Abstract
Impact of exogenous calcium and ethylene glycol tetraacetic acid (EGTA) supplement on chickpea (Cicer arietinum L.) germinating seeds exposed to cadmium stress for 6 days was studied. Ca and EGTA late treatment (3 days) alleviated growth inhibition and decreased Cd accumulation as well as lipid peroxidation and protein carbonylation in both root and shoot cells. Exogenous effector application relieved Cd-induced cell death which was associated with a constant level of ATP, which was considered as an apoptotic-like process. Redox balance was examined through the study of the redox state of pyridine nucleotide couples NAD+/NADH and NADP+/NADPH as well as their related oxidative [NAD(P)H-oxidase] and dehydrogenase (glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase and malate dehydrogenase) enzyme activities. The present research illustrated an ameliorative effect of Ca and EGTA on growth of Cd-exposed chickpea seedlings that occurs through the protection of sensitive cell sites from Cd-induced oxidation, namely membrane lipids and proteins, rather than the improvement of recycling capabilities of the cellular reducing power.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Germination is crucial in the life of a plant and is very sensitive to metal stress because the seed is the first interface of material exchange between the plant and the environment (Ernst 1998). In germination and early seedlings, key processes such as capacity of moisture, resumption of respiration, reserve mobilization, and nutrient availability are depressed under metal stress resulting in growth inhibition in several plant species (Hasan and others 2008; Karmous and others 2012; Rahoui and others 2014, 2015).
Metal-induced membrane injury in plants is exemplified by increased solute leakage in germination media (Rahoui and others 2014; Chaoui and El Ferjani 2014). Lipid degradation product enhancement is often observed in metal-exposed plants (Meng and others 2009; Chaoui and El Ferjani 2014). Increases in lipid peroxides in seedlings were reported to be related to reactive oxygen species (ROS) over-production inducing antioxidant status changes and oxidative stress establishment (Rahoui and others 2014). Cell antioxidative systems tend to maintain intracellular homeostasis, through trace element availability control in the tissues and ROS-scavenging enzyme enhancement as well as non-enzymatic antioxidant components in order to prevent ROS toxic accumulation (Hu and others 2007; Chaoui and El Ferjani 2014). Tolerance to metal toxicity is also dependent on reduced cell metabolite availability such as pyridine nucleotide coenzymes, NAD(P)H, which are recycled by dehydrogenase activities, namely glucose-6-phosphate dehydrogenase (G6PDH), 6-phosphogluconate dehydrogenase (6PGDH), malate dehydrogenase (MDH), and isocitrate dehydrogenase (ICDH) (León and others 2002). Pyridine nucleotides are essential cofactors for different antioxidative enzymes such as glutathione reductase, a key enzyme in the ascorbate–glutathione cycle (Noctor and others 2006) and NADPH-dependent thioredoxin reductase (Cha and others 2014). Besides their role as cofactors in ROS-scavenger enzymatic systems, nicotinamides are key brokers in redox signaling by modulating not only ROS generating enzymes, such as NAD(P)H oxidases, but also nitric oxide species (NOS) signaling (Noctor and others 2006).
To cope with metal phytotoxicity, numerous chemicals have been used as exogenous treatments during germination and early seedling growth (Islam and others 2009; Ali and others 2014; Chaoui and El Ferjani 2014). Calcium, as an ionic competitor, can afford a protective role against Cd (Wang and Song 2009; Tian and others 2011). In fact, Ca can be involved in the signal transduction of environmental stimuli and related gene expression in plants, increasing, thereby, plant tolerance (Bramm 1992).
On the other hand, the literature has reported that organic and synthetic chelates, namely ethylene diamine tetraacetic acid (EDTA), diethylene triamine pentaacetic acid (DTPA) and citric, malic, and oxalic acids can form chemical complexes with metallic trace elements counteracting, whereby, their availability and accumulation in plants (Xu and others 2010). However, data relating the mechanism of effects of exogenously applied Ca and ethylene glycol tetraacetic acid (EGTA), a synthetic chelating agent, on metal toxicity are scarce.
