through an Akt-dependent HO-1 pathway. J Pineal Res 53:410–416. doi:10.1111/j.1600-079X.2012.01011.x
Jang JH, Chun JN, Godo S, Wu G, Shimokawa H, Jin CZ, Jeon JH, Kim SJ, Jin ZH, Zhang YH (2015) ROS and endothelial nitric oxide synthase (eNOS)-dependent trafficking of angiotensin II type 2 receptor begets neuronal NOS in cardiac myocytes. Basic Res Cardiol 110:21. doi:10.1007/s00395-015-0477-6
Jezek P, Hlavata L (2005) Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol 37:2478–2503. doi:10.1016/j.biocel.2005.05.013
Jiang S, Zhu W, Li C, Zhang X, Lu T, Ding Z, Cao K, Liu L (2013) alpha-Lipoic acid attenuates LPS-induced cardiac dysfunction through a PI3K/Akt-dependent mechanism. Int Immunopharmacol 16:100–107. doi:10.1016/j.intimp.2013.03.024
Johansson L, Snall J, Sendi P, Linner A, Thulin P, Linder A, Treutiger CJ, Norrby-Teglund A (2014) HMGB1 in severe soft tissue infections caused by Streptococcus pyogenes. Front Cell Infect Microbiol 4:4. doi:10.3389/fcimb.2014.00004
Jonassen AK, Sack MN, Mjos OD, Yellon DM (2001) Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res 89:1191–1198
Karbownik M, Reiter RJ (2000) Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proc Soc Exp Biol Med 225:9–22
Kim TH, Kim SJ, Lee SM (2014) Stimulation of the alpha7 nicotinic acetylcholine receptor protects against sepsis by inhibiting Toll-like receptor via phosphoinositide 3-kinase activation. J Infect Dis 209:1668–1677. doi:10.1093/infdis/jit669
Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A (2008) HMGB1: endogenous danger signaling. Mol Med 14:476–484. doi:10.2119/2008-00034.Klune
Korkmaz B, Cuez T, Buharalioglu CK, Demiryurek AT, Sahan-Firat S, Sari AN, Tunctan B (2012) Contribution of MEK1/ERK1/2/iNOS pathway to oxidative stress and decreased caspase-3 activity in endotoxemic rats. Antiinflamm Antiallergy Agents Med Chem 11:243–252
Kumar A, Haery C, Parrillo JE (2000) Myocardial dysfunction in septic shock. Crit Care Clin 16:251–287
Lee I, Huttemann M (2014) Energy crisis: the role of oxidative phosphorylation in acute inflammation and sepsis. Biochim Biophys Acta 1842:1579–1586. doi:10.1016/j.bbadis.2014.05.031
Lew WY, Yasuda S, Yuan T, Hammond HK (1996) Endotoxin-induced cardiac depression is associated with decreased cardiac dihydropyridine receptors in rabbits. J Mol Cell Cardiol 28:1367–1371
Li C, Hua F, Ha T, Singh K, Lu C, Kalbfleisch J, Breuel KF, Ford T, Kao RL, Gao M, Ozment TR, Williams DL (2012) Activation of myocardial phosphoinositide-3-kinase p110alpha ameliorates cardiac dysfunction and improves survival in polymicrobial sepsis. PLoS ONE 7:e44712. doi:10.1371/journal.pone.0044712
Li L, Ling Y, Huang M, Yin T, Gou SM, Zhan NY, Xiong JX, Wu HS, Yang ZY, Wang CY (2015) Heparin inhibits the inflammatory response induced by LPS and HMGB1 by blocking the binding of HMGB1 to the surface of macrophages. Cytokine 72:36–42. doi:10.1016/j.cyto.2014.12.010
Li P, Guo Y, Bledsoe G, Yang ZR, Fan H, Chao L, Chao J (2015) Kallistatin treatment attenuates lethality and organ injury in mouse models of established sepsis. Crit Care 19:200. doi:10.1186/s13054-015-0919-4
Li Volti G, Musumeci T, Pignatello R, Murabito P, Barbagallo I, Carbone C, Gullo A, Puglisi G (2012) Antioxidant potential of different melatonin-loaded nanomedicines in an experimental model of sepsis. Exp Biol Med (Maywood) 237:670–677. doi:10.1258/ebm.2012.011425
