Abstract
Background
This study was designed to compare the long-term oncological outcome of patients with clinical T3 (cT3) prostate cancer (PCA) treated with either radical prostatectomy (RP) or external-beam radiation therapy (EBRT) and to identify predictors of oncological outcomes.
Methods
A total of 231 patients with cT3 PCA underwent either RP (n = 112) or EBRT (n = 119). Local progression-free (LPFS), distant metastasis-free (DMFS), cancer-specific (CSS), and overall survival curves were generated with the Kaplan–Meier method, and the differences in survival rates between the two groups were assessed with a log-rank test. Cox proportional stepwise multivariate analysis was used to assess the association of variables to the oncological outcomes.
Results
The median follow-up of the RP and EBRT groups was 93 and 85 months, respectively (p = 0.004).The 10-year LPFS, DMFS, and CSS rates were not statistically different between the two groups (90.2, 73.9, and 93.7 % in the RP group and 82.7, 88.2, and 85.1 % in the EBRT group; p = 0.25, 0.10, and 0.10, respectively). The Cox proportional multivariate analysis revealed that clinical T3b (cT3b) (p = 0.001) and a biopsy Gleason score of 7–10 (p = 0.043) were significant predictors of cancer-specific mortality and that cT3b was also a significant predictor of local progression and all-cause mortality.
Conclusion
In cT3 PCA, both RP and EBRT provide an excellent long-term oncological outcome. cT3b was the strongest predictor of oncological outcome for the patients with locally advanced PCA who underwent the definitive therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Optimal definitive treatment [either radical prostatectomy (RP) or external-beam radiation therapy (EBRT)] for patients with clinical T3 (cT3) prostate cancer (PCA) remains controversial because of a lack of well-conducted prospective randomized studies. It is, however, ethically very difficult to perform such randomized studies. To our knowledge, although there has been one prospective randomized study reported by Akakura et al. [1], the study cohort size was too small, and clinical T2b (which is not “true” locally advanced PCA) was included in the study eligibility. Therefore, at the present time, attending physicians have been forced to utilize the information of prior retrospective studies [2–4] to assist with patient counseling and to decide upon the best course of treatment for patients with cT3 PCA. Unfortunately, some bias existed between the RP and EBRT cohorts in these previous studies, and the follow-up periods were too short. Boorjian et al. [3] did, however, retrospectively compare the long-term survival rates after RP or EBRT for patients with high-risk PCA, but the study cohort consisted of two centers and there was a lack of a central pathology review. Moreover, the Charlson score data in the two groups were not available. Arcangeli et al. [4] also reported a retrospective comparison of oncological outcomes after EBRT and RP for patients with high-risk localized PCA. Although the study was performed at a single center, the median follow-up times of the RP and EBRT groups were only 33.8 and 38.6 months, respectively. Such a short follow-up time makes it difficult to draw accurate conclusions.
To date, most previous studies have used prostate-specific antigen (PSA) failure as a surrogate endpoint of oncological outcome for patients with PCA [5–7]. There are, however, two problems with using PSA failure to evaluate oncological outcome. First, since PSA failure does not always translate into systemic progression, PCA-related death, and all-cause mortality [8, 9], PSA failure might not be a suitable choice as a surrogate endpoint of oncological outcome. Second, it is very difficult to compare PSA failure among the different treatment groups because the definition of PSA failure varies based on the treatment method [10]. Additionally, all patients with cT3 PCA are not completely cured using only first-step treatment. Accordingly, distant metastasis-free survival (DMFS) and cancer-specific survival (CSS) rates are much more suitable endpoints of oncological outcome. Needless to say, longer follow-up times are needed to calculate DMFS and CSS rates.
In this study, we retrospectively compared the oncological outcomes, including long-term DMFS and CSS, between RP and EBRT for patients with cT3 PCA and identified the predictor of oncological outcomes. To minimize bias, all patients had been treated at a single institution from 1994 to 2005.
