Abstract
The present study aimed to investigate the potential protective role of oxalic acid (OA) against Cadmium (Cd) toxicity. Chickpea (Cicer arietinum L.) seeds were exposed to 200 µM Cd stress for 6 days or to co-treatment with 200 µM Cd + 100 µM OA for 3 days following to 3-day Cd stress. The application of OA ameliorated the growth of both roots and shoots of Cd-treated seedlings. This effect was mediated by the restriction of Cd accumulation in plant tissues. Besides, malondialdehyde (MDA) and carbonyl group contents decreased in OA-treated seedlings, suggesting that OA reversed the Cd-induced oxidative stress and its detrimental effect on cell membrane integrity. These results were further confirmed by the reduction by 2- and 1.7-fold of hydrogen peroxide (H2O2) accrual in roots and shoots, respectively, when compared to Cd-challenged seedlings. Moreover, OA corrected the Cd-imposed imbalance of the glutathione redox state, mainly via the restoration of the glutathione pool. This achievement seems to be the result of the modulation of glutathione peroxidase (GPX) and glutathione reductase (GR) activities. Likewise, OA counteracted the adverse effect of Cd on nicotinamides redox state reflected by the restoration of the balance between oxidized [NAD(P)] and reduced [NAD(P)H] forms. Taken together, our results suggest that the exogenous supply of OA to germinating seeds can be a promising alternative to improve plant tolerance to heavy metal stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is one of the most toxic heavy metals (HM) due to its high affinity to sulfur-containing ligands (Khan et al. 2016). In soil, Cd is considered as a relatively mobile element compared to lead (Pb) and copper (Cu) (Nedjimi and Daoud 2009). Several physicochemical parameters play an important role in the bioavailability of Cd in the soil solution, such as soil pH, redox potential, and abundance of organic or inorganic colloids (Bolan et al. 2013).
Stunted seedling growth is the first symptom of Cd toxicity. Overaccumulation of the metal in the seed reserve tissues, as well as the accumulation of carbohydrates (glucose, fructose, sucrose) and the reduction of their transfer to the embryonic axis could be a cause of the growth delay (Rahoui et al. 2015). The depressive effect of Cd on seedling growth may result from metal interference with cell division and/or elongation (Seregin and Ivanov 2001). The exposition to Cd leads to the reduction of the cellular plasticity and the glutathione pool, the increase of abscisic acid and ethylene synthesis, the inhibition of microtubule polymerization, the induction of chromosome aberrations, and the binding of metal ions to DNA (Seregin and Ivanov 2001). Besides, Cd acts by altering chloroplast and mitochondrial electron transport chains and/or by inhibiting antioxidant systems (Zaid and Mohammad 2018; Gratão et al. 2019), leading to oxidative stress installation (Gratão et al. 2019). Reactive oxygen species (ROS) overaccumulation may result from the raised Cd-induced NADPH oxidase activities (Sakouhi et al. 2016).
The involvement of NADPH oxidase activities in generating oxidative burst, through H2O2 overproduction, in response to numerous abiotic stresses, such as drought, salt, anoxic and HM stress has been attested (Hasanuzzaman et al. 2020; Kharbech et al. 2020).
Heavy metals can alter directly or indirectly the structure of proteins by binding to the functional groups, leading to changes in their structure and activity (Latowski et al. 2005). Moreover, metal ions can affect protein function by displacement of other divalent ions, such as calcium (Ca) and zinc (Zn) (Chmielowska-Bąk et al. 2013). Heavy metals, also, can indirectly affect protein structure by ROS induction, which could modulate protein activity by nitrosylation, carboxylation, disulfide bond formation, or glutathionylation (Romero-Puertas et al. 2013; Zagorchev et al. 2013). Thiol (–SH) groups are, oxidized by H2O2 and ·OH, leading to the inactivation of enzymes (Stadtman and Levine 2000). Furthermore, it was reported that ROS overproduction induced changes in amino acids and protein charges, fragmentation of the peptide chain, production of aggregate and increased the susceptibility to proteolysis (Pacifici and Davies 1990; Zaid and Wani 2019).
To cope with ROS-mediated detrimental impact, plants developed enzymatic and non-enzymatic antioxidant systems (Zaid and Wani 2019). In addition to the "high three" antioxidants, ascorbate, glutathione (GSH) and NAD(P)H (Ahmad et al. 2010), plants contain several redox metabolites, such as phenols, quercetin, carotenoids (Smirnoff 2000), polyamines (Martin-Tanguy 2001) and thiol-dependent systems (Hasanuzzaman et al. 2020).
The concept of a cellular redox state, which regulates stress responses, is intrinsically associated with the kinetics of stress exposure and, in particular, the balance between damage and acclimation responses (Potters et al. 2010). Besides, stress alters the redox state of metabolites, which could result from the production of H2O2 by NAD(P)H oxidases and/or peroxidases, causing either damage or tolerance response (Miller et al. 2018). The change in the cellular redox status modulates gene expression, enzyme activity, and hormone perception (Potters et al. 2010).
Several investigations have been undertaken to confirm the effectiveness of the exogenous application of organic and inorganic chemical compounds in mitigating HM phytotoxicity (Zaid and Mohammad 2018; Ben Massoud et al. 2019; Kharbech et al. 2020). Chelation of metallic ions in the soil solution or the cytosol could be an important mechanism of contaminated soil remediation (Xu et al. 2010; Sakouhi et al. 2021). Organic acids (OrAs), including OA, are potential chelating agents and may play a key role in plant tolerance to HM by reducing metals bioavailability (Zhu et al. 2011) and the enhancement of the cellular antioxidant capacity (Soyingbe et al. 2020). In a recent study, Fu et al. (2018) noticed a simultaneous accumulation of OrAs and Cd in rice plants and an increased exudation of OrAs by roots, suggesting an internal and external chelation of Cd ions by OrAs. More recently, it was opined that Cd stress inhibited the organic acid metabolism in Sedum plumbizincicola, while it led to oxidative stress and OrAs exudation in Trachyspermum ammi plants (Javed et al. 2020; Sun et al. 2020).