Li XQ, Cao W, Li T, Zeng AG, Hao LL, Zhang XN, Mei QB (2009) Amlodipine inhibits TNF-alpha production and attenuates cardiac dysfunction induced by lipopolysaccharide involving PI3K/Akt pathway. Int Immunopharmacol 9:1032–1041. doi:10.1016/j.intimp.2009.04.010
Liu L, Wang P, Liang C, He D, Yu Y, Liu X (2013) Distinct effects of Nampt inhibition on mild and severe models of lipopolysaccharide-induced myocardial impairment. Int Immunopharmacol 17:342–349. doi:10.1016/j.intimp.2013.06.017
Liu L, Xie K, Chen H, Dong X, Li Y, Yu Y, Wang G, Yu Y (2014) Inhalation of hydrogen gas attenuates brain injury in mice with cecal ligation and puncture via inhibiting neuroinflammation, oxidative stress and neuronal apoptosis. Brain Res 1589:78–92. doi:10.1016/j.brainres.2014.09.030
Liu LF, Qin Q, Qian ZH, Shi M, Deng QC, Zhu WP, Zhang H, Tao XM, Liu Y (2014) Protective effects of melatonin on ischemia-reperfusion induced myocardial damage and hemodynamic recovery in rats. Eur Rev Med Pharmacol Sci 18:3681–3686
Liu XY, Zhou XY, Hou JC, Zhu H, Wang Z, Liu JX, Zheng YQ (2015) Ginsenoside Rd promotes neurogenesis in rat brain after transient focal cerebral ischemia via activation of PI3K/Akt pathway. Acta Pharmacol Sin 36:421–428. doi:10.1038/aps.2014.156
Lorente L, Martin MM, Abreu-Gonzalez P, de la Cruz T, Ferreres J, Sole-Violan J, Labarta L, Diaz C, Jimenez A, Borreguero-Leon JM (2015) Serum melatonin levels are associated with mortality in severe septic patients. J Crit Care 30(860):e861–e866. doi:10.1016/j.jcrc.2015.03.023
Lorigados CB, Ariga SK, Batista TR, Velasco IT, Soriano FG (2015) Endotoxaemic myocardial dysfunction: the role of coronary driving pressure in subendocardial perfusion. Crit Care Resusc 17:12–22
Lu B, Antoine DJ, Kwan K, Lundback P, Wahamaa H, Schierbeck H, Robinson M, Van Zoelen MA, Yang H, Li J, Erlandsson-Harris H, Chavan SS, Wang H, Andersson U, Tracey KJ (2014) JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci USA 111:3068–3073. doi:10.1073/pnas.1316925111
Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L (2009) Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 41:82–88. doi:10.1038/ng.288
Macias M, Escames G, Leon J, Coto A, Sbihi Y, Osuna A, Acuna-Castroviejo D (2003) Calreticulin-melatonin. An unexpected relationship. Eur J Biochem 270:832–840
Mao L, Lin W, Nie T, Hui X, Gao X, Li K, Ding M, Tang X, Li P, Wang Y, Xu A, Liu P, Wu D (2014) Absence of Appl2 sensitizes endotoxin shock through activation of PI3K/Akt pathway. Cell Biosci 4:60. doi:10.1186/2045-3701-4-60
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554. doi:10.1056/NEJMoa022139
Masana MI, Doolen S, Ersahin C, Al-Ghoul WM, Duckles SP, Dubocovich ML, Krause DN (2002) MT(2) melatonin receptors are present and functional in rat caudal artery. J Pharmacol Exp Ther 302:1295–1302
Mauriz JL, Collado PS, Veneroso C, Reiter RJ, Gonzalez-Gallego J (2013) A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J Pineal Res 54:1–14. doi:10.1111/j.1600-079X.2012.01014.x
Melendez J, Maldonado V, Ortega A (1996) Effect of melatonin on beta-tubulin and MAP2 expression in NIE-115 cells. Neurochem Res 21:653–658
Merx MW, Weber C (2007) Sepsis and the heart. Circulation 116:793–802. doi:10.1161/CIRCULATIONAHA.106.678359
Miki T, Miura T, Tanno M, Nishihara M, Naitoh K, Sato T, Takahashi A, Shimamoto K (2007) Impairment of cardioprotective PI3K-Akt signaling by post-infarct ventricular remodeling is compensated by an ERK-mediated pathway. Basic Res Cardiol 102:163–170. doi:10.1007/s00395-006-0622-3