Patients and methods
Patient population
Between January 1994 and July 2005, a total of 231 Japanese men with cT3 PCA underwent either RP (n = 112) or EBRT (n = 119) at the Cancer Institute Hospital in Tokyo, Japan. Clinical staging was determined according to the 1997 TNM classification. Digital rectal examination (DRE), abdominopelvic computed tomography (CT), and bone scan were performed for all patients. Since 1999, pelvic magnetic resonance imaging (MRI) has also been carried out to determine the T-stage. All MRI and CT scan findings were determined by a single radiologist (AK). Diagnostic criteria of extraprostatic extension [clinical stage T3a (cT3a)] on MRI T2-weighted images included broad (>12 mm) tumor contact, smooth capsular bulge, irregular capsular bulge, obliteration of the rectoprostatic angle, and asymmetry or direct involvement of the neurovascular bundle [11]. In addition, diagnostic criteria for the presence of seminal vesicle involvement (SVI) [clinical T3b (cT3b)] on MRI T2-weighted images included disruption or loss of the normal architecture of the SV, focal or diffuse areas of low signal intensity within the SV, low signal intensity within the SV causing a mass effect, or direct extension of the low signal intensity of a tumor from the base of the prostate to the SV. An enhanced SV wall on dynamic T1-weighted MR images was also defined as one of the diagnostic criteria for SVI [12]. When cT3 (a or b) findings on either the MRI image or DRE were identified, the patient was diagnosed as having cT3 (a or b). PSA measurements after the definitive treatments and histopathological grading of both the biopsy and RP specimen were performed as reported previously [13–16]. In total, 187 (75 %) patients received neo-adjuvant androgen deprivation therapy (NADT) before the definitive treatments. Combined androgen blockade (consisting of a luteinizing hormone-releasing hormone agonist and a nonsteroidal anti-androgen agent) was used as the ADT in the majority of the patients in the current study.
Radical prostatectomy
During the study period, RP was performed as reported previously [13–16]. Only two of the 112 patients (1.8 %) underwent a unilateral nerve sparing procedure. Eighty-six (76.8 %) patients received NADT for a median of 8 (range 3–18) months before surgery (Table 1). Because of the retrospective nature of the study, the use and period of NADT were decided at the discretion of the attending physician. After RP, 99 patients (88.4 %) were prospectively observed without any adjuvant treatment until PSA failure was confirmed. Exceptions to this protocol were 11 patients who received salvage ADT for persistently elevated PSA following RP due to adverse pathological findings (lymph node metastasis and SVI) and two patients who concurrently underwent orchiectomy as adjuvant ADT (AADT) with RP. At last follow-up, five (4.5 %) and 35 (31.2 %) patients received salvage EBRT and salvage ADT after PSA failure, respectively. The median time from RP to the salvage treatments was 2.0 (range 0.29–12.6) years. PSA failure was defined as a PSA level of >0.2 ng/mL.
External beam radiotherapy
All patients receiving EBRT were treated at 2 Gy per fraction using an opposing bilateral 120° arc technique or three-dimensional conformal radiation therapy (3DCRT). The median dose was 70 Gy (range 60–72 Gy; <70 Gy in 6 and ≥70 Gy in 113 patients). The clinical target volume (CTV) was defined as the prostate for the patients with clinical T3a lesions and was extended to include the seminal vesicles in their entirety for those patients with cT3b lesions. The margins of the planning target volume to CTV were 6 mm posteriorly, and 10 mm in all other directions. No pelvic lymph nodes were included in the radiation field.
Of the 119 patients, 114 (95.8 %) received NADT before EBRT and concomitant with ADT for a median of 9 (range 4–34) months (Table 1); 25 (21.0 %) of the 119 patients also received AADT for a median of 27 (range 3–78) months following concomitant ADT (Table 1). Because of the retrospective nature of the study, the use and period of NADT and AADT were decided upon at the discretion of the attending physician. At last follow-up, of the 94 patients who did not receive AADT after EBRT, 32 (26.9 %) had received salvage ADT after PSA failure. The median time from EBRT to salvage ADT was 3.1 (range 0.81–10.3) years. PSA failure was defined as the PSA nadir + 2 ng/mL.