Organic acids regulate rhizosphere pH by increasing the ammonification (Sidhu et al. 2019). Javed et al. (2017) reported that the OrAs release by the anion channels was accompanied by a cation exudation. Thereby, the exudation of OrAs was found to be an important process involved in HM tolerance (Sidhu et al. 2019). Agnello et al. (2014) proposed that the Cd-OA complex reduced the accumulation of the metal ions in roots and ameliorated their translocation to shoots. By contrast, Li et al. (2014) suggested that OrAs treatment enhanced Cd uptake through apoplastic pathways and transport in Boehmeria nivea plants. Furthermore, OrAs-Cd complexes could penetrate plant tissues by breaking the root endoderm and the Casparian bands (Li et al. 2014). It seems that OrAs effect on Cd availability and translocation depends on their respective concentrations in the medium (Sabir et al. 2014). Besides, OrAs, such as citric and oxalic acids, improved growth attributes, and ameliorated root respiration and ATP synthesis (Li et al. 2014). Moreover, OrAs could alleviate HM-induced oxidative stress by protecting membrane integrity, modulating plasma membrane and vacuolar H+-ATPase activities (Zhang et al. 2019). Growing evidence argues in favor of the assumption that OrAs trigger antioxidant enzymes, such as superoxide dismutase, peroxidases, and catalase activities (Li et al. 2014; Liu et al. 2015; Kaur et al. 2018).
Oxalic acid, a dicarboxylic acid, currently linked to biotic stress. Pathogenic attack induces an increased OA accumulation, which triggers programmed cell death via ethylene synthesis (Errakhi et al. 2008). Besides, OA is involved in the maintaining of the balance between inorganic cations and anions in plants (Webb et al. 1995), and the regulation of cellular concentration of Ca via Ca-OA complex formation (Ruiz and Mansfield 1994). These complexes were suggested as Ca storage mechanism for future needs of the plant (Helper and Wayne 1985). Moreover, Soyingbe et al. (2020) provided prospective evidence that OA was a key factor in nickel (Ni) detoxification in maize plants by limiting HM accumulation and improving defense enzymes. Liu et al. (2015) reported that OA, when supplemented at low concentration (10–20 mg Kg−1 soil), drastically enhanced the Cd content in all the tissues of Phytolacca Americana plant, while a higher concentration of OA (40 mg Kg−1 soil) limited Cd accumulation in root cells.
Taking into consideration the above-mentioned properties of OA, its protective effect against Cd toxicity was expected. Thereby, the present investigation was undertaken to inspect the impact of the combination of OA and Cd on the growth and the cellular redox status of germinating chickpea seeds. In this aim, indicators of oxidative stress: H2O2, MDA and carbonyl groups were inspected. Besides, the redox balance of nicotinamides [NAD(P)/NAD(P)H], the glutathione pool and the implicated activities were studied.
Materials and Methods
Germination and Treatments' Conditions
Seeds of chickpea (Cicer arietinum L., cv. Beja 1) were disinfected with 2% sodium hypochlorite for 10 min and were immediately rinsed with distilled water. Germination was conducted in a germination chamber with controlled conditions (in the dark at 25 °C and 65(± 5)% relative humidity). The seeds are germinated on filter paper soaked with 30 mL H2O (first batch) or aqueous solution of 200 µM CdCl2 (second batch). After 3 days, seeds from the first batch continued to germinate on filter paper moistened with distilled water (control condition), or in the presence of 100 µM OA. Seeds from the second batch continued their germination in the presence of 200 µM CdCl2 (stress condition) or were transferred in distilled water (stress cessation) or the combination of 200 µM CdCl2 and 100 µM OA for 3 additional days as described in Table 1. Cadmium and OA concentrations were selected following preliminary tests indicating, respectively, a 50% decrease and the most significant recovery of the seedling growth.
Six-day-old seedlings were harvested and roots and shoots were separated and weighed. Samples were stored at − 80 °C for biochemical analyses or dried at 70 °C for dry weight (DW) measurement. Fresh plant material was used for glutathione quantification.
All the experiments were carried out from three successive germinations (Juin, 7 to 13, 14 to 20 and 21 to 27, 2016). For each replication, the measurements were performed on three lots of samples from the same treatment; using extracts from several samples and the data presented were the mean values from these independent experiments.
Determination of Cadmium Content
Seedlings were dried and subjected to wet digestion (10 ml per 0.1 g dry weight) with an acid mixture (HNO3: HClO4, 4:1). Cadmium concentrations were determined using an atomic absorption spectrophotometer (Perkin Elmer, Waltham MA, USA) and the calibration was performed by Sigma Diagnostic Standards (Cadmium Atomic Absorption Solutions, Sigma-Aldrich) solutions, diluted with 0.1 N HNO3 (65% HNO3, Sigma-Aldrich, Analytical grade). The translocation factor (TF) was determined to assess the ability of the plant to accumulate Cd in shoots.
Lipid Peroxides Estimation
Lipoperoxidation products were evaluated by the determination of MDA concentration, considered as the major thiobarbituric acid-reactive substance, following to Heath and Packer (1968) method.
Determination of Hydrogen Peroxide Content
Shoot and root tissues (1 g) were homogenized in trichloroacetic acid (TCA; 5%, w/v). After centrifugation at 10,000×g for 15 min at 4 °C, the supernatant was added to potassium phosphate buffer (25 mM, pH 7.0) and 1 M KI. The H2O2 concentration was determined by measuring the absorbance at 390 nm (Sergiev et al. 1997).
