Abstract
Purpose
No prospective data examined the effect of radical prostatectomy (RP) vs. external beam radiotherapy (EBRT) in locally advanced prostate cancer (PCa). We aimed to compare survival outcomes of RP and EBRT in patients harboring cT3N0-1 PCa.
Methods
Within the SEER database (2004–2014), we identified 5500 cT3N0-1 PCa patients. Cumulative incidence plots and competing-risks regression models (CRRs) tested cancer-specific mortality (CSM) and other cause of mortality (OCM) according to treatment type. The multivariable relationship between baseline prostate-specific antigen (PSA) values and 10-year CSM after either RP or EBRT was graphically depicted using the LOESS smoothing method. Sensitivity analyses were performed in cT3N0-only patients, after OCM propensity score matching, and through landmark analyses.
Results
Ten-year CSM and OCM rates were significantly higher after EBRT (15.8 and 28.2%) than RP (8.1 and 10.4%) (all p < 0.0001). In multivariable CRRs, RP yielded lower CSM [hazard ratio (HR): 0.64] than EBRT. Significantly lower 10-year CSM rate was recorded after RP vs. EBRT through the entire range of baseline PSA values. The same results were recorded in cT3N0 subgroup, as well as after OCM propensity score matching. Finally, landmark analyses at 6, 12, 24, and 36 months rejected the effect of favorable survival bias after RP.
Conclusions
CSM was significantly lower after RP than EBRT in cT3N0-1 PCa. A lower CSM was recorded throughout the entire range of baseline PSA and even in cT3N0 subgroup, as well as after OCM propensity score matching and landmark analyses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A small, nonetheless significant proportion of newly diagnosed non-metastatic prostate cancers (PCa) shows characteristics of locally advanced disease with or without clinical lymph node invasion [1]. Clear and concise treatment guidelines have not been defined for the management of PCa in this patient population, and the choice of radical prostatectomy (RP) or external beam radiotherapy (EBRT) remains debatable.
Several advantages and disadvantages differentiate the two procedures. In general, RP is associated with more accurate staging of the disease, prostate-specific antigen (PSA) level reliability for prediction of recurrence, and fewer bowel/rectal problems than EBRT. However, RP requires hospitalization, and it is associated with general risk of surgery and higher risk of incontinence and impotence compared to EBRT [2]. On the other hand, patients treated with EBRT do not require hospitalization or surgery, and they usually present lower risk of urinary incontinence than RP patients. Such advantages are compensated by lack of post-treatment staging information, higher rate of bladder irritation, and higher rates of bowel/rectal problems than RP patients [2].
To date, international guidelines agreed that RP as part of a multimodal therapy or EBRT plus androgen deprivation therapy (ADT) can be recommended to all locally advanced PCa patients with life expectancy more than 10 years. However, whether the choice should prefer one or the other option is not yet defined due to the absence of level 1 evidence [3].
Only few and non-randomized studies examined oncological outcomes of RP and EBRT in patients harbouring cT3 PCa. Here, such uncontrolled retrospective case series demonstrated comparable cancer-specific mortality (CSM) rates after RP (10-year CSM lower 13%) [1, 4, 5] or EBRT with ADT (10-year CSM lower 14%) [6,7,8] in locally advanced PCa (cT3).
To address the uncertainty regarding the choice of RP vs. EBRT for cT3N0-1 PCa, especially in the absence of prospective randomized phase III trials, we examined CSM rates after either RP or EBRT, within the most contemporary Surveillance Epidemiology and End Results (SEER) database, spanning years 2004–2014.
Materials and methods
Study cohorts
The current study relied on the SEER database, which samples 26% of the United States and approximates the United States in terms of demographic composition, as well as of cancer incidence and mortality [9]. We relied on the SEER research data 1973–2014, where an extensive data quality review of SEER PSA values from 2004 to 2014 has been completed [10].