The present work intended to measure growth, cadmium content, oxidative injury indicators (malondialdehyde; MDA and carbonyl groups), cell death, intracellular ATP, and changes in redox state of pyridine nucleotide coenzymes and their related recycling enzyme activities in chickpea (Cicer arietinum L.).
Materials and Methods
Germination Conditions
Seeds of chickpea (Cicer arietinum L. cv. Béja 1) were disinfected with 2 % sodium hypochlorite for 10 min and then rinsed thoroughly. Seeds were germinated at 25 °C in the dark over two sheets of filter paper moistened with distilled water or aqueous solutions of 200 µM CdCl2. After 3 days, germinating seeds were subjected to treatments as described in Table 1. Cadmium concentration was chosen on the basis of preliminary experiments showing the level at which embryo growth decreased about half of controls. For each treatment, 6-day-old embryonic axes were harvested. Shoots and roots were separated, length and fresh weight (FW) were measured, and samples were (1) stored at −80 °C for biochemical analyses or (2) dried for 15 days at 70 °C for dry weight (DW) estimation and cadmium content determination.
Cadmium Content Determination
After wet digestion of the oven-dried seedlings (10 ml per 0.1 g dry weight) with an acid mixture (HNO3:HClO4, 4:1), the concentrations of Cd were determined by an atomic absorption spectrophotometer (Perkin Elmer, Waltham MA, USA). Sigma Diagnostic Standards (Cadmium Atomic Absorption Solutions, Sigma-Aldrich) were diluted in appropriate ranges with 0.1 N HNO3 (65 % HNO3, Sigma-Aldrich, Analytical grade) and used for calibration.
Lipid Peroxides Estimation
Lipoperoxidation products were estimated by measuring the concentration of MDA, the major thiobarbituric acid-reactive substance as described by Heath and Packer (1968).
Protein Extraction
Fresh tissues were homogenized in 50 mM K-phosphate buffer pH 7.0, supplemented with 1 mM EDTA. The homogenate was centrifuged at 10,000×g for 15 min at 4 °C. The resulting supernatant was used as soluble fraction for measurement of carbonyl contents and enzyme activities. Protein contents were measured using BSA as a standard (Bradford 1976).
Carbonyl Content Determination
Carbonyl group assays were carried out according to Levine and others (1994) using a spectrophotometric DNPH (2,4-dinitrophenylhydrazine) method.
Enzyme Activities Assay
NADH-oxidase (EC 1.6.3.4) and NADPH-oxidase (EC 1.6.3.1) activities were determined in a buffer containing 100 mM sodium acetate (pH 6.5), 1 mM MnCl2, 0.5 mM p-coumaric acid, 0.5 mM NADH or 0.2 mM NADPH, respectively, and protein extract. NAD(P)H oxidation was observed considering absorbance decrease at 340 nm (ɛ = 6.22 mM−1 cm−1; Ishida and others 1987).
Glucose-6-phosphate dehydrogenase (G6PDH; EC 1.1.1.49) and 6-phosphogluconate dehydrogenase (6PGDH; EC 1.1.1.44) activities were measured according to Lozano and others (1996) by monitoring the reduction of NADP at 340 nm (ε = 6.22 mM−1 cm−1) in a buffer containing 50 mM Tris-HC1 (pH 7.9), 5 mM MgCI2, 0.25 mM NADP, 100 mM glucose-6-phosphate or 100 mM 6-phosphogluconate, respectively, and protein extract.
MDH (EC 1.1.1.37) activity was estimated following the increase of the NADH absorbance at 340 nm (ε = 6.22 mM−1 cm−1) in 400 mM hydrazine sulfate (pH 9.0) buffer containing 500 mM glycine, 2.5 mM NAD, 50 mM malate, 10 mM MgCl2, and protein extract (Bergmeyer and Bernt 1974).