Oncological outcomes
Oncological outcomes in terms of local progression-free survival (LPFS), DMFS, CSS, and overall survival (OAS) rates were evaluated. Local progression was defined as an intrapelvic recurrent mass irrespective of the histological confirmation of cancer cells in targeted biopsies at the bladder–urethral anastomosis. Distant metastasis was defined as a positive finding on radiological examinations. Cause of death was identified from death certificates or physician correspondence.
Statistical analysis
Local progression-free survival, DMFS, CSS, and OAS curves were generated with the Kaplan–Meier method, and the difference in these rates between the RP and EBRT groups was assessed with a log-rank test. The differences in clinicopathological variables between the two groups were analyzed by the chi-square test and the Mann–Whitney U test. Cox proportional stepwise multivariate analysis was used to assess the association of variables to LPFS, DMFS, CSS, and OAS. Age (continuous), Charlson score (0–1 vs. ≥2), PSA (continuous), clinical T-stage (T3a vs. T3b), biopsy Gleason score (GS) (5–6 vs. 7–10), NADT (yes vs. no), AADT (yes vs. no), and treatment type (RP vs. EBRT) were evaluated as possible predictors.
All p values were two-sided. A p value of <0.05 was considered to be statistically significant. Statistical analyses were performed with JMP ver. 5.1.1 (SAS Institute Inc., Cary, NC).
Results
Patient characteristics and pathological findings after RP
Patient characteristics are shown in Table 1. The patients in the RP group were significantly younger than those in the EBRT group (p < 0.001). Although patients in the EBRT group had higher pretreatment PSA levels (p = 0.17), higher biopsy GSs (p = 0.23), and a more advanced T-stage (p = 0.08) than patients in the RP group, there were no significant differences between the two groups. Approximately 90 % of all patients had high-grade cancers (GS ≥7) on biopsy. The median follow-up time of the RP and EBRT groups was 93 and 85 months, respectively (p = 0.004).
The pathological T-stage of the patients who underwent RP was T0, T2, T3a, T3b, and T4 in three (3 %), 41 (37 %), 34 (30 %), 32 (29 %), and two (2 %) patients, respectively. Sixteen patients (14 %) had lymph node metastasis, and 31 (28 %) were found to have positive surgical margins.
Oncological outcomes
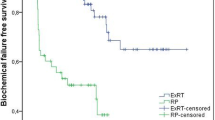
The local progression of the RP and EBRT groups was observed in ten (8.9 %) and 15 (12.6 %) patients, respectively (p = 0.37). As shown in Fig. 1a, the 10-year LPFS of the RP and EBRT groups was 90.2 and 82.7 %, respectively (p = 0.25).
Kaplan–Meier local progression-free (a), distant metastasis-free (b), cancer-specific (c) and overall (d) survival curves of patients with clinical T3 prostate cancer treated by radical prostatectomy (RP) or external-beam radiation therapy (EBRT). Numbers above X-axis (red EBRT, blue RP) Numbers of patients at risk at 0, 5, and 10 years
Twenty-three (20.5 %) patients of the RP group and 12 (10.1 %) of the EBRT group experienced a distant metastasis during the follow-up period (p = 0.03). The 10-year DMFS of the RP and EBRT groups was 73.9 and 88.2 %, respectively (p = 0.10) (Fig. 1b).
During the follow-up period, there were 39 deaths (16.9 %), including 15 PCA-related deaths (6.5 %). The RP group (93.7 %) showed only a tendency towards a better CSS rate than the EBRT group (85.1 %) (p = 0.10) (Fig. 1c), yet the 10-year OAS rate was significantly different between the RP (89.7 %) and EBRT (61.5 %) groups (p < 0.001) (Fig. 1d).