We focused on subjects diagnosed between 2004 and 2014 with histologically confirmed adenocarcinoma of the prostate [International Classification of Disease for Oncology (ICD-O-3) code 8140 of the prostate (site code C61.9)] [11]. We only considered men with locally advanced PCa (cT3N0-1, M0, PSA < 50 ng/ml) treated with RP (surgery site codes 50 or 70) [12] or EBRT (radiation code: beam radiation) [13], aged less than 79 years. According to the SEER records, clinical T stage was mainly assessed with digital rectal examination (DRE). Moreover, for the definition of the lymph node status, we relied on the SEER variables “CS Lymph Nodes” and “CS Lymph Nodes Eval” for either RP or EBRT patients [14]. Exclusion criteria consisted of unknown PSA, unknown biopsy Gleason score, unknown clinical N stage, and metastatic disease (M1), or combination of RP and EBRT. CSM was defined according to the SEER mortality code (code 28010). All other deaths were considered as other-cause mortality (OCM).
Statistics
Descriptive statistics focused on frequencies and proportions for categorical variables [year of diagnosis, race, marital status, biopsy Gleason grade group [15] (GGG), and clinical N stage]. Means, medians, and ranges were reported for continuously coded variables (age and PSA). The statistical significance of differences in medians and proportions was tested with the Kruskal–Wallis and Chi-square tests. All statistical tests were two sided with a level of significance set at p < 0.05.
Analyses consisted of seven steps. First, we evaluated the temporal local treatment trends: RP vs. EBRT. To quantify annual temporal trend differences, we relied on annual percentage change (APC) with the least-squares linear regression. Second, treatment type-specific CSM and OCM cumulative incidence rates were generated and differences were tested with the Gray test [16]. Third, univariable and multivariable (MVA) competing-risks regression (CRR) methodology was used to test the effect of treatment type (RP vs. EBRT) on CSM [17]. Covariates included age, race, marital status, year of diagnosis, PSA, GGG, and cN stage. The latter was established in all RP and EBRT patients according to pre-treatment imaging evaluation of the lymph node status. Fourth, the LOESS smoothing method was used to graphically explore the relationship between baseline PSA values and 10-year CSM rates, derived from CRR multivariable analysis and adjusted for all covariates [18]. Fifth, for the purpose of sensitivity analysis, MVA CRR, as well as LOESS analyses, was repeated in cT3N0 patients. Sixth, to account for OCM rate differences between RP and EBRT patients, we relied on 1:1 nearest neighbor (caliper 0.5; R package “matchit”) propensity score matching defined according to individual OCM risk [14, 19]. Thus, defined OCM propensity score matching was applied to the entire study population, and MVA CRR models predicting CSM were refitted and the graphical depiction of treatment type-specific 10-year CSM rates (LOESS methodology) was also generated. Last, landmark analyses were performed at 6, 12, 24, and 36 months after the time of diagnosis, to address the potential effect of immortal time bias, which may favorably affect patients treated with RP, relative to EBRT patients [20].
All statistical tests were two sided with a level of significance set at p < 0.05. Analyses were performed using the R software environment for statistical computing and graphics (version 3.3.0; http://www.r-project.org/).
Results
We identified 5500 men with locally advanced (cT3N0-1) PCa, diagnosed between 2004 and 2014. Median age was 65 years [interquartile range (IQR) 59–71]. Most were Caucasian (3996, 72.7%), married (3908, 71.1%), harbored Gleason score ≤ 7 (3093, 56.2%), and cN0 (3215, 58.5%). Median PSA value at diagnosis was 12.6 ng/ml (IQR 5.5–16.5). RP or EBRT was performed in 2507 (45.6%) and 2993 (54.4%) patients, respectively (Table 1).
Temporal trend analyses
During the study period, the proportion of EBRT patients decreased from 63.8 to 50.9% (APC − 2.38%, CI − 3.56 to − 1.10, p = 0.003) vs. an increase from 36.2 to 49.1% (APC + 3.00%, CI + 1.12 to + 1.19, p = 0.007) for RP (Fig. 1).