Cell Death Estimation
Cells viability changes were estimated spectrophotometrically as Evans Blue uptake (Romero-Puertas and others 2004). Roots and shoots were infiltrated with an aqueous solution of 0.25 % Evans Blue then boiled in 80 % ethanol for 5 min. The samples were oven-dried and then incubated in 50 % methanol with 1 % (w/v) sodium dodecyl sulfate (SDS) at 60 °C for 30 min to extract dye bound to dead cells which was quantified by absorbance at 600 nm.
ATP Assay
ATP determination was performed according to the protocol supplied by the manufacturer (Promega Corporation, Madison, USA).
Freshly harvested samples (0.5 g) were immediately homogenized with 5 mL of ice-cold 2.5 % trichloroacetic acid (TCA) supplemented with 10 mM EDTA. The extracts were centrifuged at 10,000×g for 10 min at 4 °C. The resulting supernatant was diluted with 1 mol L−1 Tris–acetate buffer (pH 7.75) for neutralization.
Intracellular ATP levels were measured using an ATP Bioluminescence Detection Kit (Promega Corporation, Madison, USA). Luminescence was measured using a Luminometer (model AB-2200-R, Atto Corporation, Japan). ATP content was calculated from an ATP standard curve and expressed as nmol mg protein−1.
NADP(H) and NAD(H) Extraction and Concentration Determination
Oxidized forms of nicotinamide (NADP+ and NAD+) were extracted in 0.2 N HCl, whereas reduced forms (NADPH and NADH) were extracted in 0.2 N NaOH. Each homogenate was heated in a boiling water bath for 5 min, cooled, and centrifuged at 10,000×g at 4 °C for 10 min (Zhao and others 1987). Supernatants were used for enzyme cycling assays with 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT) as the terminal electron acceptor. A cycling mixture of 10 mM glycine (pH 9.5), 1 mM MTT, 0.5 mM phenazine ethosulfate (PES), 100 mM ethanol, and 1 U mL−1 alcohol dehydrogenase (ADH) was used to determine NAD+ and NADH contents, whereas for NADP+ and NADPH, 5 mM glucose-6P and 0.5 U mL−1 G6PDH replaced ethanol and ADH. MTT reduction rate was recorded as absorbance at 570 nm and is proportional to the concentration of coenzyme (Matsumura and Miyachi 1980). Standard curves were performed for each nicotinamide forms.
Statistics
Significances were tested by ANOVA, and the results are expressed as the mean values ± standard error (SE) obtained from at least three replicates. A probability of P < 0.05 was considered significant.
Results and Discussion
Seedling Growth and Cd Accumulation
Cd reduced growth estimated by length (Fig. 1a) and fresh weight (Fig. 1b). Similar results were reported in legumes (Hasan and others 2008; Chaoui and El Ferjani 2014). Seedling vulnerability to metal stress was explained as the consequence of disturbance of metabolic pathways mainly reserve mobilization disorders, that is, transport to sink tissues, and impairment of reserve mobilization related activities (Karmous and others 2012; Rahoui and others 2014, 2015).
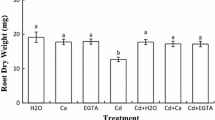
Root and shoot length (a) and fresh weight (b) of chickpea seedlings after imbibition in H2O or 200 µM CdCl2 in the presence or absence of 10 mM CaCl2 and 100 µM EGTA. Values are the averages of 20 individual measurements. Values (±SE) followed by a common letter in the same seedling part are not different at the 0.05 level of significance
The addition of Ca and EGTA to the germinating medium, in the absence of Cd, did not affect significantly seedling growth, whereas they restored considerably growth in Cd-stressed seedlings (100 % in the case of Cd + Ca/FW, Fig. 1b).