Extraction of Protein and Determination of Carbonyl Groups Contents
Protein extraction was performed in a potassium phosphate buffer (50 mM, pH 7.0) supplemented with 5 mM sodium ascorbate and 0.2 mM EDTA. The homogenate was centrifuged at 10,000×g for 15 min at 4 °C and the obtained supernatant was used for the determination of protein and carbonyl groups contents and enzyme assays. Protein contents were estimated, using bovine serum albumin as standard protein, following to Bradford (1976) method. Carbonyl groups assay was carried out according to Levine et al. (1994) using a spectrophotometric DNPH (2,4-dinitrophenylhydrazine) method.
Determination of Glutathione Contents
The extraction of the glutathione pool was performed according to Garcia et al. (2008). Thiol groups were derivated by DTNB (5,5′-dithiobis [2-nitrobenzoic acid]) as described by Katrusiak et al. (2001). The determination of glutathione pool contents was performed using a reverse-phase column (Agilent, 1100 Series, USA) connected to a UV–Visible detector fixed at 330 nm (Katrusiak et al. 2001). The mobile phase was composed of acetonitrile as mobile phase A and acidified water (pH 3.5) as mobile phase B at a flow rate of 1.2 ml min−1. The elution profile was as follows: 0–15 min, 10% A; 15–16 min, 100% A; 16–20 min, 10% A. Peaks were identified and the total (GSH + GSSG) and the reduced (GSH) forms of glutathione were quantified using calibration curves realized from DTNB-derived glutathione (Sigma) solutions. Determination of glutathione contents was carried out using the area of peaks and GSSG contents were calculated as the difference between total and reduced forms concentrations.
Nicotinamides Quantification
Oxidized forms (NADP+ and NAD+) were extracted in HCl, while reduced forms (NADPH and NADH) were extracted in NaOH, according to Zhao et al. (1987). Quantification of nicotinamides was carried out by monitoring the absorbance at 570 nm as described in our previous report (Sakouhi et al. 2016).
Enzyme Activities Assays
Glutathione peroxidase (GPX, EC 1.11.1.9) activity was assessed according to Nagalakshmi and Prasad (2001), and glutathione reductase (GR; EC 1.6.4.2) activity was determined as stated by Foyer and Halliwell (1976).
Statistics
Significances were tested by one-way analysis of variance (ANOVA) and pairwise comparisons between treatments were performed using Tukey’s HSD multiple comparisons test. The results were expressed as the mean values ± standard error (SE) obtained from three replicates. A probability of p ≤ 0.05 was considered significant in all the above-mentioned analyses. The level of correlation between tested parameters was evaluated by calculating Pearson’s coefficients using R packages (www.R-project.org) and the correlations were represented as correlograms. Data with similar patterns are grouped together and presented indicating positive or negative correlations.
Results and Discussion
Effects on Seedling Growth and Cd Accumulation
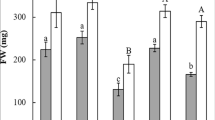
The exposure to Cd stress led to dry weight (DW) reduction by 36% and 26% in roots and shoots, respectively (Fig. 1a) and the inhibition of seedling elongation as shown in Fig. 1b. The stunted growth could be attributed to metabolic pathways disruption such as the transfer of the cotyledonary reserve to embryonic tissues due to the inhibition of proteolytic enzymes (Rahoui et al. 2015). However, the addition of OA to the germinating medium of chickpea seeds mitigated the adverse effect of Cd on root and shoot growth (28% increase vs Cd-treated only, Fig. 1a). This finding is in agreement with previous studies suggesting a protective effect of organic chelating agents on seedling growth against HM stress (Sakouhi et al. 2018; Ben Massoud et al. 2019).
Root and shoot dry weight (a) and morphological changes (b) of chickpea seedlings after seeds imbibition in H2O or 200 µM CdCl2 in the presence or absence of 100 µM OA. Values are the averages of twenty individual measurements. Values (± SE, n = 3) followed by a common letter for the same seedling part (a) are not different at the 0.05 level of significance using ANOVA followed by Tukey’s test. Bar scale (b) represents 2 cm
The protective effect of OA on growth could be attributed to the reduced Cd accumulation, as evidenced by 36% and 31% decrease in root and shoot, respectively (Table 2). Furthermore, the enrichment with OA of the Cd-contaminated germinating medium caused a 24% decrease in the TF, reaching, thereby, the relative control (Cd + H2O treatment) TF value (Table 2). This result suggested the sequestration of Cd ions in root cells, leading to the complete recovery of shoot growth (Fig. 1).
In line with our results, a decline in metallic ions accumulation subsequent to citric and oxalic acids supplement was reported in Changbai larch, pea and rice seedlings exposed to Pb, Cu and Cd stress, respectively (Xu et al. 2010; Song et al. 2018; Ben Massoud et al. 2019).