Mukherjee D, Ghosh AK, Dutta M, Mitra E, Mallick S, Saha B, Reiter RJ, Bandyopadhyay D (2015) Mechanisms of isoproterenol-induced cardiac mitochondrial damage: protective actions of melatonin. J Pineal Res 58:275–290. doi:10.1111/jpi.12213
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103. doi:10.1038/47513
Neviere R, Fauvel H, Chopin C, Formstecher P, Marchetti P (2001) Caspase inhibition prevents cardiac dysfunction and heart apoptosis in a rat model of sepsis. Am J Respir Crit Care Med 163:218–225. doi:10.1164/ajrccm.163.1.2003109
Ortiz F, Garcia JA, Acuna-Castroviejo D, Doerrier C, Lopez A, Venegas C, Volt H, Luna-Sanchez M, Lopez LC, Escames G (2014) The beneficial effects of melatonin against heart mitochondrial impairment during sepsis: inhibition of iNOS and preservation of nNOS. J Pineal Res 56:71–81. doi:10.1111/jpi.12099
Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP (1990) Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med 113:227–242
Pasqua T, Filice E, Mazza R, Quintieri AM, Carmela Cerra M, Iannacone R, Melfi D, Indiveri C, Gattuso A, Angelone T (2015) Cardiac and hepatic role of r-AtHSP70: basal effects and protection against ischemic and sepsis conditions. J Cell Mol Med. doi:10.1111/jcmm.12491
Penna C, Brancaccio M, Tullio F, Rubinetto C, Perrelli MG, Angotti C, Pagliaro P, Tarone G (2014) Overexpression of the muscle-specific protein, melusin, protects from cardiac ischemia/reperfusion injury. Basic Res Cardiol 109:418. doi:10.1007/s00395-014-0418-9
Prabhu MM, Yalakala SK, Shetty R, Thakkar A, Sitapara T (2015) Prognosis of left ventricular systolic dysfunction in septic shock patients. J Clin Diagn Res 9:OC05–OC08. doi:10.7860/JCDR/2015/10812.5640
Rajaram MV, Ganesan LP, Parsa KV, Butchar JP, Gunn JS, Tridandapani S (2006) Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol 177:6317–6324
Reiter RJ, Tan DX, Gitto E, Sainz RM, Mayo JC, Leon J, Manchester LC, Vijayalaxmi Kilic E, Kilic U (2004) Pharmacological utility of melatonin in reducing oxidative cellular and molecular damage. Pol J Pharmacol 56:159–170
Reppert SM (1997) Melatonin receptors: molecular biology of a new family of G protein-coupled receptors. J Biol Rhythms 12:528–531
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative G (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377. doi:10.1056/NEJMoa010307
Rudiger A, Singer M (2007) Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35:1599–1608. doi:10.1097/01.CCM.0000266683.64081.02
Sanfilippo F, Corredor C, Fletcher N, Landesberg G, Benedetto U, Foex P, Cecconi M (2015) Diastolic dysfunction and mortality in septic patients: a systematic review and meta-analysis. Intensive Care Med. doi:10.1007/s00134-015-3748-7
Schmidt HB, Werdan K, Muller-Werdan U (2001) Autonomic dysfunction in the ICU patient. Curr Opin Crit Care 7:314–322
Sharshar T, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A, Orlikowski D, Raphael JC, Gajdos P, Annane D (2003) Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet 362:1799–1805
Shim DW, Shin HJ, Han JW, Ji YE, Jang CH, Koppula S, Kang TB, Lee KH (2015) A novel synthetic derivative of melatonin, 5-hydroxy-2′-isobutyl-streptochlorin (HIS), inhibits inflammatory responses via regulation of TRIF-dependent signaling and inflammasome activation. Toxicol Appl Pharmacol. doi:10.1016/j.taap.2015.02.006
Siddall HK, Warrell CE, Yellon DM, Mocanu MM (2008) Ischemia-reperfusion injury and cardioprotection: investigating PTEN, the phosphatase that negatively regulates PI3K, using a congenital model of PTEN haploinsufficiency. Basic Res Cardiol 103:560–568. doi:10.1007/s00395-008-0735-y
Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G (2009) Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res 104:15–18. doi:10.1161/CIRCRESAHA.108.186429
Song JQ, Teng X, Cai Y, Tang CS, Qi YF (2009) Activation of Akt/GSK-3beta signaling pathway is involved in intermedin(1-53) protection against myocardial apoptosis induced by ischemia/reperfusion. Apoptosis 14:1061–1069. doi:10.1007/s10495-009-0382-2
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85:1093–1129. doi:10.1152/physrev.00006.2004
Steven S, Hausding M, Kroller-Schon S, Mader M, Mikhed Y, Stamm P, Zinssius E, Pfeffer A, Welschof P, Agdauletova S, Sudowe S, Li H, Oelze M, Schulz E, Klein T, Munzel T, Daiber A (2015) Gliptin and GLP-1 analog treatment improves survival and vascular inflammation/dysfunction in animals with lipopolysaccharide-induced endotoxemia. Basic Res Cardiol 110:6. doi:10.1007/s00395-015-0465-x
Sun B, Xiao J, Sun XB, Wu Y (2013) Notoginsenoside R1 attenuates cardiac dysfunction in endotoxemic mice: an insight into oestrogen receptor activation and PI3K/Akt signalling. Br J Pharmacol 168:1758–1770. doi:10.1111/bph.12063
Turner KL, Moore LJ, Todd SR, Sucher JF, Jones SA, McKinley BA, Valdivia A, Sailors RM, Moore FA (2011) Identification of cardiac dysfunction in sepsis with B-type natriuretic peptide. J Am Coll Surg 213:139–146. doi:10.1016/j.jamcollsurg.2011.03.027 (discussion 146–147)
van de Sandt AM, Windler R, Godecke A, Ohlig J, Zander S, Reinartz M, Graf J, van Faassen EE, Rassaf T, Schrader J, Kelm M, Merx MW (2013) Endothelial NOS (NOS3) impairs myocardial function in developing sepsis. Basic Res Cardiol 108:330. doi:10.1007/s00395-013-0330-8
Vanmassenhove J, Glorieux G, Lameire N, Hoste E, Dhondt A, Vanholder R, Van Biesen W (2015) Influence of severity of illness on neutrophil gelatinase-associated lipocalin performance as a marker of acute kidney injury: a prospective cohort study of patients with sepsis. BMC Nephrol 16:18. doi:10.1186/s12882-015-0003-y
Vazan R, Ravingerova T (2015) Protective effect of melatonin against myocardial injury induced by epinephrine. J Physiol Biochem 71:43–49. doi:10.1007/s13105-014-0377-5
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251
Wang X, Liu D, Chai W, Long Y, Su L, Yang R (2014) The role of uncoupling protein-2 (UCP2) during myocardial dysfunction in a canine model of endotoxin shock. Shock. doi:10.1097/SHK.0000000000000286
Whelan RS, Kaplinskiy V, Kitsis RN (2010) Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72:19–44. doi:10.1146/annurev.physiol.010908.163111
Williams DL, Ozment-Skelton T, Li C (2006) Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock 25:432–439. doi:10.1097/01.shk.0000209542.76305.55
Wu JY, Tsou MY, Chen TH, Chen SJ, Tsao CM, Wu CC (2008) Therapeutic effects of melatonin on peritonitis-induced septic shock with multiple organ dysfunction syndrome in rats. J Pineal Res 45:106–116. doi:10.1111/j.1600-079X.2008.00567.x
Wu YH, Zhou JN, Van Heerikhuize J, Jockers R, Swaab DF (2007) Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiol Aging 28:1239–1247. doi:10.1016/j.neurobiolaging.2006.06.002
Xu C, Yi C, Wang H, Bruce IC, Xia Q (2012) Mitochondrial nitric oxide synthase participates in septic shock myocardial depression by nitric oxide overproduction and mitochondrial permeability transition pore opening. Shock 37:110–115. doi:10.1097/SHK.0b013e3182391831