These results are in agreement with previous studies suggesting a protective role for Ca against metal stress (Wang and Song 2009; Farzadfar and others 2013). This finding may be attributed to metal accumulation reduction (Fig. 2). Several reports provide evidence that application of Cd resulted in a decrease of Ca cytosolic content (Wang and Song 2009), whereas the adverse effect of Cd on Ca levels was reversed by exogenous Ca application (Tian and others 2011; Farzadfar and others 2013). Furthermore, it has been reported that Ca deficiency enhanced Cd toxicity in rice seedlings (Cho and others 2012), whereas exogenous Ca application alleviated Cd stress in plants (Wan and others 2011). The antagonistic effects of cadmium and calcium on their accumulation in plant tissues were attributed to reduction of cell-surface negativity of the plasma membrane by displacement of the metal by Ca2+ (Kinraide 1998) and to competition for Ca channels (Perfus-Barbeoch and others 2002) because Cd accumulation was inhibited by Ca channel blockers such as nifedipine, diltiazem, verapamil, and nitrendipine (Blazka and Shaikh 1991).
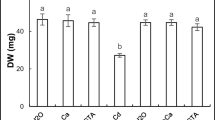
Root and shoot cadmium content of chickpea seedlings after imbibition in 200 µM CdCl2 in the presence or absence of 10 mM CaCl2 and 100 µM EGTA. Values are the averages of four individual measurements. Each measurement was performed in an extract obtained from several seedlings. Values (±SE) followed by a common letter in the same seedling part are not different at the 0.05 level of significance
Chelating agents have been used in phytoremediation by phytoextraction to decontaminate soils using trace element accumulator plants. In this context, Van Engelen and others (2007) reported an increase in trace element accumulation in plant tissues following the addition of chelates such as malic acid, oxalic acid, EGTA, and EDTA.
However, our results showed a decrease in Cd accumulation subsequent to EGTA addition (Fig. 2) protecting thereby seedlings from Cd-induced growth damage (Fig. 1). In agreement with our finding, it has been reported that Cd concentration decreased with increasing concentration of organic chelators (EDTA and DTPA) suggesting that Cd and chelators form a Cd-chelator complex with high molecular weight and stability, leading to reduced metal exchange and thereby restraining Cd availability (Sun and others 2005; Xu and others 2010).
The controversial role of chelating agents in Cd accumulation can be attributed to differences relating to metals, plant species, and applied doses.
Oxidative Stress Markers: MDA and CO Groups
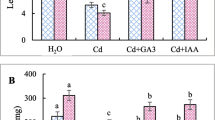
Cd induced a huge increase of the levels of MDA and protein carbonyl groups (by 64 and 72 % for MDA and by 360 and 647 % for CO groups in roots and shoots, respectively) (Fig. 3).
MDA (a) and carbonyl groups (b) contents in roots and shoots of chickpea seedlings after imbibition in H2O or 200 µM CdCl2 in the presence or absence of 10 mM CaCl2 and 100 µM EGTA. Values are the averages of four individual measurements. Each measurement was performed in an extract obtained from several seedlings. Values (±SE) followed by a common letter in the same seedling part are not different at the 0.05 level of significance
This increase has significantly reduced in the presence of effectors that act in the same way, almost; MDA levels were similar to control roots (Fig. 3a) and CO groups were almost 38 % of Cd-treated seedlings content (Fig. 3b). However, Ca and EGTA treatments applied alone have no significant effect on either parameter.
A similar concomitant increase of MDA and CO groups has been reported under metal stress, suggesting oxidative stress establishment as a result of ROS accumulation (Chaoui and El Ferjani 2014). In fact, ROS can affect protein functionality either by amino acid oxidation or by secondary reactions with lipid peroxidation aldehydic products (Reinheckel and others 1998). Thereby, carbonyl groups and MDA production are considered evidence of oxidative stress.