Owing to its high affinity to metallic ions, OA is involved in metal complexation leading to a decreased metal availability (Ma et al. 2020). Thus, the release of OrAs, including OA, was reported to be an efficient mechanism to enhance HM tolerance (Montiel-Rozas et al. 2016). Recently, Bittencourt et al. (2020) associated the release of OA with the decreased Al uptake by Styrax camporum plantlets, suggesting an Al exclusion mechanism. Moreover, Najeeb et al. (2011) suggested the involvement of OrAs, such as citric, malic and oxalic acids, in the vacuolar sequestration of metallic ions reducing, thereby, their mobility within the cell and consequently their toxicity. Likewise, Collins et al. (2003) reported that organic ligands could chelate Cd in the soil, thus, reducing its uptake. Moreover, Zhu et al. (2011) reported that Cd treatment raised OA exudation from tomato roots, thus, preventing metal entry and the exogenous OA supply improved plant tolerance to Cd. The authors confirmed this idea by the increased Cd accumulation and toxicity following to the inhibition of OA secretion by the application of anions channels inhibitors. Contrariwise, Luo et al. (2014) suggested that OA exudation controlled Cd mobilization. The internal likely OrAs-induced mechanism is the sequestration and the vacuolar compartmentalization of Cd as suggested by Chaffai et al. (2006). Indeed, OrAs may chelate with free metal ions in the cytoplasm, enhancing their ability to diffuse to the vacuoles, and reducing, thereby, free harmful metal ions (Chen et al. 2003). By contrast, several reports confirmed that OrAs could be effective in soil remediation through the enhancement of metallic ions phytoextraction (Zaheer et al. 2015). The controversial effect of OrAs on HM accumulation seems to be dependent on metal concentration and the plant species (Montiel-Rozas et al. 2016). Moreover, the effect of OrAs on HM bioavailability depends on the HM-OrAs complex solubility. Cadmium-oxalate, being partially soluble, led to a decreased Cd uptake (Zhu et al. 2011).
Effects on Oxidative Stress Biomarkers
As predicted, Cd stress caused a considerable rise in MDA (63% and 73% increase of control in roots and shoots, respectively; Fig. 2a) and protein carbonyl groups contents (4.4- and 7.5-fold increase as compared to control in roots and shoots, respectively; Fig. 2b). The adverse effects of Cd on cell membrane integrity and proteins should be attributed to oxidative stress establishment, argued by the concomitant increase of H2O2 contents (2.5- and twofold increase as compared to control in roots and shoots, respectively; Fig. 2c).
MDA (a), carbonyl groups (b) and H2O2 (c) contents in roots and shoots of chickpea seedlings after seeds imbibition in H2O or 200 µM CdCl2 in the presence or absence of 100 µM OA. Values (± SE, n = 3) followed by a common letter for the same seedling part are not different at the 0.05 level of significance using ANOVA followed by Tukey’s test
A drastic increase in oxidative stress markers was reported under HM stress (Gratão et al. 2019). Exogenous OA alleviated completely he Cd-mediated enhancement of oxidative stress markers (Fig. 2), except for MDA shoot content (Fig. 2a) which exhibited the same recovery observed in the case of Cd stress relieve after 3 days exposure (Cd + H2O treatment; 26% lesser than Cd-stressed shoots; Fig. 2a). It should be emphasized that the OA improving effect was achieved despite the presence of over 63% of the metal amounts in root and shoot cells (Table 2). However, when applied alone, OA had no significant effects on MDA, carbonyl groups and H2O2 contents (Fig. 2). These results corroborate with previous studies suggesting that rice seedlings treatment and seeds priming with OrAs protected cell membrane from Cd-induced deterioration (Guo et al. 2007; Xu et al. 2010). Similarly, the addition of citric acid to the Cu-enriched pea seeds germination medium resulted in decreased levels of H2O2, MDA, and carbonyl groups testifying, thereby, the ability of OrAs to improve plant tolerance to HM (Ben Massoud et al. 2018). The protective effects of OrAs against Cd-generated cell damages could be assigned to at least two mechanisms. The first one is the change in metallic ions speciation via the occurring of steady complexes (Ma et al. 2020) and the second mechanism could be the improvement of antioxidant enzyme activities as reported by Li et al. (2014). The positive effect of OrAs on ROS accumulation was further confirmed by the recent studies of Ben Massoud et al. (2018, 2019).
Effects on Cellular Redox State
Oxidative burst results from prooxidant metabolism enhancement, redox state disruption and/or alteration of antioxidant system function. Recently, special attention was focused on the assessment of cellular redox state, which is connected with various metabolic pathways, including the expression of a large number of genes, energy production in mitochondria and chloroplasts, oxidation and reduction reactions, as well as reactions of primary and secondary metabolism (Potters et al. 2010). In stress conditions, cellular redox status tends to shift in favor of prooxidant metabolites. Glutathione, owing to its high intracellular content and reducing power, plays a crucial role in the conservation of cellular redox homeostasis (Foyer and Noctor 2011).
The exposure of germinating seeds to Cd stress caused 28% and 36% decrease of the GSH/GSSG ratio in roots and shoots, respectively, as compared to controls (Table 3). The imbalance of the glutathione redox state in shoot cells seems to be related to the depletion of GSH form (-37% compared to control) concomitant with the non-modified amounts of GSSG form (Table 3). In root cells, the shift of glutathione redox state to the oxidized form resulted from the increase in contents of GSSG (+ 80% compared to control), more important than that of GSH (+ 26% compared to control; Table 3). In non-stressful conditions, the balance of the glutathione pool is maintained through the coordination between GR and GPX, respectively, a GSH recycling and consumer activities. However, Cd stress caused modulation of both activities, as revealed by the raised GPX (62% and 77% in roots and shoots, respectively; Fig. 3a) and GR (506% and 77% in roots and shoots, respectively; Fig. 3b) activities. However, the elevated GR activity seems insufficient to regenerate the required level of GSH. Moreover, GSH depletion was reported to be related to stress acclimation via phytochelatins (PCs) synthesis (Singh et al. 2016).
In the absence of Cd, the addition of OA to the germinating medium affected neither the glutathione pool content nor its redox status (Table 3). By contrast, OA annihilated the disturbing effect of Cd on glutathione redox state (Table 3). This achievement was consequent to the correction of GSH and GSSG cell contents (Table 3). The maintaining of GSH redox homeostasis was attended with the restoration of GPX (Fig. 3a) and GR (Fig. 3b) activities.