Yang J, Zong X, Wu G, Lin S, Feng Y, Hu J (2015) Taurine increases testicular function in aged rats by inhibiting oxidative stress and apoptosis. Amino Acids. doi:10.1007/s00726-015-1995-0
Yang Y, Jiang S, Dong Y, Fan C, Zhao L, Yang X, Li J, Di S, Yue L, Liang G, Reiter RJ, Qu Y (2015) Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J Pineal Res 58:61–70. doi:10.1111/jpi.12193
Yao K, Ye P, Zhang L, Tan J, Tang X, Zhang Y (2008) Epigallocatechin gallate protects against oxidative stress-induced mitochondria-dependent apoptosis in human lens epithelial cells. Mol Vis 14:217–223
Yeung HM, Hung MW, Lau CF, Fung ML (2015) Cardioprotective effects of melatonin against myocardial injuries induced by chronic intermittent hypoxia in rats. J Pineal Res 58:12–25. doi:10.1111/jpi.12190
You W, Min X, Zhang X, Qian B, Pang S, Ding Z, Li C, Gao X, Di R, Cheng Y, Liu L (2009) Cardiac-specific expression of heat shock protein 27 attenuated endotoxin-induced cardiac dysfunction and mortality in mice through a PI3K/Akt-dependent mechanism. Shock 32:108–117. doi:10.1097/SHK.0b013e318199165d
Yu L, Sun Y, Cheng L, Jin Z, Yang Y, Zhai M, Pei H, Wang X, Zhang H, Meng Q, Zhang Y, Yu S, Duan W (2014) Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J Pineal Res 57:228–238. doi:10.1111/jpi.12161
Zhang B, Liu Y, Zhang JS, Zhang XH, Chen WJ, Yin XH, Qi YF (2015) Cortistatin protects myocardium from endoplasmic reticulum stress induced apoptosis during sepsis. Mol Cell Endocrinol 406:40–48. doi:10.1016/j.mce.2015.02.016
Zhang H, Liu D, Wang X, Chen X, Long Y, Chai W, Zhou X, Rui X, Zhang Q, Wang H, Yang Q (2013) Melatonin improved rat cardiac mitochondria and survival rate in septic heart injury. J Pineal Res 55:1–6. doi:10.1111/jpi.12033
Zhang HM, Zhang Y (2014) Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 57:131–146. doi:10.1111/jpi.12162
Zhang T, Lu X, Li J, Chidiac P, Sims SM, Feng Q (2012) Inhibition of Na/K-ATPase promotes myocardial tumor necrosis factor-alpha protein expression and cardiac dysfunction via calcium/mTOR signaling in endotoxemia. Basic Res Cardiol 107:254. doi:10.1007/s00395-012-0254-8
Zhang WJ, Wei H, Hagen T, Frei B (2007) Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci USA 104:4077–4082. doi:10.1073/pnas.0700305104
Zhang Z (2015) Biomarkers, diagnosis and management of sepsis-induced acute kidney injury: a narrative review. Heart Lung Vessel 7:64–73
Zhong J, Hwang TC, Adams HR, Rubin LJ (1997) Reduced L-type calcium current in ventricular myocytes from endotoxemic guinea pigs. Am J Physiol 273:H2312–H2324
Zhou H, Qian J, Li C, Li J, Zhang X, Ding Z, Gao X, Han Z, Cheng Y, Liu L (2011) Attenuation of cardiac dysfunction by HSPA12B in endotoxin-induced sepsis in mice through a PI3K-dependent mechanism. Cardiovasc Res 89:109–118. doi:10.1093/cvr/cvq268
Zweier JL, Flaherty JT, Weisfeldt ML (1987) Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA 84:1404–1407
Acknowledgments
This study was supported by Grants from the National Natural Science Foundation of China (81170185).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
R. An and L. Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
An, R., Zhao, L., Xi, C. et al. Melatonin attenuates sepsis-induced cardiac dysfunction via a PI3K/Akt-dependent mechanism. Basic Res Cardiol 111, 8 (2016). https://doi.org/10.1007/s00395-015-0526-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-015-0526-1