Ca and EGTA addition significantly counteracted lipoperoxide and carbonyl groups rising in both parts of Cd-poisoned seedlings (Fig. 3). Similar ameliorative effects of exogenous Ca, under Cd stress on membrane integrity have been reported in Trifolium repens (Wang and Song 2009), Matricaria chamomilla (Farzadfar and others 2013), and Sedum alfredii (Tian and others 2011). Triticum aestivum exhibited a significant MDA accumulation decrease after organic chelator additions to Cd-treated solutions (Sun and others 2005).
Cell Death and ATP Content
The increase of oxidative stress indicators in Cd-stressed chickpea seedlings (Fig. 3) was associated with an increase by 78 and 70 % of control in roots and shoots, respectively, in Evans Blue accumulation indicating dead cell number rise (Fig. 4a), whereas both Ca and EGTA application counteracted the damaging effect of Cd on cell viability. Cell death may be the result of two processes, either necrosis (Van Breusegem and Dat 2006) or apoptosis (Lam 2004). Leist and others (1997) suggested that the determination of the intracellular ATP content may indicate which process in cell death is occurring. Indeed, unlike necrosis, apoptosis required constant ATP levels (Atlante and others 2005).
Cell death (a) estimated as Blue Evans uptake (expressed as percent from control) and intracellular ATP levels (b) in roots and shoots of chickpea seedlings after imbibition in H2O or 200 µM CdCl2 in the presence or absence of 10 mM CaCl2 and 100 µM EGTA. Values are the averages of four individual measurements. Each measurement was performed in an extract obtained from several seedlings. Values (±SE) followed by a common letter in the same seedling part (a) and all values of intracellular ATP content (b) are not different at the 0.05 level of significance
The constant level of ATP, irrespective of the presence or the absence of Cd, Ca, and EGTA, noticed in chickpea tissues (Fig. 4b) suggests that the observed cell death was an apoptotic-like process. In line with the present investigation, Casolo and others (2005) reported in soybean suspension cells subjected to H2O2 and NO− stresses a constant level of ATP in apoptotic cells, whereas it decreased in necrotic cells.
Redox State and Related Enzyme Activities
To cope with potential damage that could result from ROS over-production, plants have developed antioxidant defenses including enzymatic and non-enzymatic scavengers. Additionally, plant cell tolerance to oxidative stress depends on its ability to generate redox-active metabolites. Thus, there is great interest in the study of cell redox homeostasis.
The pyridine nucleotides NADH and NADPH and their oxidized forms NAD+ and NADP+ are important redox-active components in plant cells acting as coenzymes in numerous cellular processes such as energy metabolism (Krebs cycle, Calvin cycle, glycolysis), biosynthesis (fatty acid synthesis), and defense against oxidative stress (Potters and others 2010). Several studies suggested that NADP(H) and NAD(H) are involved in redox signaling in animals, plants, and fungi (Ziegler 2005) through regulation of ROS-producing such as NADP(H) oxidases and ROS-consuming systems like glutathione-ascorbate cycle (Kapoor and others 2015).
The present study showed that Cd stimulates both oxidase (Fig. 5) and reductase activities, except MDH in shoots (Fig. 6). Thus, the redox ratio remains unchanged under Cd stress (Fig. 7b) as well as total coenzymes in shoots, whereas this parameter increases slightly in roots (Fig. 7a).