In conjunction with glutathione, nicotinamides are redox-active compounds with a crucial role in oxidative stress tolerance in plant cells. Nicotinamides are implicated in the regulation of NADPH oxidase activities and the glutathione-ascorbate cycle, besides their involvement in energy metabolism (Calvin cycle, Krebs cycle, glycolysis), as coenzymes, and fatty acid synthesis (Potters et al. 2010; Kapoor et al. 2015).
The present investigation showed that Cd did not affect nicotinamides redox state, evaluated by oxidized forms (NAD+ + NADP+) to reduced forms (NADH + NADPH), in root cells, while the metal elevated this parameter in shoot cells by 37% (Fig. 4a), suggesting an acceleration in nicotinamides oxidation. Exogenous OA counteracted the adverse effect of Cd on root nicotinamides homeostasis, manifested by the restoration of the balance between oxidized and reduced forms (Fig. 4a) with the same extent as the case of Cd stress abruption (Cd + H2O). Generally, the total amounts of nicotinamide coenzymes were unchanged independently of the applied treatment, except for the increased content in root cells under Cd and Cd + OA (+ 23 and + 45% compared to control; Fig. 4b). This could be explained by de novo synthesis of nicotinamides and changes in their degradation. A similar increase in total nicotinamides was observed in Cd-stressed chickpea seedlings subjected to an exogenous supply of EGTA, a synthetic chelating agent (Sakouhi et al. 2016). The noticed disruption of nicotinamides redox state after Cd treatment could be assigned to the potential rise of prooxidant activities such as NAD(P)H oxidases (Cha et al. 2014) and, as herein, the increased GR activity (Fig. 3b), known as NAD(P)H consumer (Fig. 3a). A similar corrective effect on coenzymes redox state was noticed after citric acid application to pea seedlings subjected to Cu stress (Ben Massoud et al. 2019). The authors ascribed this positive effect to the restoration of the balance between consuming NAD(P)H oxidases and recycling (dehydrogenases) coenzymes activities.
Nicotinamide redox ratio, evaluated as oxidized forms to total (a) and total nicotinamides content [NAD(H) + NADP(H)] (b) in roots and shoots of chickpea seedlings after seeds imbibition in H2O or 200 µM Cd in the presence or absence of 100 µM OA. Values (± SE, n = 3) followed by a common letter in the same seedling part are not different at the 0.05 level of significance using ANOVA followed by Tukey’s test
To illustrate correlations among growth and metabolic attributes between different treatments, Pearson’s coefficients were presented in a correlogram, as shown in Fig. 5. The obtained correlogram indicated that growth, estimated by the dry weight (DW), manifested a high negative correlation with Cd content in roots (r = − 0.93; p < 0.05) and shoots (r = − 0.92; p < 0.05). Cd content, in turn, was highly correlated to H2O2 accumulation in shoots (r = 0.95; p < 0.05) but no significant correlation was observed in roots (r = 0.87; p > 0.05). Besides, this analysis indicated that protein carbonylation (–CO) was proportional to the Cd content in roots (r = 0.90; p < 0.05), while in shoots, the relationship between Cd and lipid peroxidation (MDA, r = 0.91; p < 0.05) was conspicuous. As judged by these results, it becomes clear that the ameliorative effect of OA on seedling growth, at least in part, was mediated by the reduction of Cd accumulation, leading to the decrease of ROS accrual and the protection of biomolecules from oxidation.
Correlograms of Pearson’s correlation coefficients between studied parameters in roots and shoots from the control (H2O), OA, Cd, Cd + H2O and Cd + OA treatment’s group. Data with similar patterns are grouped together and presented in the form of circles with color intensity in proportion to the Pearson’s coefficient values. Blue colored circles indicate positive correlations, while red colored circles indicate negative correlations. Abbreviation: Co., coenzymes
Interestingly, Pearson’s correlations revealed an interconnection between H2O2, GPX, GR, GSH and coenzymes redox ratio. Hydrogen peroxide was significantly negatively correlated to GSH/GSSG ratio (r = − 0.88; p < 0.05), GSH content (r = − 0.95; p < 0.01) and nicotinamides ratio (r = 0.90; p < 0.05) in shoots, while it was positively correlated with GPX (r = 0.85; p < 0.05), GR (r = 0.87; p < 0.001) in roots. This finding may be explained by the involvement of GPX activity in the transformation of H2O2 to H2O using GSH as a substrate (Mittova et al. 2003). Concomitantly, GR activity, accompanied by oxidation of NADPH, was enhanced to regenerate GSH (Gill et al. 2013). Moreover, it has been suggested that glutathione redox change, as a cellular redox state cue, may play an essential role in ROS signaling pathway (Foyer and Noctor 2011).
Conclusions
The present study fills a gap in understanding the OA-mediated mechanisms to ameliorate plant tolerance to HM stress. Our results suggested that OA acts to limit penetration of the metal into the plant tissues and mitigates oxidative stress by maintaining the redox homeostasis of glutathione and nicotinamides. Nevertheless, thorough investigations are needed to elucidate the precise pathways triggered by OA to improve plant resistance to adverse conditions and, notably, extend the assays to soil conditions to unravel a prospective soil-OA interaction.