Cumulative incidence analyses
Overall, 10-year CSM rates were 15.8 for EBRT patients vs. 8.1% for RP patients (p < 0.0001). 10-year OCM rates were 28.2 for EBRT patients vs. 10.4% for RP patients (p < 0.0001) (Fig. 2). Similarly, 10-year CSM and 10-year OCM rates were recorded in cT3N0, respectively, 15.5 vs. 3.9% (p < 0.0001) and 27.8 vs. 5.9% (p < 0.0001).
Competing-risks analyses
In MVA CRR models, that were stratified according to treatment type, RP yielded lower CSM evidenced by hazard ratio (HR) of 0.62 [confidence interval (CI) 0.45–0.86] (Table 2). In cT3N0 patient subset, RP also yields lower CSM evidenced by HR of 0.45 (CI 0.25–0.81, Supplementary Table 1).
Graphical depiction of MVA adjusted CSM rate according to treatment type (LOESS)
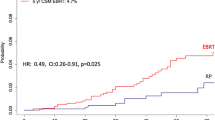
Significantly lower 10-year CSM rates were recorded after RP vs. EBRT through the entire range of baseline PSA values (Fig. 3). The same results were recorded in cT3N0 patient subgroup.
Sensitivity analyses
For the purpose of propensity score adjustment for potential OCM differences, analyses predicting OCM for the entire study period were fitted (Supplementary Table 2). Based on the propensity score cohort of 933 RP and 933 EBRT patients, we refitted MVA CRR models that focused on CSM. Here, significantly lower 10-year CSM rates were recorded after RP vs. EBRT, through the entire range of baseline PSA values (Supplementary Fig. 1). To confirm the validity of our approach, virtually, the same 10-year OCM rates (16 vs. 15.9%, p = 0.8, Supplementary Fig. 2) were recorded and validated the correctness of OCM propensity score matching with a HR of 0.98 (CI 0.68–1.39), indicative of no meaningful OCM rate difference. Finally, we performed landmark analyses to reject the hypothesis of immortal time bias. Here, at 6, 12, 24, and 36 months after the diagnosis of PCa, the decrease in CSM recorded after RP remained unchanged relative to “naïve” analyses, where the potentially favorable survival bias toward individuals who benefited from RP was unaccounted for.
Discussion
To date, no randomized controlled phase III trial has compared RP with EBRT in locally advanced non-metastatic PCa. Only historical (1988–2004) [7] and relatively small-scale [6] retrospective studies compared RP with EBRT in cT3. In consequence, the choice of RP or EBRT in this setting of patients remains controversial.
To address this void, we relied on a large population-based dataset (the SEER database) to compare CSM in 5500 cT3 PCa patients according to treatment type: RP vs. EBRT. To control for differences in baseline patient characteristics, we restricted the analyses to patients aged 79 years or less and with baseline PSA values less than 50 ng/ml. We then repeated the analyses after OCM propensity score matching, as well as in cT3N0 patient subset, which represents the vast majority of the study population. Our study yielded several noteworthy findings.
First, despite significantly higher rate of EBRT at the beginning of the study period, RP rate increased and EBRT rate decreased over time. In consequence, the proportion of patients treated with RP vs. EBRT changed over time. Initially, EBRT was favored. However, within the five most recent study years, RP and EBRT rates were virtually the same. This finding validates the confidence in RP as increasingly selected treatment modality for cT3N0-1 PCa. Our findings also agree with a contemporary report by Hager et al. [21], where the investigators compared cT3-T4 PCa treatment rates in the United States.
Second, several consecutive analytic steps demonstrated lower CSM rates after RP was compared to EBRT. Specifically, 10-year CSM rate was 7.7% lower for RP vs. EBRT. Lower CSM was confirmed in MVA CRR analyses and was evidenced by a 38% HR reduction. Lower 10-year CSM rates were also graphically depicted across the entire range of baseline PSA values in LOESS smoothed plots that focused on RP vs. EBRT, after multivariable adjustment for all covariates. Moreover, lower CSM rates were also identified after RP vs. EBRT in the subset of cT3N0 PCa patients. Similarly, as in the entire population, also these subset analyses showed lower 10-year CSM rates across the entire range of PSA values, after adjustment for all covariates. The aforementioned subset analyses allowed the examination of CSM rates in a more homogeneous and selected patient subgroup population (cT3N0). Furthermore, lower CSM rates were also recorded after additional OCM propensity score matching, which focused on potential bias that could result from higher OCM rates in EBRT patients. The results of all CSM analyses clearly demonstrated lower CSM rates after RP, regardless of analytical approach or adjustment type or patient cohort that was examined. Last but not least, landmark analyses performed at 6, 12, 24, and 36 months after PCa diagnosis failed to show that patients selected for RP have benefited from a favorable survival bias.