Predictors of the oncological outcomes in the entire cohort
Table 2 shows the Cox proportional stepwise multivariate analysis of the association between the eight variables and the oncological outcomes. Among the eight variables, cT3b (p = 0.018) and non-NADT (p = 0.016) were identified as significant predictors of local progression. cT3b (p = 0.001) and a biopsy GS of 7–10 (p = 0.043) were identified as predictors of a significantly increased risk of cancer-specific mortality, and EBRT (p = 0.013), older age (p = 0.005), and cT3b (p = 0.002) were also identified as significant predictors of all-cause mortality.
Discussion
Our retrospective study revealed two important findings.
First, patients with cT3 PCA treated with either RP or EBRT were able to obtain an excellent long-term oncological outcome. To date, there have been several published reports on the long-term oncological outcome of RP and EBRT series for patients with cT3 PCA [17–21]. Ward et al. [18] reported the long-term oncological outcome of RP in the largest ever cT3 PCA cohort. In this study, at a median follow-up of 10.3 years, the 10- and 15-year CSS rates of the 841 men with cT3 PCA who underwent RP were 90 and 79 %, respectively, yet because half of the patients in the cohort immediately received additional forms of treatment after surgery, the direct impact of RP on patients with cT3 PCA was unclear [18]. More recently, Hsu et al. [19] also reported that, at a median follow-up of 8.3 years, the 10-year CSS and OAS rates of 164 men with cT3 PCA who underwent RP were 80 and 67 %, respectively. Although the CSS and OAS rates among our patient cohort were slightly better than those of these previous studies [17–19], in general, the 10-year CSS and OAS rates of RP for patients with cT3 PCA range from 80 to 90 %, and values ranging from 70 to 80 % were obtained in the previous studies [17–19] and in our study. Furthermore, some studies have showed the benefit of adjuvant radiation therapy after RP for patients with adverse pathological features; however, it may be controversial to perform adjuvant radiation therapy routinely [20, 21]. In contrast, Zelefsky et al. [22] reported that the 10-year CSS and OAS rates of 296 patients with cT3 PCA treated with 3DCRT or intensity modulated radiotherapy (IMRT) were 83 and 65 %, respectively. Alicikus et al. [23] also reported the oncological outcome of 170 patients with PCA treated with 81 Gy IMRT. In their study, the 10-year CSS rate of patients with high-risk PCA was 86 %, and the 10-year actuarial risks of these men later developing greater than Grade 2 genitourinary and gastrointestinal toxicities were only 17 and 3.7 %, respectively. The oncological outcomes of these two studies [22, 23] are nearly comparable to those of the previous RP series [17–19] for cT3 PCA.
To our knowledge, there are only three retrospective studies which have compared the oncological outcome between RP and EBRT for patients with either high-risk or locally advanced PCA [2–4]. Zelefsky et al. [2] reported the oncological outcome of 1,318 patients with cT1c-3b PCA who underwent RP and 1,062 patients with cT1c-3b PCA who underwent IMRT at the Memorial Sloan-Kettering Cancer Center. The 8-year CSS rates of RP and IMRT for all patients were 98.6 and 95.3 %, respectively, yet the 8-year cancer-related mortality rates of RP and IMRT in patients with high-risk PCA were 3.8 and 9.5 %, respectively. These authors therefore concluded that the CSS rate of RP was superior to that of IMRT in cases of high-risk PCA. Boorijan et al. [3] conducted a comparison of the long-term survival between RP and EBRT for patients with high-risk PCA and concluded that RP is an independent positive predictor of OAS. These authors suggested that one potential explanation for this result might be an imbalance between the two treatment groups in terms of medical co-morbidities and unmeasured confounding variables. They also suggested that another potential explanation for the result might be an adverse impact of ADT on patients who received EBRT because ADT might result in adult diseases, such as cardiac disease, diabetes, and hypercholesterolemia. In our study, while treatment type was also identified as a significant predictor of all-cause mortality, it was not a significant predictor of CSS. One possible explanation for this finding may be that there was bias in terms of age between the two groups and that the EBRT group had more advanced features than the RP group, regardless of a lack of statistical significance. Also, only approximately 20 % of the EBRT group received adjuvant ADT after EBRT in our cohort, and only one patient died of heart failure in our entire patient cohort. We therefore believe that any adverse impact resulting from ADT use was not associated with the oncological outcome in our study.