References
Agnello AC, Huguenot D, Hullebusch EDV, Esposito G (2014) Enhanced phytoremediation: a review of low molecular weight organic acids and surfactants used as amendments. Crit Rev Environ Sci Technol 44:2531–2576. https://doi.org/10.1080/10643389.2013.829764
Ahmad P, Jaleel CA, Salem MA et al (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol. https://doi.org/10.3109/07388550903524243
Ben Massoud M, Karmous I, Ferjani EE, Chaoui A (2018) Alleviation of copper toxicity in germinating pea seeds by IAA, GA3, Ca and citric acid. J Plant Int 13:21–29. https://doi.org/10.1080/17429145.2017.1410733
Ben Massoud M, Sakouhi L, Chaoui A (2019) Effect of plant growth regulators, calcium and citric acid on copper toxicity in pea seedlings. J Plant Nutr 42:1230–1242. https://doi.org/10.1080/01904167.2019.1609506
Bittencourt BMDO, Da Silva CMS, Filho SZ, Habermann G (2020) Aluminum (Al)-induced organic acid exudation in an Al-accumulating species from the Brazilian savanna. Trees 34:155–162. https://doi.org/10.1007/s00468-019-01907-5
Bolan N, Mahimairaja S, Kunhikrishnan A, Naidu R (2013) Sorption–bioavailability nexus of arsenic and cadmium in variable-charge soils. J Hazard Mater 261:725–732. https://doi.org/10.1016/j.jhazmat.2012.09.074
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cha JY, Kim JY, Jung IJ, Kim MR, Melencion A, Alam SS, Yun DJ, Lee SY, Kim MG, Kim WY (2014) NADPH-dependent thioredoxin reductase A (NTRA) confers elevated tolerance to oxidative stress and drought. Plant Physiol Biochem 80:184–191. https://doi.org/10.1016/j.plaphy.2014.04.008
Chaffai R, Elhammadi MA, Seybou TN, Tekitek A, Ferjani EE (2006) Aluminum-induced changes in organic acid and lipid content in maize (Zea mays L.). Res J Bot 1:52–63. https://doi.org/10.3923/rjb.2006.52.63
Chen YX, Lin Q, Luo YM, He YF, Zhen SJ, Yu YL et al (2003) The role of citric acid on the phytoremediation of heavy metal contaminated soil. Chemosphere 50:807–811. https://doi.org/10.1016/S0045-6535(02)00223-0
Chmielowska-Bąk J, Lefèvre I, Lutts S, Deckert J (2013) Short term signaling responses in roots of young soybean seedlings exposed to cadmium stress. J Plant Physiol 170:1585–1594. https://doi.org/10.1016/j.jplph.2013.06.019
Collins RN, Merrington G, McLaughlin MJ, Morel JL (2003) Organic ligand and pH effects on isotopically exchangeable cadmium in polluted soils. Soil Sci Soc Am J 67:112. https://doi.org/10.2136/sssaj2003.1120
Errakhi R, Meimoun P, Lehner A, Vidal G, Briand J, Corbineau F et al (2008) Anion channel activity is necessary to induce ethylene synthesis and programmed cell death in response to oxalic acid. J Exp Bot 59:3121–3129. https://doi.org/10.1093/jxb/ern166
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25. https://doi.org/10.1007/BF00386001
Foyer CH, Noctor G (2011) Ascorbate and glutathione: The heart of the redox hub. Plant Physiol 155:2–18
Fu H, Yu H, Li T, Zhang X (2018) Influence of cadmium stress on root exudates of high cadmium accumulating rice line (Oryza sativa L.). Ecotoxicol Environ Saf 150:168–175. https://doi.org/10.1016/j.ecoenv.2017.12.014
Garcia SC, Schott K, Charão M, Moro A, Bulcão R, Grotto D, Valentini J, Bohrer D, Cardoso S, Pomblum V (2008) Quantification of reduced glutathione by HPLC-UV in erythrocytes of hemodialysis patients. Biomed Chromatogr 22:460–468. https://doi.org/10.1002/bmc.954
Gill SS, Anjum NA, Hasanuzzaman M et al (2013) Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiol Biochem 70:204–212. https://doi.org/10.1016/j.plaphy.2013.05.032
Gratão PL, Alves LR, Lima LW (2019) Heavy metal toxicity and plant productivity: role of metal scavengers. In: Srivastava S, Srivastava AK, Suprasanna P (eds) Plant-metal interactions. Springer International Publishing, Cham, pp 49–60
Guo B, Liang YC, Zhu YG, Zhao FJ (2007) Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ Pollut 147:743–749. https://doi.org/10.1016/j.envpol.2006.09.007
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F et al (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681. https://doi.org/10.3390/antiox9080681
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Helper PK, Wayne RO (1985) Calcium and plant development. Annu Rev Plant Physiol 36:397–439. https://doi.org/10.1146/annurev.pp.36.060185.002145
Javed MT, Akram MS, Tanwir K et al (2017) Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol Environ Saf 141:216–225. https://doi.org/10.1016/j.ecoenv.2017.03.027
Javed MT, Saleem MH, Aslam S et al (2020) Elucidating silicon-mediated distinct morpho-physio-biochemical attributes and organic acid exudation patterns of cadmium stressed Ajwain (Trachyspermum ammi L.). Plant Physiol Biochem 157:23–37. https://doi.org/10.1016/j.plaphy.2020.10.010
Kapoor D, Sharma R, Handa N, Kaur H, Rattan A, Yadav P, Gautam V, Kaur R, Bhardwaj R (2015) Redox homeostasis in plants under abiotic stress: Role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front Environ Sci 3:13. https://doi.org/10.3389/fenvs.2015.00013