The incremental complexity of hypothesis testing with multiple adjustment levels and subgroup analyses serve the purpose of rejecting the possibility that the null hypothesis of no differences was incorrectly rejected. In consequence, it appears justified to conclude that based on thorough, albeit retrospective analyses, a survival benefit might exist when RP was selected instead of EBRT. Nonetheless, lack of randomization and the retrospective nature of the current analyses reduce our findings to lesser evidence level (level III) than if our findings originated from a randomized controlled trial (level IB or II).
Similar to our study, others have previously compared RP to EBRT in high-risk PCa. However, none focused specifically on cT3 PCa [7, 22,23,24,25]. For example, Boorjian et al. [7] relied on a historical cohort (1988–2005), that included a subset of 411 cT3 PCa patients, as well as 817 cT1-2 PCa, and they found no differences in CSM rates according to treatment type: RP vs. EBRT.
Recently, a retrospective single-institution study attempted to identify the best treatment approach in cT3-only PCa patients [6]. The investigators relied on 231 PCa treated with either RP or EBRT. Despite lower CSM rate (7%) after RP vs. EBRT (15%), Yamamoto et al. [6] did not identify a statistically significant difference because of the sample size limitation.
Our study does have some limitations that merit discussion. First, the SEER database does not include baseline performance status (Eastern Cooperative Oncology Group) and comorbidities. In that regard, we attempted to obviate this limitation by relying on CRR that accounts for OCM. In addition, we also relied on OCM propensity score matching to maximally reduce the potential OCM rate differences between RP and EBRT patients. Second, according to the SEER records, clinical T stage and clinical lymph node stage were assessed using DRE and CT scan, respectively. However, both presented low accuracy and limited reliability [26]. On the other hand, magnetic resonance imaging or positron emission tomography (PET)/CT scan was neither diffused nor approved for these specific purposes during the study period. In consequence, DRE and CT scan represented the only albeit inaccurate staging methodologies that could be used between years 2004 and 2014. Third, ADT and chemotherapy data are not recorded in the SEER database. Such missing data might have affected the results of our study. Moreover, concomitant ADT during EBRT, as well as timing of ADT or adjuvant chemotherapy, also might have affected the results of our study. Fourth, it should be noted that clinical N status data were missing for a significant proportion (40%) of patients. Unfortunately, this limitation is shared with all SEER-based analyses that stratify patients treated with EBRT or RP according to clinical lymph node status. Fifth, lack of standardized preoperative imaging evaluation might have hampered the uniformity of our patient population by including also patients with metastatic disease (M1). However, such possibility was partially compensated by relying on an upper PSA threshold of 50 ng/ml, which might have excluded the vast majority of patients with metastatic PCa. Sixth, the SEER database also lacks of information about RT dose (Gy) and regiments, as well as data on relapse and follow-up treatments. In consequence, our analyses were not adjusted for such covariates. Seventh, changes in the Gleason-grading system according to the International Society of Urologic Pathology Conference 2005 could have influenced our findings. These changes may have resulted in reporting of higher Gleason score and more patients with high-risk characteristics within more recent study years. Last but not least, even with the most sophisticated statistics, our study is limited by its retrospective design. This said, prospective randomized data are neither available nor will be available due to the lack of ongoing trial on cT3 PCa. In consequence, our study represents the largest and most contemporary source of evidences that support RP instead of EBRT in cT3 PCa patients.