The second significant finding was that the cT3b stage was determined to be a very strong predictor of local progression and cancer-specific mortality, which meant that patients with cT3b PCA had a poor prognosis regardless of treatment method. To date, there have been few reports of definitive treatment for cT3b PCA. Joniau et al. [24] reported that the 10-year clinical progression-free survival and CSS rates of their 51 patients with cT3b-4 treated with RP were 73 and 92 %, respectively, leading them to the conclusion that RP was a reasonable first step in selected patients with cT3b-4 PCA and no tumor fixation to the pelvic wall or invasion into the urethral sphincter. Zelefsky et al. [24] reported that the 10-year PSA failure-free survival and DMFS of the cT3b PCA patients treated with EBRT were 32 and 32 %, respectively, and that the oncological outcome of cT3b PCA patients was significantly worse than that of cT3a PCA patients. Based on the findings of the previous studies and our current study, the development of any effective additional treatment after definitive treatment is urgently needed for cT3b PCA patients in the near future.
There were several limitations to the present study: First, this was a relatively small retrospective study, so there were quite a few biases, such as age and follow-up period. Although it is very difficult to perform such randomized prospective studies due to ethical concerns, this study is one of the largest studies conducted to date in an Asian population, and the findings of this study resulted from multivariate analysis; we therefore believe our overall findings are of value. Second, because many patients received NADT before the definitive treatments, it is unclear whether they were true cT3 PCA cases. The results of this study, however, are at least comparable to those of other studies, and as it has been reported that approximately 70–80 % of men diagnosed with cT3-4 disease show a concordance between the clinical stage and the pathological stage [25], we believe that only a few patients without cT3 disease were included in our cohort. As a rule, we have been performing RP without NADT for locally advanced PCA since 2008 because it has been demonstrated that NADT before RP does not improve OAS or disease-free survival [26]. Third, AADT was not performed on all patients of the EBRT group, regardless of the existence of cT3PCA, and even if the patients received AADT, the ADT periods were possibly too short. Fourth, the radiation dose might not have been sufficient to eradicate cT3 PCA. At present, all patients with locally advanced PCA are treated by IMRT with 78 Gy in our hospital. Lastly, the quality of life after the treatments was not evaluated.
In conclusion, despite some limitations, RP as well as EBRT for men with cT3 PCA provide an excellent long-term oncological outcome. cT3b was a very strong predictor of the oncological outcome our patients with locally advanced PCA who underwent the definitive treatment. Finally, it is necessary to add some forms of additional treatment to the definitive treatment regimen for cT3b PCA patients.