Katrusiak AE, Paterson PG, Kamencic H, Shoker A, Lyon AW (2001) Pre-column derivatization high-performance liquid chromatographic method for determination of cysteine, cysteinyl–glycine, homocysteine and glutathione in plasma and cell extracts. J Chromatogr B 758:207–212. https://doi.org/10.1016/S0378-4347(01)00182-7
Kaur R, Yadav P, Thukral AK et al (2018) Castasterone and citric acid supplementation alleviates cadmium toxicity by modifying antioxidants and organic acids in Brassica juncea. J Plant Growth Regul 37:286–299. https://doi.org/10.1007/s00344-017-9727-1
Khan MIR, Iqbal N, Masood A, Mobin M, Anjum NA, Khan NA (2016) Modulation and significance of nitrogen and sulfur metabolism in cadmium challenged plants. Plant Growth Regul 78:1–11. https://doi.org/10.1007/s10725-015-0071-9
Kharbech O, Ben Massoud M, Sakouhi L et al (2020) Exogenous application of hydrogen sulfide reduces chromium toxicity in maize seedlings by suppressing NADPH oxidase activities and methylglyoxal accumulation. Plant Physiol Biochem 154:646–656. https://doi.org/10.1016/j.plaphy.2020.06.002
Latowski D, Kruk J, Strzałka K (2005) Inhibition of zeaxanthin epoxidase activity by cadmium ions in higher plants. J Inorg Biochem 99:2081–2087. https://doi.org/10.1016/j.jinorgbio.2005.07.012
Levine RL, Williams J, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357. https://doi.org/10.1016/S0076-6879(94)33040-9
Li H, Liu Y, Zeng G et al (2014) Enhanced efficiency of cadmium removal by Boehmeria nivea (L.) Gaud. in the presence of exogenous citric and oxalic acids. J Environ Sci 26:2508–2516. https://doi.org/10.1016/j.jes.2014.05.031
Liu K, Lv J, He W et al (2015) Major factors influencing cadmium uptake from the soil into wheat plants. Ecotoxicol Environ Saf 113:207–213. https://doi.org/10.1016/j.ecoenv.2014.12.005
Luo Q, Sun L, Hu X, Zhou R (2014) The variation of root exudates from the hyperaccumulator Sedum alfredii under cadmium stress: metabonomics analysis. PLoS ONE 9(12):e115581. https://doi.org/10.1371/journal.pone.0115581
Ma H, Li X, Wei M, Zeng G, Hou S, Li D, Xu H (2020) Elucidation of the mechanisms into effects of organic acids on soil fertility, cadmium speciation and ecotoxicity in contaminated soil. Chemosphere 239:124706. https://doi.org/10.1016/j.chemosphere.2019.124706
Martin-Tanguy J (2001) Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul 34:135–148. https://doi.org/10.1023/A:1013343106574
Miller G, Coutu J, Shulaev V, Mittler R (2018) Reactive oxygen signaling in plants. Annu Plant Rev Online 33:189–201. https://doi.org/10.1002/9781119312994.apr0353
Mittova V, Theodoulou FL, Kiddle G et al (2003) Coordinate induction of glutathione biosynthesis and glutathione-metabolizing enzymes is correlated with salt tolerance in tomato. FEBS Lett 554:417–421. https://doi.org/10.1016/S0014-5793(03)01214-6
Montiel-Rozas MM, Madejón E, Madejón P (2016) Effect of heavy metals and organic matter on root exudates (low molecular weight organic acids) of herbaceous species: an assessment in sand and soil conditions under different levels of contamination. Environ Pollut 216:273–281. https://doi.org/10.1016/j.envpol.2016.05.080
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160(2):291–299. https://doi.org/10.1016/S0168-9452(00)00392-7
Najeeb U, Jilani G, Ali S, Sarwar M, Xu L, Zhou W (2011) Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J Hazard Mater 186:565–574. https://doi.org/10.1016/j.jhazmat.2010.11.037
Nedjimi B, Daoud Y (2009) Cadmium accumulation in Atriplex halimus subsp. schweinfurthii and its influence on growth, proline, root hydraulic conductivity and nutrient uptake. Flora Morphol Distrib Funct Ecol Plants 204:316–324. https://doi.org/10.1016/j.flora.2008.03.004
Pacifici RE, Davies KJ (1990) Protein degradation as an index of oxidative stress. Meth Enzymol 186:485–502. https://doi.org/10.1016/0076-6879(90)86143-J
Potters G, Horemans N, Jansen MA (2010) The cellular redox state in plant stress biology: a charging concept. Plant Physiol Biochem 48:292–300. https://doi.org/10.1016/j.plaphy.2009.12.007
Rahoui S, Chaoui A, Ben C, Rickauer M, Gentzbittel L, El Ferjani E (2015) Effect of cadmium pollution on mobilization of embryo reserves in seedlings of six contrasted Medicago truncatula lines. Phytochemistry 111:98–106. https://doi.org/10.1016/j.phytochem.2014.12.002
Romero-Puertas MC, Rodríguez-Serrano M, Sandalio LM (2013) Protein S-nitrosylation in plants under abiotic stress: an overview. Front Plant Sci. https://doi.org/10.3389/fpls.2013.00373
Ruiz LP, Mansfield TA (1994) A postulated role for calcium oxalate in the regulation of calcium ion in the vicinity of stomatal guard cells. New Phytol 127:473–781. https://doi.org/10.1111/j.1469-8137.1994.tb03965.x
Sabir M, Hanafi MM, Zia-Ur-Rehman M et al (2014) Comparison of low-molecular-weight organic acids and ethylenediaminetetraacetic acid to enhance phytoextraction of heavy metals by maize. Commun Soil Sci Plant 45:42–52. https://doi.org/10.1080/00103624.2013.848879
Sakouhi L, Kharbech O, Massoud MB et al (2021) Calcium and ethylene glycol tetraacetic acid mitigate toxicity and alteration of gene expression associated with cadmium stress in chickpea (Cicer arietinum L.) shoots. Protoplasma. https://doi.org/10.1007/s00709-020-01605-x