Conclusions
Taken together our study showed that CSM was significantly lower after RP than EBRT in cT3N0-1 PCa. A lower CSM was recorded throughout the entire range of baseline PSA values and in cT3N0 subgroup, as well as after OCM propensity score matching. Survival bias did not affect the observed lower CSM rate after RP vs. EBRT. Despite its design limitation, our findings qualify for being considered an important signal that should prompt the design of randomized controlled trial comparing RP to EBRT in cT3N0-1 PCa. Until such trial is completed and its results yield mature observations, similar analyses should be performed in other large-scale databases to corroborated or refute our findings. In the absence of contradictory findings, our study represents the largest and most contemporary source of evidence prompting the use of RP in cT3 patients. Although it would be tempting to generalize such practice to all cT3N0-1 PCa patients, we must restrict our interpretation to cT3N0 patients, since sample size limitations precluded us from performing valid comparisons in cT3N1 patient subgroup.
References
Ward JF, Slezak JM, Blute ML et al (2005) Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int 95:751–756. https://doi.org/10.1111/j.1464-410X.2005.05394.x
Lardas M, Liew M, van den Bergh RC et al (2017) Quality of life outcomes after primary treatment for clinically localised prostate cancer: a systematic review. Eur Urol 72:869–885. https://doi.org/10.1016/j.eururo.2017.06.035
Professionals SO Prostate cancer. In: Uroweb. http://uroweb.org/guideline/prostate-cancer/#5. Accessed 25 Mar 2018
Mitchell CR, Boorjian SA, Umbreit EC et al (2012) 20-Year survival after radical prostatectomy as initial treatment for cT3 prostate cancer. BJU Int 110:1709–1713. https://doi.org/10.1111/j.1464-410X.2012.11372.x
Hsu CY, Wildhagen MF, Van Poppel H, Bangma CH (2010) Prognostic factors for and outcome of locally advanced prostate cancer after radical prostatectomy. BJU Int 105:1536–1540. https://doi.org/10.1111/j.1464-410X.2009.09054.x
Yamamoto S, Kawakami S, Yonese J et al (2014) Long-term oncological outcome in men with T3 prostate cancer: radical prostatectomy versus external-beam radiation therapy at a single institution. Int J Clin Oncol 19:1085–1091. https://doi.org/10.1007/s10147-013-0654-2
Boorjian SA, Karnes RJ, Viterbo R et al (2011) Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer 117:2883–2891. https://doi.org/10.1002/cncr.25900
Pilepich MV, Winter K, Lawton CA et al (2005) Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma–long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys 61:1285–1290. https://doi.org/10.1016/j.ijrobp.2004.08.047
Noone AM, Cronin KA, Altekruse SF et al (2017) Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidemiol Biomark Prev 26:632–641. https://doi.org/10.1158/1055-9965.EPI-16-0520
National cancer institute surveillance, epidemiology, and end results prostate-specific antigen working group, Adamo MP, Boten JA et al (2017) Validation of prostate-specific antigen laboratory values recorded in surveillance, epidemiology, and end results registries. Cancer 123:697–703. https://doi.org/10.1002/cncr.30401
Bandini M, Pompe RS, Marchioni M et al (2017) Radical prostatectomy or radiotherapy reduce prostate cancer mortality in elderly patients: a population-based propensity score adjusted analysis. World J Urol. https://doi.org/10.1007/s00345-017-2102-9
Leyh-Bannurah SR, Budäus L, Zaffuto E et al (2017) Adherence to pelvic lymph node dissection recommendations according to the national comprehensive cancer network pelvic lymph node dissection guideline and the D’Amico lymph node invasion risk stratification. Urol Oncol. https://doi.org/10.1016/j.urolonc.2017.10.022
Rusthoven CG, Waxweiler TV, DeWitt PE et al (2015) Gleason stratifications prognostic for survival in men receiving definitive external beam radiation therapy for localized prostate cancer. Urol Oncol 33(71):e11–e19. https://doi.org/10.1016/j.urolonc.2014.07.010
Bandini M, Preisser F, Nazzani S et al (2018) Contemporary trends and survival outcomes after aborted radical prostatectomy in lymph node metastatic prostate cancer patients. Eur Urol Focus. https://doi.org/10.1016/j.euf.2018.01.009