References
Akakura K, Suzuki H, Ichikawa T et al (2006) A randomized trial comparing radical prostatectomy plus endocrine therapy versus external beam radiotherapy plus endocrine therapy for locally advanced prostate cancer: results at median follow-up of 102 months. Jpn J Clin Oncol 36:789–793
Zelefsky MJ, Eastham JA, Cronin AM et al (2010) Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol 28:1508–1513
Boorjian SA, Kanes RJ, Viterbo R et al (2011) Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer 117:2883–2891
Arcangeli G, Strigari L, Arcangeli S et al (2009) Retrospective comparison of external beam radiotherapy and radical prostatectomy in high-risk, clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 75:975–982
D’Amico AV, Whittington R, Kaplan I et al (1997) Equivalent biochemical failure-free survival after external beam radiation therapy of radical prostatectomy in patients with a pretreatment prostate specific antigen of >4–20 ng/ml. Int J Radiat Oncol Biol Phys 15:1053–1058
D’Amico AV, Whittington R, Malkowicz SB et al (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969–974
Kupelian PA, Elshaikh M, Reddy CA et al (2002) Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: a large single institution experience with radical prostatectomy and external beam radiotherapy. J Clin Oncol 20:3376–3385
Ward JF, Blute MI, Slezak J et al (2003) The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol 170:1872–1876
Roehl KA, Han M, Ramos CG et al (2004) Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3478 consecutive patients: long-term results. J Urol 172:910–914
Nielsen ME, Makarov DV, Humphreys E et al (2008) Is it possible to compare PSA recurrence-free survival after surgery and radiotherapy using revised ASTRO criterion-“nadir +2”? Urology 72:389–395
Yu KK, Hricak H, Alagappan R et al (1997) Detection of extracapsular extension of prostate carcinoma with endorectal and phased-array coil MR imaging: multivariate feature analysis. Radiology 202:697–702
Kim CK, Choi D, Park BK et al (2008) Diffusion-weighted MR imaging for the evaluation of seminal vesicle invasion in prostate cancer: initial results. J Magn Reson Imaging 28:963–969
Yamamoto S, Yonese J, Kawakami S et al (2007) Preoperative serum testosterone level as an independent predictor of treatment failure following radical prostatectomy. Eur Urol 52:696–701
Yamamoto S, Kawakami S, Yonese J et al (2009) Risk stratification of high-grade prostate cancer treated with antegrade radical prostatectomy with intended wide resection. Jpn J Clin Oncol 39:387–393
Yamamoto S, Kawakami S, Yonese J et al (2008) Lymphovascular invasion is an independent predictor of prostate-specific antigen failure after radical prostatectomy in patients with pT3aN0 prostate cancer. Int J Urol 15:895–899
Yamamoto S, Kawakami S, Yonese J et al (2012) Long-term oncological outcome and risk stratification in men with high-risk prostate cancer treated with radical prostatectomy. Jpn J Clin Oncol 42:541–547
Freedland SJ, Partin AW, Humphreys EB et al (2007) Radical prostatectomy for clinical T3a disease. Cancer 109:1273–1278
Ward JF, Slezak JM, Blute ML et al (2005) Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int 95:751–756
Hsu CY, Wilhagen MF, Poppel HV et al (2009) Prognostic factors for and outcome of locally advanced prostate cancer after radical prostatectomy. BJU Int 183:1536–1540
Thompson M Jr, Tangen CM, Paradelo J et al (2006) Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA 296:2329–2335
Wiegel T, Bottke D, Steiner U et al (2009) Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol 27:292402930
Zelefsky MJ, Yamada Y, Kollmeier MA et al (2008) Long-term outcome following three-dimensional conformal/intensity-modulated external-beam radiotherapy for clinical stage T3 prostate cancer. Eur Urol 53:1172–1179
Alicikus ZA, Yamada Y, Zhang Z et al (2011) Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer 117:1429–1437
Joniau S, Hsu CY, Gontero P et al (2012) Radical prostatectomy in very high-risk localized prostate cancer: long-term outcomes and outcome predictors. Scand J Urol Nephrol 46:164–171
Schreiber D, Rineer J, Sura S et al (2011) Radical prostatectomy for cT3-4 disease: an evaluation of the pathological outcomes and patterns of care for adjuvant radiation in a national cohort. BJU Int 108:360–365
Shelley MD, Kumar S, Wit T et al (2009) A systematic review and meta-analysis of randomized trials of neo-adjuvant hormone therapy for localized and locally advanced prostate carcinoma. Cancer Treat Rev 35:9–17
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yamamoto, S., Kawakami, S., Yonese, J. et al. Long-term oncological outcome in men with T3 prostate cancer: radical prostatectomy versus external-beam radiation therapy at a single institution. Int J Clin Oncol 19, 1085–1091 (2014). https://doi.org/10.1007/s10147-013-0654-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-013-0654-2