Sakouhi L, Rahoui S, Gharsallah C, Munemasa S, El Ferjani E, Murata Y, Chaoui A (2018) Effects of calcium and EGTA on thiol homeostasis and defense-related enzymes in Cd-exposed chickpea roots. Acta Physiol Plant 40(1):20. https://doi.org/10.1007/s11738-017-2596-1
Sakouhi L, Rahoui S, Massoud MB, Munemasa S, Ferjani EE, Murata Y, Chaoui A (2016) Calcium and EGTA alleviate cadmium toxicity in germinating chickpea seeds. J Plant Growth Regul 35:1064–1073. https://doi.org/10.1007/s00344-016-9605-2
Seregin IV, Ivanov VB (2001) Physiological aspects of cadmium and lead toxic effects on higher plants. Russ J Plant Physiol 48:523–544. https://doi.org/10.1023/A:1016719901147
Sergiev I, Alexieva V, Karanov E (1997) Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt Rend Acad Bulg Sci 51:121–124
Sidhu GPS, Bali AS, Bhardwaj R (2019) Role of organic acids in mitigating cadmium toxicity in plants. In: Hasanuzzaman M, Vara Prasad MN, Nahar K (eds) Cadmium tolerance in plants. Academic Press, Cambridge, pp 255–279
Singh S, Tripathi DK, Chauhan DK, Dubey NK (2016) Glutathione and phytochelatins mediated redox homeostasis and stress signal transduction in plants: an integrated overview. In: Ahmad P (ed) Plant metal interaction. Elsevier, Amsterdam, pp 285–310
Smirnoff N (2000) Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc B 355:1455–1464. https://doi.org/10.1098/rstb.2000.0706
Song JF, Markewitz D, Wu S, Sang Y, Duan C, Cui XY (2018) Exogenous oxalic acid and citric acid improve lead (Pb) tolerance of Larix olgensis A. Henry Seed For 9:510. https://doi.org/10.3390/f9090510
Soyingbe OS, Ntanzi C, Makhafola TJ, Kappo AP (2020) Ameliorative effect of exogenously applied oxalic acid on nickel (heavy metal) induced stress in Zea mays. Pak J Bot 52(2):413–418. https://doi.org/10.30848/PJB2020-2(14)
Stadtman ER, Levine RL (2000) Protein oxidation. Ann N Y Acad Sci 899:191–208. https://doi.org/10.1111/j.1749-6632.2000.tb06187.x
Sun L, Cao X, Tan C et al (2020) Analysis of the effect of cadmium stress on root exudates of Sedum plumbizincicola based on metabolomics. Ecotoxicol Environ Saf 205:111152. https://doi.org/10.1016/j.ecoenv.2020.111152
Xu W, Li Y, He J, Ma Q, Zhang X, Chen G, Wang H, Zhang H (2010) Cd uptake in rice cultivars treated with organic acids and EDTA. J Environ Sci 22(3):441–447. https://doi.org/10.1016/S1001-0742(09)60127-3
Zagorchev L, Seal CE, Kranner I, Odjakova M (2013) A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci 14:7405–7432. https://doi.org/10.3390/ijms14047405
Zaheer IE, Ali S, Rizwan M, Farid M, Shakoor MB, Gill RA, Najeeb U, Iqbal N, Ahmad R (2015) Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol Environ Saf 120:310–317. https://doi.org/10.1016/j.ecoenv.2015.06.020
Zaid A, Mohammad F (2018) Methyl jasmonate and nitrogen interact to alleviate cadmium stress in Mentha arvensis by regulating physio-biochemical damages and ROS detoxification. J Plant Growth Regul 37:1331–1348. https://doi.org/10.1007/s00344-018-9854-3
Zaid A, Wanni SH (2019) Reactive oxygen species generation, scavenging and signaling in plant defense responses. In: Jogaiah S, Abdelrahman M (eds) Bioactive molecules in plant defense signaling in growth and stress. Springer Nature, Dordrecht
Zhang X, Chen J, Liu X et al (2019) The relief effects of organic acids on Scirpus triqueter L. under pyrene–lead stress. Environ Sci Pollut Res 26:15828–15837. https://doi.org/10.1007/s11356-019-04976-8
Zhao Z, Hu X, Ross CW (1987) Comparison of tissue preparation methods for assay of nicotinamide coenzymes. Plant Physiol 84(4):987–988. https://doi.org/10.1104/pp.84.4.987
Zhu XF, Zheng CL, Hu YT, Jiang T, Liu Y, Dong NY et al (2011) Cadmium-induced oxalate secretion from root apex is associated with cadmium exclusion and resistance in Lycopersicon esulentum. Plant Cell Environ 34:1055–1064. https://doi.org/10.1111/j.1365-3040.2011.02304.x
Webb MA, Cavaletto JM, Carpita NC, Lopez LE, Amott HJ (1995) The intravacuolar organic matrix associated with calcium oxalate crystals in the leaves of Vitis. Plant J 7:633–648. https://doi.org/10.1046/j.1365-313X.1995.7040633.x
Acknowledgements
This work was financially supported by the Tunisian Ministry of High Education and Scientific Research (LR18ES38). The authors acknowledge Mr. Abbes Oucherine for technical assistance.
Author information
Authors and Affiliations
Contributions
LS performed all laboratory experiments, statistical analyzed the data and wrote the manuscript; OK and MBM helped in manuscript drafting and revised the language and the statistical analysis; SM and YM revised critically the manuscript; AC supervised the experiments, helped in the physiological analyses and revision step. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Handling Editor: Rhonda peavy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakouhi, L., Kharbech, O., Massoud, M.B. et al. Oxalic Acid Mitigates Cadmium Toxicity in Cicer arietinum L. Germinating Seeds by Maintaining the Cellular Redox Homeostasis. J Plant Growth Regul 41, 697–709 (2022). https://doi.org/10.1007/s00344-021-10334-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10334-1