NADH-oxidase (a) and NADPH-oxidase (b) activities in roots and shoots of chickpea seedlings after imbibition in H2O or 200 µM CdCl2 in the presence or absence of 10 mM CaCl2 and 100 µM EGTA. One unit of enzyme was defined as the amount necessary to decompose 1 µmol of NAD(P)H per min at 25 °C. Values are the averages of four individual measurements (±SE). Each measurement was performed in an extract obtained from several seedlings. Values (±SE) followed by a common letter in the same seedling part are not different at the 0.05 level of significance
6PGDH (a), G6PDH (b) and MDH (c) activities in roots and shoots of germinating chickpea seeds after imbibition in H2O or 200 µM CdCl2 in the presence or absence of 10 mM CaCl2 and 100 µM EGTA. One unit of enzyme was defined as the amount necessary to produce 1 µmol of NAD(P)H per min at 25 °C. Values are the averages of four individual measurements (±SE). Each measurement was performed in an extract obtained from several seedlings. Values (±SE) followed by a common letter in the same seedling part (a, b) and values of MDH activity in shoots (c) are not different at the 0.05 level of significance
Total nicotinamide content [NAD(H) + NADP(H)] (a) and nicotinamide redox ratio, evaluated as oxidized forms to total (b) in roots and shoots of chickpea seedlings after imbibition in H2O or 200 µM Cd in the presence or absence of 10 mM CaCl2 and 100 µM EGTA. Values are the averages of four individual measurements. Each measurement was performed in an extract obtained from several seedlings. Values (±SE) followed by a common letter in the same seedling part (b) and values of total nicotinamides content in shoots (a) are not different at the 0.05 level of significance
A similar response pattern was observed in Cd-stressed seedlings subjected to Ca and EGTA treatments. Total coenzyme content is constant in shoots, whereas it increases even more in roots by about 45 % relative to controls (Fig. 7a). Likewise, the redox ratio is constant in roots, whereas in shoots it increases by 45 % for Cd + Ca and 50 % for Cd + EGTA compared to Cd (Fig. 7b). This rise occurred, despite the concomitant decrease of oxidases (Fig. 5) and reductases (Fig. 6) except MDH activity which was unchanged for all the treatments (Fig. 6c).
Under Cd stress, maintenance of redox homeostasis, can be attributed, at least in part, to an increase in NADPH-recycling activity, mainly G6PDH and 6PGDH, in spite of the concomitant increase of oxidase activities.
NADP-dehydrogenase activity stimulation was reported in different species and under several stresses, namely in olive plant (Valderrama and others 2006) and pepper plant (León and others 2002) exposed to salt and cadmium stress, respectively. Scharte and others (2009), using a genetic approach involving G6PDH isozyme over-expression, demonstrated an improvement in NADPH provision for NADPH-oxidases during the early oxidative burst subsequent to Phytophthora nicotianae pathogen infection in tobacco leaves.
Increased redox ratios after Ca and EGTA treatments, suggesting a decrease in reduced coenzyme forms, could be attributed to the potential increase of the antioxidant enzyme activity NAD(P)H consumer such as glutathione reductase. Data relating exogenous effector impact to redox status are scarce or even non-existent. Therefore, our research work presents a novelty in metal stress-alleviation studies that necessitates further investigations.
Conclusions
The present study exemplified that the protective effect of Ca and EGTA against the detrimental impact of Cd on growth, could not be mediated by improvement of recycling capabilities of the cellular reducing power, but rather by a possible protection of sensitive cell sites to oxidation imposed by Cd, namely, membrane lipids and proteins.