Epstein JI, Zelefsky MJ, Sjoberg DD et al (2016) A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol 69:428–435. https://doi.org/10.1016/j.eururo.2015.06.046
Logan BR, Zhang MJ (2013) The use of group sequential designs with common competing risks tests. Stat Med 32:899–913. https://doi.org/10.1002/sim.5597
Scrucca L, Santucci A, Aversa F (2010) Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant 45:1388–1395. https://doi.org/10.1038/bmt.2009.359
Cleveland WS, Devlin SJ (1988) Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc 83:596–610. https://doi.org/10.1080/01621459.1988.10478639
Mnatzaganian G, Davidson DC, Hiller JE, Ryan P (2015) Propensity score matching and randomization. J Clin Epidemiol 68:760–768. https://doi.org/10.1016/j.jclinepi.2015.01.002
Anderson JR, Cain KC, Gelber RD (1983) Analysis of survival by tumor response. J Clin Oncol 1:710–719. https://doi.org/10.1200/JCO.1983.1.11.710
Hager B, Kraywinkel K, Keck B et al (2017) Increasing use of radical prostatectomy for locally advanced prostate cancer in the USA and Germany: a comparative population-based study. Prostate Cancer Prostatic Dis 20:61–66. https://doi.org/10.1038/pcan.2016.43
Zelefsky MJ, Eastham JA, Cronin AM et al (2010) Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol 28:1508–1513. https://doi.org/10.1200/JCO.2009.22.2265
Kupelian PA, Elshaikh M, Reddy CA et al (2002) Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: a large single-institution experience with radical prostatectomy and external-beam radiotherapy. J Clin Oncol 20:3376–3385. https://doi.org/10.1200/JCO.2002.01.150
Akakura K, Suzuki H, Ichikawa T et al (2006) A randomized trial comparing radical prostatectomy plus endocrine therapy versus external beam radiotherapy plus endocrine therapy for locally advanced prostate cancer: results at median follow-up of 102 months. Jpn J Clin Oncol 36:789–793. https://doi.org/10.1093/jjco/hyl115
Arcangeli G, Strigari L, Arcangeli S et al (2009) Retrospective comparison of external beam radiotherapy and radical prostatectomy in high-risk, clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 75:975–982. https://doi.org/10.1016/j.ijrobp.2008.12.045
Gabriele D, Collura D, Oderda M et al (2016) Is there still a role for computed tomography and bone scintigraphy in prostate cancer staging? An analysis from the EUREKA-1 database. World J Urol 34:517–523. https://doi.org/10.1007/s00345-015-1669-2
Author information
Authors and Affiliations
Contributions
MB: protocol/project development, data collection or management, data analysis, manuscript writing/editing. FP: data collection or management. MM: manuscript writing/editing. ZT: data analysis. EZ: manuscript writing/editing. DT: data analysis. FM: protocol/project development. SFS: data collection or management. AB: protocol/project development. FS: manuscript writing/editing. PIK: manuscript writing/editing, protocol/project development.
Corresponding author
Ethics declarations
Conflict of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors have stated that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
345_2018_2310_MOESM1_ESM.tiff
Supplementary Figure 1. Graphical depiction of multivariable adjusted cancer specific mortality rate (LOESS) in 1,866 cT3N0-1 prostate cancer after other cause mortality propensity score matching according to treatment received: radical prostatectomy vs. external beam radiotherapy. 1 (TIFF 902 kb)
345_2018_2310_MOESM2_ESM.tiff
Supplementary Figure 2. Cumulative incidence plots depicting other cause mortality rates in 1,866 cT3N0-1 prostate cancer after other cause mortality propensity score matching stratified according to treatment received: radical prostatectomy vs. external beam radiotherapy. 2 (TIFF 902 kb)
Rights and permissions
About this article
Cite this article
Bandini, M., Marchioni, M., Preisser, F. et al. Survival after radical prostatectomy or radiotherapy for locally advanced (cT3) prostate cancer. World J Urol 36, 1399–1407 (2018). https://doi.org/10.1007/s00345-018-2310-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2310-y