References
Ali B, Xu X, Gill RA, Yang S, Ali S, Tahir M, Zhou W (2014) Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Ind Crop Prod 52:617–626
Atlante A, Giannattasio S, Bobba A, Gagliardi S, Petragallo V, Calissano P, Marra E, Passarella S (2005) An increase in the ATP levels occurs in cerebellar granule cells en route to apoptosis in which ATP derives from both oxidative phosphorylation and anaerobic glycolysis. Biochim Biophys Acta 1708(1):50–62
Bergmeyer HU, Bernt E (1974) Malate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press Inc, New York, pp 1577–1580
Blazka ME, Shaikh ZA (1991) Differences in cadmium and mercury uptakes by hepatocytes: role of calcium channels. Toxicol Appl Pharmacol 110:355–363
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Bramm J (1992) Regulated expression of the calmodulin-related TCH genes in cultured Arabidopsis cells: induction by calcium and heat shock. Proc Natl Acad Sci USA 89:3213–3216
Casolo V, Petrussa E, Krajnáková J, Macri F, Vianello A (2005) Involvement of the mitochondrial K+ ATP channel in H2O2- or NO-induced programmed death of soybean suspension cell cultures. J Exp Bot 56:997–1006
Cha JY, Kim JY, Jung IJ, Kim MR, Melencion A, Alam SS, Yun DJ, Lee SY, Kim MG, Kim WY (2014) NADPH-dependent thioredoxin reductase A (NTRA) confers elevated tolerance to oxidative stress and drought. Plant Physiol Biochem 80:184–191
Chaoui A, El Ferjani E (2014) Heavy metal-induced oxidative damage is reduced by β -Estradiol application in lentil seedlings. Plant Growth Regul 74:1–9
Cho SC, Chao YY, Kao CH (2012) Calcium deficiency increases Cd toxicity and Ca is required for heat-shock induced Cd tolerance in rice seedlings. J Plant Physiol 169:892–898
Ernst W (1998) Effects of heavy metals in plants at the cellular and organismic level ecotoxicology. In: Gerrit S, Bernd M III (eds) Bioaccumulation and biological effects of chemicals. Wiley and Spektrum Akademisher Verlag, Berlin, pp 587–620
Farzadfar S, Zarinkamar F, Modarres-Sanavy SAM, Hojati M (2013) Exogenously applied calcium alleviates cadmium toxicity in Matricaria chamomilla L. plants. Environ Sci Pollut Res 20:1413–1422
Hasan SA, Hayat S, Ali B, Ahmad A (2008) 28-homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidant. Environ Pollut 151:60–66
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hu KD, Hu LY, Li YH, Zhang FQ, Zhang H (2007) Protective roles of nitric oxide on germination and antioxidant metabolism in wheat seeds under copper stress. Plant Growth Regul 53:173–183
Ishida A, Ookubo K, Ono K (1987) Formation of hydrogen peroxide by NAD(P)H oxidation with isolated cell wall-associated peroxidase from cultured liverwort cells, Marchantia polymorpha L. Plant Cell Physiol 28:723–726
Islam MM, Hoque MA, Okuma E, Banu MNA, Shimoishi Y, Nakamura Y, Murata Y (2009) Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol 166(15):1587–1597
Kapoor D, Sharma R, Handa N, Kaur H, Rattan A, Yadav P, Gautam V, Kaur R, Bhardwaj R (2015) Redox homeostasis in plants under abiotic stress: role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front environ Sci 3:13
Karmous I, Jaouani K, Chaoui A, El Ferjani E (2012) Proteolytic activities in Phaseolus vulgaris cotyledons under copper stress. Physiol Mol Biol Plants 18(4):337–343
Kinraide TB (1998) Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol 118(2):513–520
Lam E (2004) Controlled cell death, plant survival and development. Nat Rev Mol Cell Biol 5:305–315
Leist M, Single B, Castoldi AF, Kühnle S, Nicotera P (1997) Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med 185(8):1481–1486
Levine RL, Williams J, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Leόn AM, Palma JM, Corpas FJ, Gόmez M, Romero-Puertas MC, Chatterjee D, Mateos RM, Del Río LA, Sandalio LM (2002) Antioxidative enzymes in cultivars of pepper plants with different sensitivity to cadmium. Plant Physiol Biochem 40:813–820
Lozano RM, Wong JH, Yee BC, Peters A, Kobrehel K, Buchanan BB (1996) New evidence for a role for thioredoxin h in germination and seedling development. Planta 200:100–106
Matsumura H, Miyachi S (1980) Cycling assay for nicotinamide adenine dinucleotides. Methods Enzymol 69:465–470
Meng H, Hua S, Shamsi IH, Jilani G, Li Y, Jiang L (2009) Cadmium-induced stress on the seed germination and seedling growth of Brassica napus L., and its alleviation through exogenous plant growth regulators. Plant Growth Regul 58:47–59
Noctor G, Queval G, Gakière B (2006) NAD(P) synthesis and pyridine nucleotide cycling in plants and their potential importance in stress conditions. J Exp Bot 57(8):1603–1620
Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J 32:539–548
Potters G, Horemans N, Jansen MA (2010) The cellular redox state in plant stress biology: a charging concept. Plant Physiol Biochem 48:292–300
Rahoui S, Ben C, Chaoui A, Martinez Y, Yamchi A, Rickauer M, Gentzbittel L, El Ferjani E (2014) Oxidative injury and antioxidant genes regulation in cadmium-exposed radicles of six contrasted Medicago truncatula genotypes. Environ Sci Pollut Res 21(13):8070–8083
Rahoui S, Chaoui A, Ben C, Rickauer M, Gentzbittel L, El Ferjani E (2015) Effect of cadmium pollution on mobilization of embryo reserves in seedlings of six contrasted Medicago truncatula lines. Phytochemistry 111:98–106
Reinheckel T, Sitte N, Ullrich O, Kuckelkorn U, Davies KJ, Grune T (1998) Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J 335:637–642
Romero-Puertas MC, Rodríguez-Serrano M, Corpas FJ, Gómez M, Del Río LA, Sandalio LM (2004) Cadmium-induced subcellular accumulation of O ·−2 and H2O2 in pea leaves. Plant Cell Environ 27:1122–1134
Scharte J, Schön H, Tjaden Z, Weis E, Von Schaewen A (2009) Isoenzyme replacement of glucose-6-phosphate dehydrogenase in cytosol improves stress tolerance in plants. Proc Natl Acad Sci USA 106(19):8061–8066
Sun Q, Wang XR, Ding SM, Yuan XF (2005) Effects of exogenous organic chelators on phytochelatins production and its relationship with cadmium toxicity in wheat (Triticum aestivum L.) under cadmium stress. Chemosphere 60:22–31
Tian S, Lu L, Zhang J, Wang K, Brown P, He Z, Liang J, Yang X (2011) Calcium protects roots of Sedum alfredii H. against cadmium-induced oxidative stress. Chemosphere 84:63–69
Valderrama R, Corpas FJ, Carreras A, Gómez-Rodríguez MV, Chaki M, Pedrajas JR, Fernández-Ocaña A, Del Río LA, Barroso JB (2006) The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant Cell Environ 29(7):1449–1459
Van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141:384–390
Van Engelen DL, Sharpe-Pedler RC, Moorhead KK (2007) Effect of chelating agents and solubility of cadmium complexes on uptake from soil by Brassica juncea. Chemosphere 68:401–408
Wan G, Najeeb U, Jilani G, Naeem MS, Zhou W (2011) Calcium invigorates the cadmium-stressed Brassica napus L. plants by strengthening their photosynthetic system. Environ Sci Pollut Res 18(9):1478–1486
Wang CQ, Song H (2009) Calcium protects Trifolium repens L. seedlings against cadmium stress. Plant Cell Rep 28:1341–1349
Xu W, Li Y, He J, Ma Q, Zhang X, Chen G, Wang H, Zhang H (2010) Cd uptake in rice cultivars treated with organic acids and EDTA. J Environ Sci 22(3):441–447
Zhao Z, Hu X, Ross CW (1987) Comparison of tissue preparation methods for assay of nicotinamide coenzymes. Plant Physiol 84(4):987–988
Ziegler M (2005) A vital link between energy and signal transduction. Regulatory functions of NAD(P). FEBS J 272(18):4561–4564
Acknowledgments
This work was financially supported by the Tunisian Ministry of Higher Education and Scientific Research (UR11ES32) and by Graduate School of Environmental and Life Science, Okayama University (Japan). The authors wish to thank Mr. Bechir Azib and Mr. Abbes Oucherine for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakouhi, L., Rahoui, S., Ben Massoud, M. et al. Calcium and EGTA Alleviate Cadmium Toxicity in Germinating Chickpea Seeds. J Plant Growth Regul 35, 1064–1073 (2016). https://doi.org/10.1007/s00344-016-9605-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9605-2